QUIZ SECTION
A Woman with Red Oedematous Erythema of the Face: A Quiz
Hong-yang ZHANG1 and Zheng-xiu LI2*
1Department of Otolaryngological Medicine, and 2Department of Dermatology, The First Hospital of China Medical University, 155N Nanjing Street, Heping District, Shenyang 110001, China. *E-mail: lizx_cmu@foxmail.com
Citation: Acta Derm Venereol 2024; 104: adv40766. DOI https://doi.org/10.2340/actadv.v104.40766.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Published: August 7, 2024
Competing interests and funding: This study was supported by a grant from the National Science Foundation of China (No.82103747).
A woman in her 40s presented with a 2-month history of oedematous erythema, exudation, crusting, and scales distributed symmetrically on her superciliary arch with evidence of pruritus. She reported that these lesions initially presented 2 months previously as small erythematous papules on her eyelid bilaterally (Fig. 1). She had visited a local clinic where she received a diagnosis of allergic dermatitis. She was given antihistamines and topical steroids, but this treatment failed to help. Her lesions rapidly progressed to the oedematous erythema, exudation, crusts, and scales observed on presentation, with exudation being evident 1 month previously.
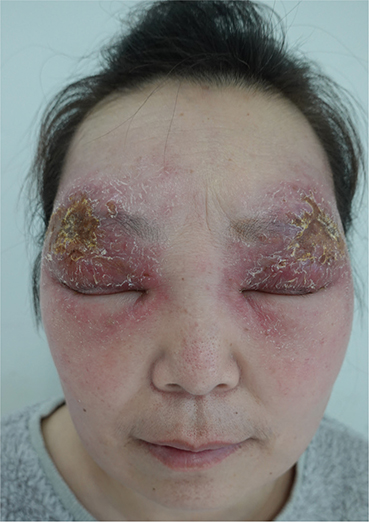
Fig. 1. Oedematous, exudative erythema with thin yellowish crusts and scales bilaterally on the eyebrow arches, eyelids, and temples.
Approximately 3 years before presentation, the patient began to experience mild shortness of breath when fatigued, but she did not receive any associated diagnosis.
Physical examination revealed oedematous, exudative erythema with thin yellowish crusts and scales bilaterally on the eyebrow arches, eyelids, and temples. Palpebral fissure narrowing was evident, together with enlargement of the bilateral cervical and inguinal lymph nodes that was painless on palpation.
Laboratory testing revealed elevated angiotensin-converting enzyme (ACE) levels of 222.0 U/L (Reference range: 5–52 U/L), while her liver enzymes were within normal ranges, as were both antinuclear antibody and TSPOT. TB test results were negative. Serology results were negative for both treponemal and non-treponemal tests. Chest computed tomography (CT) revealed nodular interstitial lung disease and hilar lymphadenopathy.
Histopathologic examination revealed nodular aggregations of epithelioid histiocytes with a mild surrounding lymphocytic infiltrate, extending from the deep dermis into the subcutis (Fig. 2). Periodic acid-Schiff stains were negative. The culture from skin biopsy specimen with microbial stains for bacteria, fungal culture, and acid-fast bacilli was negative. A biopsy of the right cervical lymph node reveals granulomatous nodules with epithelioid cells and multinucleated giant cells. Acid-fast staining was negative. Findings from sputum acid-fast bacilli stains were also negative.
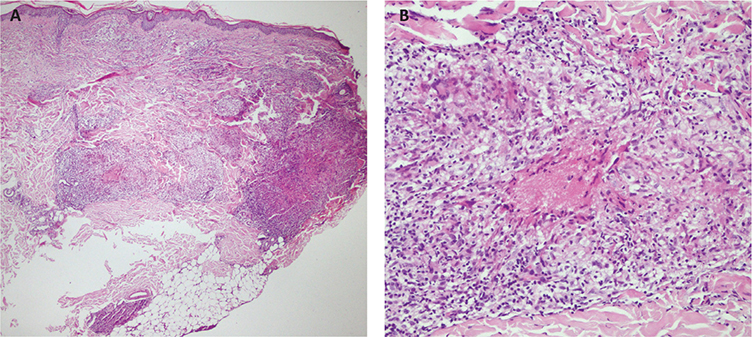
Fig. 2. Nodular aggregations of epithelioid histiocytes with a mild surrounding lymphocytic infiltrate, extending from the deep dermis into the subcutis.
What is your diagnosis?
Differential diagnosis 1: Cutaneous sarcoidosis
Differential diagnosis 2: Cutaneous lymphoma
Differential diagnosis 3: Atypical mycobacterial infection
Differential diagnosis 4: Discoid lupus erythematosus (DLE)
See next page for answer.
ANSWERS TO QUIZ
A Woman with Red Oedematous Erythema of the Face: A Commentary
Diagnosis: Cutaneous sarcoidosis
Sarcoidosis is a multisystemic granulomatous disease of unknown origin that results in the development of non-caseating granulomatous lesions within impacted tissues and organs. The most commonly affected sites include the spleen, liver, skin, lymph nodes, and eyes. Sarcoidosis is more common among women and its onset peaks in individuals 25–40 years of age as well as in individuals over 50 years of age (1). Approximately 30% of patients initially present with cutaneous lesions that ultimately affect an estimated 20–35% of individuals with sarcoidosis (2). There are several important reasons why sarcoidosis should be taken into consideration for the differential diagnosis of patients with consistent symptoms. For one thing, cutaneous sarcoidosis is often an early symptom of this disease and its presence underscores the need for further testing to assess the potential for systemic involvement irrespective of the extent of cutaneous involvement. The skin can also readily be sampled to enable histologic diagnosis, allowing affected patients to be more efficiently and reliably diagnosed than patients with other subtypes of sarcoidosis. Second, the presentation of cutaneous sarcoidosis may have implications for patient treatment and prognosis, as certain forms of this disease are related to subsequent systemic disease progression, particularly in cases of erythema nodosum. Lastly, sarcoidosis has the potential to be associated with certain forms of malignancies such that a detailed diagnostic workup is warranted (3).
The presentation of cutaneous sarcoidosis can be highly variable, such that it can imitate a wide array of other skin diseases. As a result, diagnosing atypical cases can be a clinical challenge. The presence or absence of classic granulomas is used to classify cutaneous sarcoidosis lesions histopathologically into those that are specific and non-specific. Non-specific lesions lack classic granulomas and most commonly present in the form of reactive erythema nodosum, although other presentations can include erythema multiforme, nail clubbing, prurigo, or calcifications. Specific lesions can include plaques, papules, maculopapules, nodules, alopecia, ulcerative lesions, infiltrative scars, subcutaneous nodules, lupus pernio, or hypopigmentation.
Histopathological evaluation remains the gold standard means of diagnosing cutaneous sarcoidosis, with affected patients generally exhibiting classic non-caseating granulomatous inflammation of the dermal or subcutaneous tissue together with limited lymphocytic infiltration (3). As sarcoidosis is ultimately an exclusionary diagnosis, other granulomatous diseases must be excluded. In one study, tuberculosis patients were found to exhibit an 8.08-fold increased risk of sarcoidosis development relative to the general populace, such that the potential for comorbid sarcoidosis and tuberculosis cannot be excluded (4). Approximately 26% of biopsies exhibit foreign bodies such that these foreign bodies cannot be used to exclude cutaneous sarcoidosis as a potential diagnosis (5). Ultimately, clinical, imaging, and histologic findings are necessary to support a sarcoidosis diagnosis, together with the exclusion of other potential diseases, with affected patients exhibiting non-caseating granulomatous inflammation affecting at least 1 organ system.
Patients who present with only cutaneous symptoms of sarcoidosis are relatively rare, with 1 report estimating that at least 70% of patients with cutaneous sarcoidosis also exhibited systemic sarcoidosis without even taking into account those patients who subsequently exhibited systemic involvement at later time points (6). When patients exhibit suspicious lesions or receive a diagnosis of cutaneous sarcoidosis, chest CT, pulmonary, lymph node, and eye exams are warranted, as is the testing of serum ACE levels as a means of assessing potential systemic disease. When patients with cutaneous symptoms do not exhibit any systemic symptoms, the long-term monitoring of these patients is appropriate. While elevated ACE levels are insufficient to support the diagnosis of sarcoidosis, they are nonetheless correlated with systemic involvement as evident in the present case and prior reports. Combining these ACE levels and other factors can provide more robust support to diagnose sarcoidosis (7). In prior reports, CD163 immunostaining has also been shown to offer value as a predictor of systemic sarcoidosis in individuals exhibiting epithelioid granuloma lesions (8).
In our case, the patient exhibited an atypical presentation consisting of oedematous exudative erythema with thin yellowish crusts and scales that resembled those associated with many other cutaneous conditions including facial eczema and discoid lupus erythematosus. Tertiary syphilis should also be considered when dealing with granulomatous lesions. Syphilitic nodules may mimic granulomatous disease. Syphilis should also be considered when approaching granulomatous lesions of unclear nature. In our case, serologic tests for syphilis were negative, thus we ruled out syphilis (9). The patient was diagnosed based on histopathological results, and her lesions responded well to oral corticosteroid treatment that was gradually tapered and discontinued after 18 months.
ACKNOWLEDGEMENT
IRB approval status: Our patient has already signed the patient consent.
REFERENCES
- Karadağ AS, Parish LC. Sarcoidosis: a great imitator. Clin Dermatol 2019; 37: 240–254. https://doi.org/10.1016/j.clindermatol.2019.01.005
- El Jammal T, Jamilloux Y, Gerfaud-Valentin M, Valeyre D, Sève P. Refractory sarcoidosis: a review. Ther Clin Risk Manag 2020; 16: 323–345. https://doi.org/10.2147/TCRM.S192922
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol 2007; 25: 276-287. https://doi.org/10.1016/j.clindermatol.2007.03.004
- Wang SH, Chung CH, Huang TW, Tsai WC, Peng CK, Huang KL, et al. Bidirectional association between tuberculosis and sarcoidosis. Respirology 2019; 24: 467–474. https://doi.org/10.1111/resp.13482
- Mangas C, Fernández-Figueras MT, Fité E, Fernández-Chico N, Sàbat M, Ferrándiz C. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol 2006; 33: 772–777. https://doi.org/10.1111/j.1600-0560.2006.00563.x
- Mañá J, Marcoval J, Graells J, Salazar A, Peyrí J, Pujol R. Cutaneous involvement in sarcoidosis: relationship to systemic disease. Arch Dermatol 1997; 133: 882–888. https://doi.org/10.1001/archderm.1997.03890430098013
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, Pujol RM. The natural history of cutaneous sarcoidosis: clinical spectrum and histological analysis of 40 cases. Int J Dermatol 2019; 58: 178–184. https://doi.org/10.1111/ijd.14218
- Isohisa T, Asai J, Kanemaru M, Arita T, Tsutsumi M, Kaneko Y, et al. CD163-positive macrophage infiltration predicts systemic involvement in sarcoidosis. J Cutan Pathol 2020; 47: 584–591. https://doi.org/10.1111/cup.13675
- Cozzani E, Gasparini G, Ciccarese G, Drago F, Trave I, Vellone V, et al. Concurrent benign tertiary syphilis and asymptomatic neurosyphilis in an immunocompetent patient. J Eur Acad Dermatol Venereol 2021; 35: e151–e152. https://doi.org/10.1111/jdv.16882