ORIGINAL REPORT
Evaluating the Real-World Effectiveness of Systemic Treatments in Atopic Dermatitis Using the Atopic Dermatitis Control Tool (ADCT): A Multi-Centre, Prospective Study
Hyun Ji LEE1, Yuri WOO2, Young Bok LEE3, Ji Hye LEE4, Jung Eun KIM5, Ji Hyun LEE6 and Sang Hyun CHO2
1Department of Dermatology, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 2Department of Dermatology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 3Department of Dermatology, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 4Department of Dermatology, St Vincent’s Hospital, College of Medicine, The Catholic University of Korea, 5Department of Dermatology, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, and 6Department of Dermatology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Atopic dermatitis is a chronic skin disease affecting quality of life, sleep, and mental health. Traditional evaluation methods focus on clinical assessments, but there is a growing need for tools that incorporate patient-reported outcomes (PROs). To evaluate the effectiveness of the Atopic Dermatitis Control Tool (ADCT) in assessing disease severity in patients with moderate to severe atopic dermatitis and to compare the efficacy of systemic immunosuppressants and dupilumab in patients with moderate to severe atopic dermatitis. A prospective, observational study was conducted across seven centres in Korea, involving 112 patients with moderate to severe atopic dermatitis. The ADCT, Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI) were used for assessing atopic dermatitis severity. In addition, the study assessed the effectiveness of immunosuppressants and dupilumab over the course of one year. The study found significant correlations between ADCT scores and other severity measures (EASI, DLQI). The correlation coefficients were 0.54 (p < 0.0001) for ADCT vs EASI and 0.83 (p < 0.0001) for ADCT vs DLQI. Furthermore, patients treated with dupilumab exhibited greater improvement compared with those on cyclosporine, as measured by the ADCT (adjusted OR [95% CI]); 6.98 [2.49, 19.58]). The ADCT effectively captures subjective aspects compared with the EASI and can be used practically and effectively in clinical settings of atopic dermatitis.
SIGNIFICANCE
This study highlights the importance of listening to patients’ experiences in managing atopic dermatitis, a skin condition that affects daily life, sleep, and mental well-being. By using the Atopic Dermatitis Control Tool, which captures how patients feel about their symptoms, we can better understand and track the effectiveness of treatments. Our findings show that the Atopic Dermatitis Control Tool is a practical tool for evaluating atopic dermatitis severity and that dupilumab treatment offers significant benefits over other options. This research can help improve care for people with atopic dermatitis by emphasizing treatment options that support both clinical outcomes and patient quality of life.
Key words: atopic dermatitis; Atopic Dermatitis Control Tool; dupilumab; cyclosporine.
Citation: Acta Derm Venereol 2025; 105: adv40704. DOI: https://doi.org/10.2340/actadv.v105.40704.
Copyright: © 2025 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: May 2, 2024; Accepted after revision: Nov 13, 2024; Published: Feb 12, 2025.
Corr: Ji Hyun Lee, Department of Dermatology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea and Sang Hyun Cho, Department of Dermatology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. E-mails: ejee@catholic.ac.kr; drchosh@hotmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
This study was supported by Sanofi Korea.
INTRODUCTION
A topic dermatitis (AD) is a chronic, relapsing inflammatory skin disease with increasing prevalence and significant impact on health-related quality of life (HRQoL), sleep disturbance, and mental health (1). Considering the impact of AD on the quality of life for patients, their family, and their community, holistic measurements of AD severity including patient-reported outcomes (PRO) have been significantly important to control AD disease (2, 3). In addition, to overcome the chronic nature of AD, communication between clinicians and patients with discussion of objective measurements from clinicians and subjective reporting from patients is important in AD control (4).
In clinical practice, objective assessments like the Eczema Area and Severity Index (EASI), SCORing Atopic Dermatitis (SCORAD), or Investigator’s Global Assessment (IGA) are commonly used. PRO tools, such as the Dermatology Life Quality Index (DLQI) and Patient-Oriented Eczema Measure (POEM), are less frequently employed due to their complexity and time-consuming nature. This gap highlights the need for simpler, more efficient PRO tools like the Atopic Dermatitis Control Tool (ADCT), developed in 2019 (4). That tool features 6 questions that assess the overall severity of symptoms, frequency of severe itching episodes, level of discomfort, sleep disturbances, disruption to daily activities, and emotional or mood impacts (https://www.adcontroltool.com/). The total possible score on the ADCT can vary from 0 to 24. Scores of 7n or greater signify patients who are “not in control” (5).
Moderate to severe AD presents a significant challenge in dermatological practice. In the United States, the American Academy of Dermatology (AAD) emphasizes a stepwise treatment protocol, starting with robust skin care routines and topical treatments, including corticosteroids and calcineurin inhibitors. For cases resistant to topical therapies, systemic treatments such as immunosuppressants (IS) (cyclosporine, methotrexate, mycophenolate mofetil, and azathioprine) and the biologic agent dupilumab are recommended. Phototherapy may also be considered in cases of extensive AD or when other treatments fail (6). The European guidelines similarly advocate for foundational use of emollients and topical treatments, with an added emphasis on the critical role of patient education and psychological support. The updated European guidelines from 2018 discuss systemic treatment options that include conventional immunosuppressive drugs (such as azathioprine, cyclosporine, glucocorticosteroids, methotrexate, and mycophenolate mofetil), biologics (including dupilumab, lebrikizumab, nemolizumab, omalizumab, and tralokinumab), and Janus kinase inhibitors (abrocitinib, baricitinib, and upadacitinib) as recommended treatments (7). In 2019, the Korean Atopic Dermatitis Association (KADA) published Consensus Korean Diagnostic Guidelines to define severity classifications, with moderate to severe AD including objective and subjective assessments (8). Systemic treatments such as IS, biologics, and JAK inhibitors are the standard of care for managing moderate to severe AD (9). In the 2021 updated Japanese guidelines, dupilumab and baricitinib were newly added for treatment of severe AD (10). In recent US guidelines, the systemic therapies that have been strongly recommended include dupilumab, tralokinumab, abrocitinib, baricitinib, and upadacitinib, while conventional treatments including cyclosporine were conditionally recommended (11). Due to considerations of cost-effectiveness and insurance coverage, along with preferences of both patients and physicians, cyclosporine and dupilumab have been the most frequently used treatment in Korea. The clinical efficacy of IS in moderate to severe AD was supported in several studies (12). However, systemic IS, while they have a broad spectrum of action compared with targeted therapies such as biologics, may lead to serious side effects such as liver and kidney dysfunction. These side effects or concerns about them can reduce patient treatment satisfaction and treatment compliance.
There is a clear unmet need for academic evidence comparing the effectiveness of these systemic treatments in real-world practice, particularly reflecting PRO. Therefore, this study aims to assess and to evaluate real-world effectiveness by leveraging the ADCT in Korean patients with moderate to severe AD.
METHODS
Study design
A prospective, multi-centre, non-controlled, open-label, non-randomized, longitudinal observational study was conducted across 7 centres in Korea. The study involved 112 patients diagnosed with moderate to severe AD to assess the effectiveness of systemic therapy, especially cyclosporine and dupilumab for one year.
Study population
Subjects were Korean patients older than 12 years, diagnosed with moderate to severe AD as per the 2019 Korean Consensus AD Diagnostic Guidelines. Moderate AD is defined as EASI score ≥16 or EASI score < 16 with at least 1 of the following: itch with NRS score ≥7 or DLQI >10. Severe AD is defined as EASI score ≥23 or 16 ≤EASI score ≥23 with at least 1 of the following: Itch with NRS score ≥7 or DLQI >10.
Inclusion and exclusion criteria
Inclusion criteria comprised patients diagnosed with moderate to severe AD by dermatologists before enrolment as per the 2019 Korean Consensus AD Diagnostic Guidelines, the ability to read and write Korean, and the ability to provide written informed consent. Exclusion criteria were patients younger than 11 years; other active, severe skin disease or major comorbidity other than eczema; and uncertain AD diagnosis. Patients who had initiated treatment with dupilumab or who were previously enrolled in other interventional trials also were excluded.
Variables and data collection
This study involved a 12-month prescription period of AD treatment for eligible patients, including options of cyclosporine for systemic IS or dupilumab (200–300 mg administered subcutaneously every 2 weeks). Subjects underwent measurements of baseline demographics (including age, gender, and age at AD diagnosis); clinical characteristics (such as atopic comorbidities, medical history before and after dupilumab or cyclosporine initiation, and any concurrent therapies); and scores for EASI, DLQI, and ADCT during their first visit. Subsequently, they visited every 4 months to repeat EASI, DLQI, and ADCT assessment, with a total of 4 measurement dates for each score. In cases where a patient switched from the dupilumab treatment group to the cyclosporine treatment group, or vice versa, the date of medication change was defined as an ad hoc visit, and the following visit 4 months later will be defined as the next visit, with a total of 5 measurements dates. The evaluation criteria for severity of AD encompassed a range of data collection methods: patient self-assessment using the ADCT (0–24, higher scores indicating poorer management of the disease), DLQI (0–30, higher scores indicating worse HRQoL), and physician assessments with the EASI (0–72, higher scores indicating more severe eczema), both at baseline and at each subsequent visit.
Statistical analysis
The meaningful improvement of AD evaluated by the ADCT was analysed using logistic regression. Changes in each evaluation tool during subject visits were analysed with a p for trend. The correlations between ADCT and the EASI and between ADCT and the DLQI were measured using Pearson’s correlation coefficient. A value closer to 1 indicates a stronger correlation. A p-value less than 0.05 was considered statistically significant. All data analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC, USA).
RESULTS
Study subjects and patient characteristics
Initially, 111 patients were registered for the study. After the first visit, 18 cases were lost to follow-up. Additionally, 2 cases were excluded due to missing data. Ultimately, 91 patients were enrolled in the final study. Of these, 43 patients were treated with dupilumab and 48 were administered cyclosporine. There were no significant differences between the 2 treatment groups in terms of age, gender, BMI, or atopic comorbidities (Table I).
Severity measures and correlations between EASI, DLQI, and ADCT
Our study’s primary focus was to evaluate the effectiveness of the ADCT in assessing disease severity and control in patients with moderate to severe AD. Fig. 1 examines the correlations between EASI and ADCT and between DLQI and ADCT. The correlation coefficients were 0.54 (p < 0.0001) for ADCT vs EASI and 0.83 (p < 0.0001) for ADCT vs DLQI, both of which were significant.
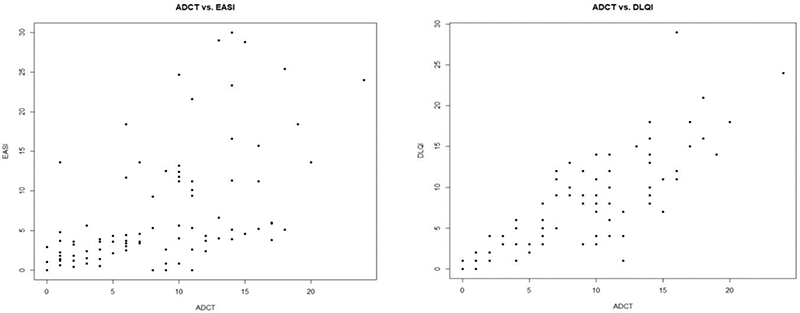
Fig. 1. Correlation between Eczema Area and Severity Index (EASI) and Atopic Dermatitis Control Tool (ADCT), and correlation between Dermatology Life Quality Index (DLQI) and ADCT.
Fig. 2 highlights the meaningful improvement of AD evaluated by ADCT. Notably, the proportion of patients experiencing significant improvement in AD, as defined by ADCT, was considerably higher in the dupilumab treatment group compared with the cyclosporine treatment group. The odds ratio (95% CI) was 6.98 (2.49, 19.58) after adjustments for age, sex, and atopic comorbidities. Meaningful improvement was defined as ADCT < 7 at the final visit or a decrease of 5 or more points in ADCT score from the first visit. Fig. S1 shows the percentage of patients reporting improvement in individual ADCT categories evaluated by χ2 test following treatment with either cyclosporine or dupilumab. The results are based solely on the initial assessment of discomfort using these categories. χ2 testing revealed significantly better outcomes for dupilumab with adjusted OR (95% CI) as follows: overall severity of symptoms (p = 0.04), level of discomfort (p < 0.01), sleep disturbances (p = 0.02), disruption to daily activities (p < 0.01), and emotional or mood impacts (p < 0.01), underscoring the enhanced effectiveness of dupilumab in the management of AD.
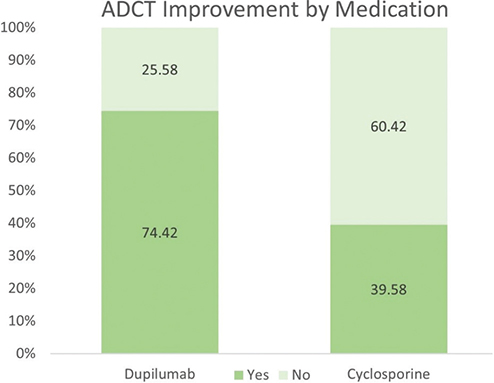
Fig. 2. Meaningful improvement of atopic dermatitis (AD) evaluated by Atopic Dermatitis Control Tool (ADCT). The proportion of patients with significant AD improvement measured by ADCT was markedly higher in the dupilumab treatment group than in the cyclosporine treatment group. Odd ratio (95% CI) = 6.98 (2.49,19.58), adjusted by age, sex, and atopic comorbidities. Meaningful improvement of AD is defined by ADCT < 7 at last visit or decreasing 5 or more on ADCT from first visit.
Fig. 3 presents the mean scores of EASI, DLQI, and ADCT at each study visit. The graph shows the higher effectiveness of dupilumab compared with that of cyclosporine. When measured with EASI, the dupilumab group exhibited an improvement of 82.8% by the fourth visit compared with the first visit, whereas the cyclosporine group demonstrated an improvement of 53.2%. When measured with DLQI, the dupilumab group improved by 51.7% and the cyclosporine group by 36.8%. Measured with ADCT, the dupilumab group improved by 52.6%, while the cyclosporine group improved by 32.1%. Fig. 4 illustrates the proportion of patients achieving EASI-50, EASI-75, and ADCT < 7 in each treatment group at each visit. In the dupilumab treatment group, the p-values for trend for EASI-50, EASI-75, and ADCT were < 0.01, < 0.01, and 0.41, respectively. In the cyclosporine treatment group, these values were < 0.01, < 0.01, and 0.03, respectively.
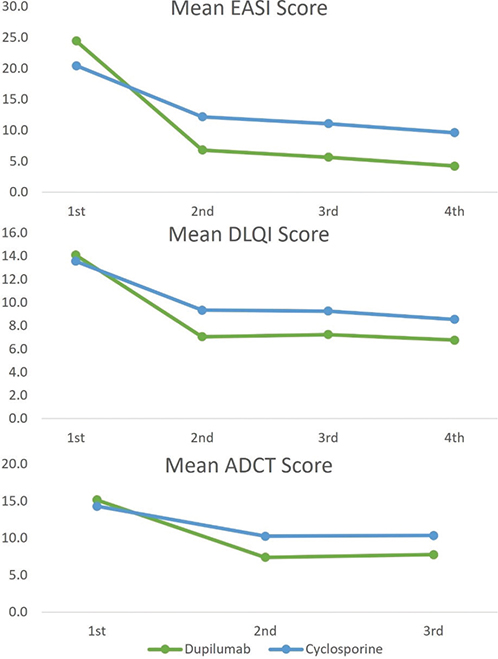
Fig. 3. Mean Eczema Area and Severity Index (EASI), Dermatology Life Quality Index (DLQI), and Atopic Dermatitis Control Tool (ADCT) score at each visit.
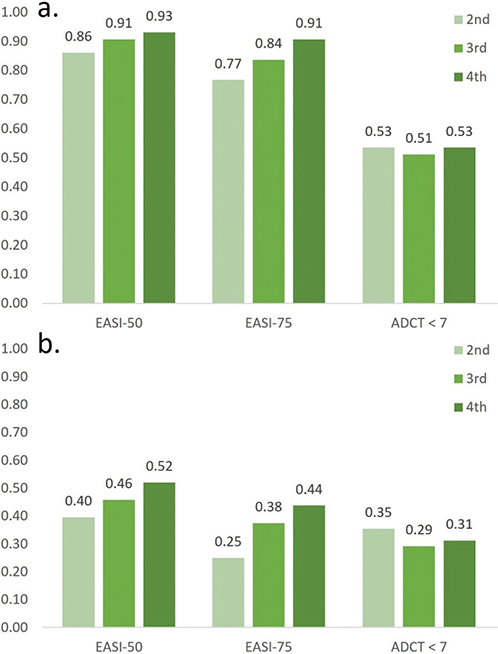
Fig. 4. Proportion of patients achieving EASI-50, EASI-75 and ADCT < 7 at each visit. (a) Dupilumab treatment group. The p for trend of EASI-50, EASI-75, and ADCT is < 0.01, < 0.01, and 0.41, respectively. (b) Cyclosporine treatment group. The p for trend of EASI-50, EASI-75, and ADCT is < 0.01, < 0.01, and 0.03, respectively.
DISCUSSION
Our study’s key contribution lies in the establishment of the ADCT as a reliable tool that correlates with the widely used severity evaluation tools EASI and DLQI while also being simple and effective for evaluating the severity of AD, especially in moderate to severe AD patients with systemic treatments. The correlation coefficients were 0.54 (p < 0.01) for ADCT vs EASI and 0.83 (p < 0.01) for ADCT vs DLQI, indicating stronger significant correlation of ADCT with DLQI. This result suggests that the ADCT is particularly effective in reflecting patient quality of life and disease control.
According to available studies, the effectiveness of the ADCT is its capture of multidimensional aspects of disease burden that can influence AD, further validating its comprehensive application in clinical settings (4, 5, 13). Examining the studies published so far that validate the ADCT, Kido Nakahara et al. (14) demonstrated ADCT correlations with other clinical measures of the POEM, the itch-NRS, and the EASI. The reported correlation coefficients were r = 0.885, p < 0.001; r = 0.860, p < 0.001; and r = 0.568, p < 0.001, respectively, The high correlations between ADCT and the POEM and the Itch-NRS indicate their effectiveness in reflecting the subjective symptoms of patients. Similarly, in our study, we observed a higher correlation with the DLQI, another PROM that captures subjective symptoms. This underscores consistent findings across studies: subjective measurements of POEM, Itch-NRS, and DLQI are highly correlated with ADCT. These results affirm that patient-reported ADCT alone is sufficient when assessing and managing AD and underscores its value in clinical practice.
In a parallel investigation in Japan, an online cross-sectional observational study was conducted to evaluate the ADCT among individuals with physician-diagnosed AD (15). The study, involving 5,970 participants from a pool of 14,097, revealed significant correlations between the ADCT and DLQI, POEM, Worst Itch Numerical Rating Scale (WI-NRS), and Global Questions (GQ) scores. Notably, the ADCT demonstrated strong correlations with the DLQI, POEM, and WI-NRS (all r = 0.74) and a moderate correlation with GQ score (r = 0.61). This finding echoes the results of our study, emphasizing the consistent reliability of ADCT across populations. Complementing these findings, a study at Peking University First Hospital involving 114 patients validated the Chinese version of the ADCT (16). This research used a range of assessment tools, including the ADCT, POEM, PP-NRS, and DLQI. The correlation coefficients between the ADCT and these measurements – 0.805 with POEM, 0.861 with PP-NRS, and 0.709 with DLQI – align closely with those observed in both our study and the Japanese study. These studies all support the ADCT as a simple yet effective evaluation tool, offering a valid, consistent, and reliable method to evaluate AD control, combining both clinician and patient perspectives.
In addition, studies have shown that patients treated with dupilumab exhibit significantly greater improvements in AD severity as measured by ADCT, EASI, and DLQI compared with patients treated with cyclosporine, demonstrating that dupilumab is an effective treatment. As far as we know, there has been no head-to-head comparison study between the efficacy of cyclosporine and dupilumab (17). In our real-world study, when measured by ADCT, the results of the logistic regression analysis demonstrated that the odds ratio of the improvement in the dupilumab group compared with the cyclosporine group was 6.98 (95% CI: 2.49, 19.58). The proportion of patients achieving EASI-75 was 91% in the dupilumab group and 44% in the cyclosporine group, a significant difference. When measured with DLQI, the dupilumab group improved by 51.7% and the cyclosporine group by 36.8%.
The superior efficacy of dupilumab over cyclosporine in treating moderate-to-severe AD can be attributed to several factors. In terms of efficacy over time, cyclosporine provides a rapid initial response at a high dose (5 mg/kg/day). However, as the dose is gradually tapered to a maintenance level (2–3 mg/kg/day) after 3–6 weeks, a decline in efficacy can be often observed. This dose reduction is necessary to mitigate potential side effects but may result in reduced disease control. In contrast, dupilumab is administered at a stable dosage of 300 mg every 2 weeks without the need for dose adjustments. This consistent dosing regimen ensures sustained efficacy over time, contributing to superior long-term outcomes compared with cyclosporine. Moreover, dupilumab offers a distinct advantage over cyclosporine in modulating the skin microbiome. Recent studies have demonstrated that dupilumab, but not cyclosporine, shifts the skin microbiome of patients with moderate-to-severe AD toward a healthier composition (18). Dupilumab treatment significantly reduces the relative abundance of Staphylococcus aureus – a known driver of AD inflammation – while increasing the prevalence of Staphylococcus hominis, a beneficial commensal species. This suggests that dupilumab’s targeting of the IL-4 and IL-13 pathways not only controls disease severity but also helps restore a microbial balance that resembles the skin flora of healthy individuals. In contrast, cyclosporine did not produce such changes, further highlighting dupilumab’s broader immunomodulatory effects and its potential to improve the underlying pathogenic mechanisms of AD beyond clinical symptom reduction.
ADCT is an efficient tool that can differentiate the degree of improvement between items. Among the ADCT items, overall severity of symptoms, level of discomfort, sleep disturbances, disruption of daily activities, and emotional or mood impacts exhibited significant improvement in the dupilumab group compared with the cyclosporine group. The results suggest that dupilumab not only improves overall disease severity, but may also have differential impacts on specific aspects of the disease, such as alleviating sleep disturbances (19, 20), improving health-related quality of life (21, 22), or eliminating psychiatric issues (23), consistent with previous studies. This could imply a broader, more holistic improvement in the quality of life for patients treated with dupilumab.
While this study presents significant insights into the efficacy of the ADCT, it is important to acknowledge the study limitations. One primary limitation is the non-randomized design of the study, which may introduce selection bias. Additionally, the study’s focus on the Korean population may limit the generalizability of the findings to other ethnic or demographic groups. Furthermore, the observational nature of the study may not fully control for all confounding factors that could influence the outcomes. Despite these limitations, our study possesses several notable strengths. It provides a head-to-head comparison between dupilumab and cyclosporine treatments, offering direct comparative data that are often lacking in other studies. This approach allows a more nuanced understanding of the relative efficacy of these treatments in real-world settings. Moreover, our study targets moderate to severe AD patients, ensuring that the findings are particularly relevant to this more severely affected patient population.
Critically, the results demonstrate that the ADCT is an effective tool for assessing disease control in this demographic. Its ability to capture both objective clinical measures and subjective PROs makes it uniquely suited for a holistic evaluation of AD. By providing a comprehensive assessment tool that resonates with both clinicians and patients, the ADCT stands out as a crucial instrument in optimizing treatment strategies and enhancing patient care in AD.
ACKNOWLEDGEMENTS
IRB approval status: Reviewed and approved by the Institutional Review Board of the Catholic University of Korea. Approval # XC21QIDV0070.
The patients in this study have given written informed consent to publication of their case details.
REFERENCES
- Fishbein AB, Silverberg JI, Wilson EJ, Ong PY. Update on atopic dermatitis: diagnosis, severity assessment, and treatment selection. J Allergy Clin Immunol Pract 2020; 8: 91–101. https://doi.org/10.1016/j.jaip.2019.06.044
- Wei W, Anderson P, Gadkari A, Blackburn S, Moon R, Piercy J, et al. Discordance between physician- and patient-reported disease severity in adults with atopic dermatitis: a US cross-sectional survey. Am J Clin Dermatol 2017; 18: 825–835. https://doi.org/10.1007/s40257-017-0284-y
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014; 134: 800–807. https://doi.org/10.1016/j.jaci.2014.07.043
- Pariser DM, Simpson EL, Gadkari A, Bieber T, Margolis DJ, Brown M, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr Med Res Opin 2020; 36: 367–376. https://doi.org/10.1080/03007995.2019.1699516
- Simpson E, Eckert L, Gadkari A, Mallya UG, Yang M, Nelson L, et al. Validation of the Atopic Dermatitis Control Tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol 2019; 19: 15. https://doi.org/10.1186/s12895-019-0095-3
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. https://doi.org/10.1016/j.jaad.2014.03.023
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. https://doi.org/10.1111/jdv.14891
- Kim JE, Shin MK, Park GH, Lee UH, Lee JH, Han TY, et al. 2019 Consensus Korean diagnostic guidelines to define severity classification and treatment refractoriness for atopic dermatitis: objective and subjective assessment of severity. Ann Dermatol 2019; 31: 654–661. https://doi.org/10.5021/ad.2019.31.6.654
- Lee JH, Kim JE, Park GH, Bae JM, Byun JY, Shin MK, et al. Consensus update for systemic treatment of atopic dermatitis. Ann Dermatol 2021; 33: 497–514. https://doi.org/10.5021/ad.2021.33.6.497
- Saeki H, Ohya Y, Furuta J, Arakawa H, Ichiyama S, Katsunuma T, et al. English version of Clinical Practice Guidelines for the Management of Atopic Dermatitis 2021. J Dermatol 2022; 49: e315–e375. https://doi.org/10.1111/1346-8138.16527
- Davis DMR, Drucker AM, Alikhan A, Bercovitch L, Cohen DE, Darr JM, et al. Executive summary: Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol 2024; 90: 342–345. https://doi.org/10.1016/j.jaad.2023.08.103
- Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014; 133: 429–438. https://doi.org/10.1016/j.jaci.2013.07.049
- Staumont-Sallé D, Taieb C, Merhand S, Shourick J. The Atopic Dermatitis Control Tool: a high-performance tool for optimal support. Acta Derm Venereol 2021; 101: adv00618. https://doi.org/10.2340/actadv.v101.750
- Kido-Nakahara M, Yokote G, Yoshida M, Furue M, Nakahara T. Atopic Dermatitis Control Tool (ADCT): a useful tool for self-evaluation in patients with atopic dermatitis. J Dermatol 2021; 48: 1951–1952. https://doi.org/10.1111/1346–8138.16176
- Nakahara T, Fujita H, Tajima Y, Arima K. Evaluating the usefulness of the Atopic Dermatitis Control Tool for assessing disease control in individuals with atopic dermatitis in Japan. Br J Dermatol 2023; 190: 123–125. https://doi.org/10.1093/bjd/ljad344
- Luan TT, Peng CY, Song XT, Liao SL, Zhao ZT. [Validation study of the Chinese version of atopic dermatitis control tool]. Zhonghua Yu Fang Yi Xue Za Zhi 2023; 57: 422–426.
- Wallace DV. Treatment options for moderate to severe atopic dermatitis. Allergy Asthma Proc 2022; 43: 474–493. https://doi.org/10.2500/aap.2022.43.220076
- Hartmann J, Moitinho-Silva L, Sander N, Harder I, Häsler R, Rodriguez E, et al. Dupilumab but not cyclosporine treatment shifts the microbiome toward a healthy skin flora in patients with moderate-to-severe atopic dermatitis. Allergy 2023; 78: 2290–2300. https://doi.org/10.1111/all.15742
- Merola JF, Chiou AS, During E, Costanzo A, Foley P, Alfalasi A, et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: results from the 12-week placebo-controlled period of the 24-week phase IV randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol 2023; 189: 685–694. https://doi.org/10.1093/bjd/ljad284
- Milanesi N, Gola M, Cartocci A, Tronconi G, Bruzziches F, Flori ML, et al. Effect of dupilumab on sleep disturbances in adult patients with severe atopic dermatitis. Ital J Dermatol Venerol 2022; 157: 142–145. https://doi.org/10.23736/S2784-8671.21.07072-9
- Simpson EL. Dupilumab improves general health-related quality-of-life in patients with moderate-to-severe atopic dermatitis: pooled results from two randomized, controlled phase 3 clinical trials. Dermatol Ther (Heidelb) 2017; 7: 243–248. https://doi.org/10.1007/s13555-017-0181–6
- Lee SE, Hopkins C, Mullol J, Msihid J, Guillemin I, Amin N, et al. Dupilumab improves health related quality of life: results from the phase 3 SINUS studies. Allergy 2022; 77: 2211–2221. https://doi.org/10.1111/all.15222
- Miniotti M, Lazzarin G, Ortoncelli M, Mastorino L, Ribero S, Leombruni P. Impact on health-related quality of life and symptoms of anxiety and depression after 32 weeks of dupilumab treatment for moderate-to-severe atopic dermatitis. Dermatol Ther 2022; 35: e15407. https://doi.org/10.1111/dth.15407