REVIEW ARTICLE
Efficacy of Intralesional Candida Injection in the Treatment of Cutaneous Warts: A Systematic Review and Meta-Analysis
Chin-Hsuan CHANG1#, Zih-Yi SUNG1# and Yu-Chen HUANG2–4
1School of Medicine, College of Medicine, Taipei Medical University, Taipei, 2Research Center of Big Data and Meta-analysis, Wan Fang Hospital, Taipei Medical University, Taipei, 3Department of Dermatology, Wan Fang Hospital, Taipei Medical University, Taipei, and 4Department of Dermatology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
#These authors shared co-first authorship, and contributed equally to this work.
Recent studies that examined the treatment efficacy of Candida antigen injection for both non-genital and genital warts yield inconsistent results. To address this, a systematic review and meta-analysis was conducted, comparing the treatment response between Candida antigen injection therapy and other intralesional immunotherapies across all types of warts. PubMed, Cochrane Library, and Embase were searched for relevant randomized controlled trials (RCTs) from inception to 16 September 2023, and 24 eligible RCTs were identified. A protocol was developed using the PRISM A-P checklist. In terms of complete clearance, intralesional Candida injection therapy demonstrated a significant improvement compared with saline (risk ratio [RR] 5.39; 95% confidence interval [CI] 3.49–8.33; I2=0%). However, no statistically significant differences were observed when compared with other therapies such as mumps–measles–rubella vaccines, purified protein derivative, vitamin D3, bivalent human papillomavirus vaccine, and zinc sulphate. Adverse effects associated with intralesional Candida therapy were generally reported as mild and manageable. In conclusion, intralesional Candida injection therapy for cutaneous warts may exhibit a superior complete and distant response rate. Nevertheless, owing to a limited sample size and other limitations, future research should aim for larger studies to provide more conclusive evidence.
Key words: warts; verruca; immunotherapy; candida; meta-analysis.
SIGNIFICANCE
Recent studies on Candida antigen injections for treating warts have shown mixed results. This review compared Candida injections with other treatments by analysing 24 studies. Candida injections were better than saline injections for clearing warts but similar to treatments like MMR vaccines and vitamin D3 injections. Side effects were generally mild and manageable. Candida injections might be as effective as other available treatments. Overall, Candida injections might be a good option for treating warts.
Citation: Acta Derm Venereol 2024; 104: adv40819. DOI https://doi.org/10.2340/actadv.v104.40819.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: May 18, 2024; Accepted after revision: Sep 3, 2024; Published: Oct 18, 2024
Corr: Yu-Chen Huang, Department of Dermatology, School of Medicine, College of Medicine, Taipei Medical University, No. 111, Sec. 3, Xinglong Rd., Wenshan Dist., Taipei City 116079 , Taiwan (ROC). E-mail: dhist2002@yahoo.com.tw
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Warts are common benign epidermal tumours secondary to human papillomavirus (HPV) infection and may occur on different areas of the body. The primary types of warts include common, flat, plantar, filiform, periungual, mosaic, and genital warts (1).
Currently, various treatment options are available (2). Traditional wart treatments, such as cryotherapy, electrodesiccation, and salicylic acid, are commonly employed; however, the warts have a high recurrence rate. Conversely, current alternative medical wart treatments include injectable immunotherapy, vitamin D3, antiviral medication (cidofovir), topical immunotherapy, chemotherapeutics (bleomycin, 5-fluorouracil), and device-based treatments such as photodynamic therapy and pulsed dye laser. These alternative treatments are more effective and are better tolerated than traditional treatments. Nonetheless, treatment with a 100% cure rate is currently non-existent. Thus, ongoing research is investigating emerging treatments such as nanopulse stimulation technology, ionic contra-viral therapy, and cold atmospheric pressure plasma. The objective is to further increase cure rates while minimizing side effects.
Intralesional immunotherapy is highly effective for treating recalcitrant or multiple warts (3). Delivering antigens directly into the lesion or throughout the body triggers an immune response that promotes clearance of injected warts and aids clearance of distant warts. The most extensively researched agents include Candida antigen, measles, mumps, and rubella (MMR) vaccine, purified protein derivative (PPD), and HPV vaccine. Among these, the most well-known intralesional immunotherapy is possibly Candida antigen injection, as it requires only one injection site to clear warts in distant areas, exhibiting the lowest rate of recurrence and new wart development (4). A comparative study by Fawzy et al. (5) underscored the efficacy and safety of Candida antigen, MMR, and PPD for intralesional immunotherapy of flat warts. The findings indicated a higher rate of complete clearance with Candida antigen. Conversely, a network meta-analysis by Salman et al. (6) concluded that PPD and MMR were the most effective treatments for both complete primary and distant recovery of warts. Notably, only 2 out of the 17 studies in the analysis by Salman et al. (6) focused on Candida antigen, which suggests that there may not be sufficient robust evidence to draw definitive conclusions concerning its efficacy. Moreover, the studies included in the analysis by Salman et al. (6) encompassed diverse wart types without directly comparing treatment outcomes for each type. This variability may have contributed to inconsistent results. A recent meta-analysis by Ju et al. (7) focused specifically on the efficacy and safety of intralesional immunotherapy for nongenital warts, revealing a lack of systematic investigation into the treatment response of Candida antigen injection for both nongenital and genital warts.
Thus, we conducted a systematic review and meta-analysis of all relevant randomized controlled trials (RCTs) to compare the treatment response, recurrence rate, and safety between Candida antigen injection therapy and other intralesional immunotherapies across all types of warts.
MATERIALS AND METHODS
We conducted a systematic review and meta-analyses following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement guidelines (Table SI) (8). The protocol of this review was registered on PROSPERO (CRD42023484966).
Data sources and search strategy
A systematic electronic search of PubMed, the Cochrane Library, and Embase was conducted from inception to 16 September 2023. We used the following key terms to search the literature (Fig. S1): ([intralesional] AND [Candida]) AND (warts OR verruca OR human papilloma virus). English restrictions were employed. Animal studies were excluded. We also searched for additional studies from the reference lists of primary articles and relevant reviews to find relevant publications not retrieved through the electronic search.
Inclusion and exclusion criteria of the articles
The inclusion criteria were as follows: (1) RCTs; (2) participants in all age groups who have been diagnosed with cutaneous warts who were allowed to receive previous treatment; (3) studies involving the comparison of at least 2 groups of intralesional injection agents, with 1 group requiring the exclusive administration of intralesional Candida injection therapy; (4) data were sufficient for conducting an analysis.
The exclusion criteria were as follows: (1) case reports, reviews, retrospective medical analysis, single-arm study, comments, letters, or conference abstracts; (2) duplicated articles; (3) outcomes not relevant; and (4) RCTs that were underway (unpublished articles).
Two investigators (C-H Chang, Z-Y Sung) independently reviewed the titles and abstracts yielded by this comprehensive search and subsequently selected articles according to the predetermined inclusion and exclusion criteria (9). A third reviewer (Y-C Huang) would serve as an arbitrator to address inconsistent viewpoints or disagreements.
Outcomes
The primary outcome of this study was the treatment response rate of intralesional immunotherapy in patients with cutaneous warts, specifically evaluating complete and partial responses. Complete responses were defined as the complete clearance (100%) of cutaneous wart lesions, whereas partial responses were defined as the clearance of 50–99% of wart lesions. The secondary outcomes included clinical response in distant warts and adverse effects.
Data extraction
Data extracted included enrolled patient numbers from each included study, age, number of lesions, type and duration of warts, type of intralesional immunotherapeutic agents, interval, and maximum number of treatment sessions. Wart clearance rate, and adverse effects of treatment were also recorded (Tables SII–SIV). If warranted, we contacted the corresponding authors of the obtained studies to request additional information.
Quality assessment and risk of bias
The quality assessment and risk of bias for this study were conducted following the guidelines outlined in the Cochrane Reviewers’ Handbook (3). The Cochrane Risk of Bias Tool 2.0 (RoB 2.0) was used to assess bias risk across 5 specific domains: (i) allocation bias, (ii) performance bias, (iii) attrition bias, (iv) detection bias, and (v) reporting bias. Each domain was evaluated for low, high, or unclear risk of bias. The overall bias was determined through the highest bias rating. Any discrepancies between the 2 investigators were discussed with a third reviewer.
Statistical analysis
Studies that reported the use of the same type of intralesional injection agents were pooled for meta-analysis. Consequently, the meta-analysis was performed in 6 groups: Candida vs placebo (saline group), MMR, PPD, vitamin D3, bivalent HPV vaccine, and zinc sulphate.
Efficacy analysis outcomes were calculated as risk ratios (RR) with 95% confidence intervals (CIs). Heterogeneity was evaluated using p- and I2 values and subset analyses (10). Under more cautious consideration, we uniformly employed the inverse variance random-effects model for dichotomous outcomes.
Possible publication bias was assessed in the case of ≥ 10 studies using funnel plots. We also used Egger’s regression test and the trim and fill test to determine whether publication bias had influenced the results of the meta-analyses (11, 12). All analyses were conducted using RevMan version 5.4 and Comprehensive Meta-Analysis Version 3 (Biostat, Inc, Englewood, NJ, US).
Evaluation of quality of evidence
The assessment of evidence quality for each outcome was performed using GRADEpro GDT (Guideline Development Tool; https://www.gradepro.org/). Consensus was achieved by 2 authors following the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system. In this system, evidence from RCTs is initially rated “high” quality, with the possibility of downgrading according to 5 domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The levels of evidence are categorized on a 4-level scale: very low, low, moderate, or high.
RESULTS
Search results and trial characteristics
Based on the search terms used, 269 eligible articles were retrieved. After removing 76 duplicates, we proceeded to select and review the titles and abstracts of the remaining articles. After the removal of 1 non-English trial, 7 unpublished/non-full-text research articles and 140 unrelated articles, 45 articles underwent further review. Finally, after excluding 21 articles that did not satisfy the inclusion criteria, 24 articles were included in the meta-analysis (5, 13–35). The screening process for the literature search is shown in Fig. 1.
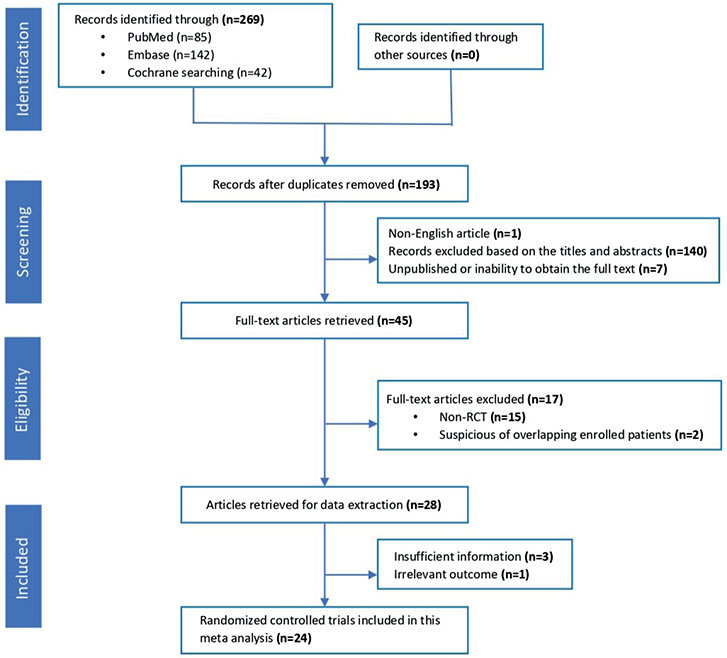
Fig. 1. Flow diagram for selection of eligible studies included in the systematic review and meta-analysis.
In our final quantitative analysis, a total of 24 RCTs with 1,982 participants were included. Among these, 11 articles compared saline, 6 with MMR, 7 with PPD, 6 with vitamin D3, 2 with bivalent HPV vaccine, and 2 with zinc sulphate. The participant age ranged from 4 to 75 years. The majority of the included trials were conducted in Egypt, with one originating in India. The studies were published between 2019 and 2023. In the studies we included, the most common types of warts were common, genital, plantar, and periungual. The characteristics of studies are shown in Table I.
| Study | Country | Arm | Age, years | Lesions (n) | Duration of warts, months | Type of warts | No. of immunotherapy sessions | Interval between sessions |
| Nasr et al., 2023 (23) | Egypt | Candida | 26.7 ± 5.86 | Median 5 | 8.97 ± 8.27 | Common warts, palmo-plantar warts, periungual warts, plane warts, filiform warts, genital warts | Maximum 5 | Week |
| Vitamin D3 | 29.1 ± 7.53 | Median 5 | 8.4 ± 5.73 | |||||
| Digoxin and furosemide | 29.5 ± 7.63 | Median 5 | 7.88 ± 4.82 | |||||
| Fawzy et al., 2023 (19) | Egypt | Candida | 42.2 ± 13 | 18 ± 6.96 | 9.8 ± 8.1 | Anogenital warts | Maximum 3 | 2 weeks |
| Candida + Cervarix | 41.8 ± 11.9 | 20.8 ± 8.6 | 11 ± 8 | Maximum 5 (3 of Candida and 2 of Cervarix) | 1 week | |||
| Candida + Gardasil | 32.6 ± 12.8 | 15.8 ± 10.1 | 5.2 ± 3.9 | Maximum 5 (3 of Candida and 2 of Gardasil) | 1 week | |||
| Saline | 39.4 ± 12.1 | 15 ± 4.6 | 7.8 ± 3.9 | Maximum 3 | 2 weeks | |||
| Chaudhary et al., 2023 (16) | India | MMR | 18–75 | Multiple (> 5) | A total of 53% of the patients had a disease duration of fewer than 6 months | Cutaneous warts | Maximum 3 | 3 weeks |
| PPD | ||||||||
| Candida | ||||||||
| Vitamin D3 | ||||||||
| Youssef et al., 2023 (35) | Egypt | Candida (1/100 concentration) | 4–49 | 20.09 ± 24.12 | 14.49 ± 13.80 | Common warts, plantar warts, plane warts | Maximum 6 | 2 weeks |
| Candida (1/1000 concentration) | 19.97 ± 25.63 | 13.00 ± 13.10 | ||||||
| Zinc sulphate | 22.40 ± 23.04 | 15.69 ± 13.14 | ||||||
| Tawfik et al., 2022 (34) | Egypt | PPD | 35.77 ± 10.19 | 18.54 ± 9.78 | 6.47 ± 6.34 | Genital warts | Maximum 4 | 2 weeks |
| Candida | 38.68 ± 6.81 | 15.0 ± 11.07 | 6.02 ± 4.33 | |||||
| Nofal et al., 2022[a] (27) | Egypt | Candida | 18–54 | 5–50 | 2–7 (yrs) | Multiple (> 5) recalcitrant plantar warts | Maximum 5 | 2 weeks |
| PPD | ||||||||
| Saline | ||||||||
| Eldahshan et al., 2022 (17) | Egypt | MMR | 34.6 ± 9.7 | 2.6 ± 0.89 | 2.6 ± 1.28 (yrs) | Extragenital warts | Maximum 5 | 2 weeks |
| BCG | 35.7 ± 11.05 | 2.77 ± 1.07 | 2.7 ± 1.1 (yrs) | |||||
| Candida | 35.2 ± 9.07 | 2.9 ± 1.06 | 2.65 ± 1.19 (yrs) | |||||
| Nassar et al., 2022[a] (24) | Egypt | Candida | 30.33 ± 17.88 | Multiple | 11.2 ± 5.35 | Common warts | ||
| Bivalent HPV vaccine | 29.96 ± 18.85 | 10.7 ± 3.94 | ||||||
| Cryotherapy | 31.73 ± 17.80 | 10.9 ± 3.89 | ||||||
| Saline | 31.93 ± 17.58 | 10.5 ± 3.73 | ||||||
| Nassar et al., 2022[b] (26) | Egypt | Candida | 32.8 ± 12.74 | Multiple | - | Common warts | Maximum 5 | 2 weeks |
| Saline | 30.76 ± 12.08 | |||||||
| Nofal et al., 2022[b] (28) | Egypt | MMR | 6.5 ± 7.77 | 23.5 ± 13.435 | 7 ± 5.676 | Anogenital warts | Maximum 5 | 2 weeks |
| Candida | 6.5 ± 6.36 | 21.5 ± 9.192 | 9 ± 7.071 | |||||
| Saline | 5 ± 5.65 | 19.5 ± 7.778 | 6 ± 4.242 | |||||
| Nofal et al., 2022[c] (30) | Egypt | Zinc sulphate | 25.21 ± 11.74 | 2 ± 1.27 | 3.63 ± 1.86 (yrs) | Recalcitrant plantar warts | Maximum 4 | 3 weeks |
| Vitamin D3 | 27.89 ± 12.66 | 2.89 ± 2.3 | 3.42 ± 2.12 (yrs) | |||||
| Candida | 26.32 ± 11.87 | 2.5 ± 1.9 | 3.33 ± 1.92 (yrs) | |||||
| Saline | 25.64 ± 12.23 | 2.2 ± 1.8 | 3.52 ± 2.03 (yrs) | |||||
| Abdel Razik et al., 2021 (13) | Egypt | Candida | 19.50–35.50 | 3.0–10.0 | 17.64 ± 3.0 | Common warts | Maximum 4 | 3 weeks |
| Vitamin D3 | 20.0–30.0 | 4.0–7.0 | 17.38 ± 3.29 | |||||
| Saline | 21.0–29.0 | 4.0–5.0 | 16.82 ± 2.74 | |||||
| Abdelaal et al., 2021(14) | Egypt | Vitamin D3 | 30.4 ± 8.6 | 2.2 ± 0.9 | 4.4 ± 1.6 | Plantar warts | Maximum 3 | 3 weeks |
| Candida | 31.9 ± 9.7 | 2.6 ± 1.1 | 4.3 ± 1.7 | |||||
| Rageh et al., 2021 (33) | Egypt | Candida | 31.6 ± 11.3 | Single: 6 Multiple: 24 |
6.66 ± 3.22 | Plantar warts | Maximum 5 | 3 weeks |
| MMR vaccine | 32.2 ± 11.1 | Single: 8 Multiple: 22 |
10.66 ± 8.89 | |||||
| Nofal et al., 2021 (29) | Egypt | PPD | 11.3 ± 7.64 | 2.21 ± 1.22 | 1.88 ± 2.13 (yrs) | Periungual warts | Maximum 5 | 2 weeks |
| Candida | 14.8 ± 9.2 | 3.98 ± 1.77 | 1.16 ± 1.13 (yrs) | |||||
| MMR | 16.5 ± 12.7 | 4.81 ± 1.32 | 1.02 ± 1.5 (yrs) | |||||
| Amer et al., 2021 (15) | Egypt | Candida | 26.39 ± 8.58 | 3–15 | 1–5 (yrs) | Plantar warts, genital warts, plane warts | Maximum 4 | 2 weeks |
| Varicella zoster vaccine | 29.78 ± 9.31 | |||||||
| Hodeib et al., 2021 (20) | Egypt | Candida | 18.9 ± 7.7 | 1–5 (n): 9 6–10 (n): 4 > 10 (n): 7 |
– | Plane warts (face, upper limb) | Maximum 4 | 2 weeks |
| Bleomycin | 25.1 ± 9.4 | 1–5 (n): 12 6–10 (n): 6 > 10 (n): 2 |
||||||
| 5 –FU | 22.95 ± 10.7 | 1–5 (n): 10 6–10 (n): 7 > 10 (n): 2 |
– | |||||
| Marei et al., 2020[a] (21) | Egypt | Candida | 50.7 ± 4.9 | 5–12 | 2 ± 0.75 (yrs) | Common, plantar, periungual, genital | Maximum 5 | 2 weeks |
| Saline | 52.6 ± 3.7 | 3–10 | 1.7 ± 0.34 (yrs) | |||||
| Nassar et al., 2020 (25) | Egypt | Methylene blue and intense pulsed light | 15.8 ± 11.1 | 23 ± 21.4 | 3–60 | Plane warts | Maximum 3 | 2 weeks |
| Candida | 16.5 ± 9.7 | 18.4 ± 13.1 | 3–36 | Maximum 5 | ||||
| Saline | 15.8 ± 11.1 | 19.5 ± 12.4 | 3–36 | Maximum 5 | ||||
| Fawzy et al., 2020 (5) | Egypt | PPD | 12.3 ± 8.65 | 19.21 ± 3.22 | 1.88 ± 2.13 (yrs) | Multiple plane warts (face, hand) | Maximum 5 | 2 weeks |
| Candida | 14.8 ± 9.2 | 17.89 ± 5.77 | 1.16 ± 1.13 (yr) | |||||
| MMR | 19.5 ± 11.6 | 14.81 ± 5.32 | 1.97 ± 1.02 (yrs) | |||||
| Nofal et al., 2020[a] (32) | Egypt | PPD | 21.2 ± 9.78 | 7.05 ± 4.90 | 1.97 ± 1.02 | Common warts | Maximum 6 | 2 weeks |
| Candida | 23.9 ± 12.3 | 9.21 ± 7.67 | 2.26 ± 1.24 | |||||
| Alternating therapy of PPD and Candida | 22.4 ± 10.7 | 9.70 ± 8.25 | 2.5 ± 2.30 | |||||
| Saline | 22.5 ± 10.1 | 7.4 ± 4.42 | 2.3 ± 1.4 | |||||
| Nofal et al., 2020[b] (31) | Egypt | PPD | 24.4 ± 10.7 | 8.56 ± 7.17 | 5.5 ± 8.9 (yrs) | Multiple recalcitrant genital warts | Maximum 5 | 2 weeks |
| Candida | 30.7 ± 12.5 | 9.225 ± 8.55 | 4.9 ± 6.7 | |||||
| Marei et al., 2020[b] (22) | Egypt | Candida | 31 ± 12.9 | 9.8 ± 4.75 | 2.58 ± 1.12 | Recalcitrant warts | Maximum 5 | 2 weeks |
| Combined therapy Candida + Cervarix vaccine |
29 ± 8.47 | 11.2 ± 3.62 | 3.22 ± 2.53 | |||||
| Fathy et al., 2019 (18) | Egypt | Cholecalciferol (vit D3) | responders/non responders 28.86 ± 6.05 32.00 ± 7.21 |
10.00 ± 5.48 15.33 ± 9.03 |
21.14 ± 22.52 53.00 ± 35.68 |
Multiple recalcitrant warts plantar warts | Maximum 3 | 3 weeks |
| Candida | 28.11 ± 4.70 24.55 ± 4.16 |
8.22 ± 2.86 14.00 ± 7.96 |
24.11 ± 12.55 37.64 ± 19.73 |
|||||
| Saline | 20–40 | – | – |
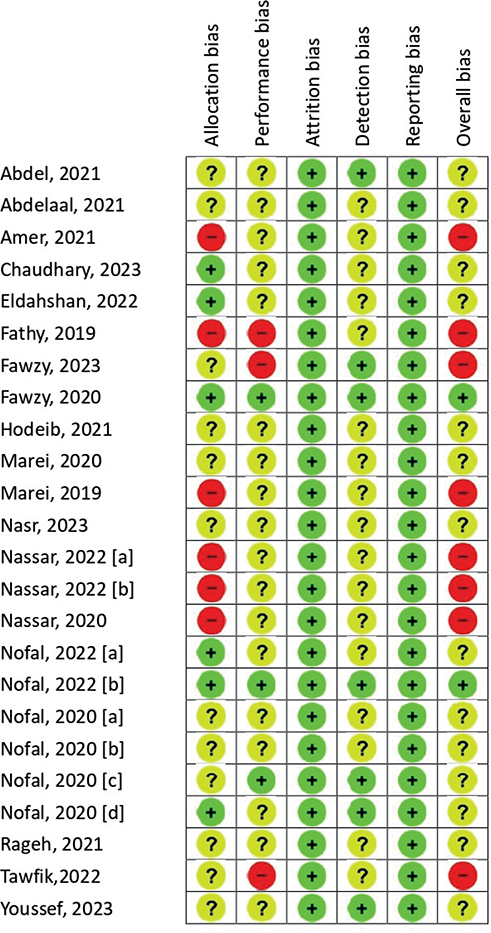
The result of the risk of bias is presented in Fig. 2. In most evaluated studies, the RoB 2.0 quality assessments exposed an unclear risk of bias across various domains. Six of the 24 included studies exhibited a high risk of bias in the domains of allocation concealment and random sequence, with no mention of randomization in patient grouping in these studies (15, 18, 22, 24–26). Furthermore, a high risk of bias in the single blinding of results, participants, and/or professionals was identified in 3 out of the 24 included studies (18, 19, 34), raising concerns regarding potential sources of bias in the reported outcomes.
Clinical effectiveness
First, in the complete clearance group (Fig. 3), 6 subgroups were established to examine complete clearance. The control groups were saline, MMR, PPD, vitamin D3, bivalent HPV vaccine, and zinc sulphate. Intralesional Candida injection therapy showed a significant improvement compared with saline (RR 5.39, 95% CI 3.49–8.33; I2=0%). However, when compared with MMR (RR 1.07, 95% CI 0.78–1.47; I2=76%), PPD (RR 1.08, 95% CI 0.90–1.29; I2=56%), vitamin D3 (RR 1.13, 95% CI 0.75–1.71; I2=78%), bivalent HPV vaccine (RR 1.36, 95% CI 0.90–2.06; I2=0%), and zinc sulphate (RR 1.14, 95% CI 0.91–1.45; I2=0%), no statistically significant differences were found in the complete response rate for wart treatment.
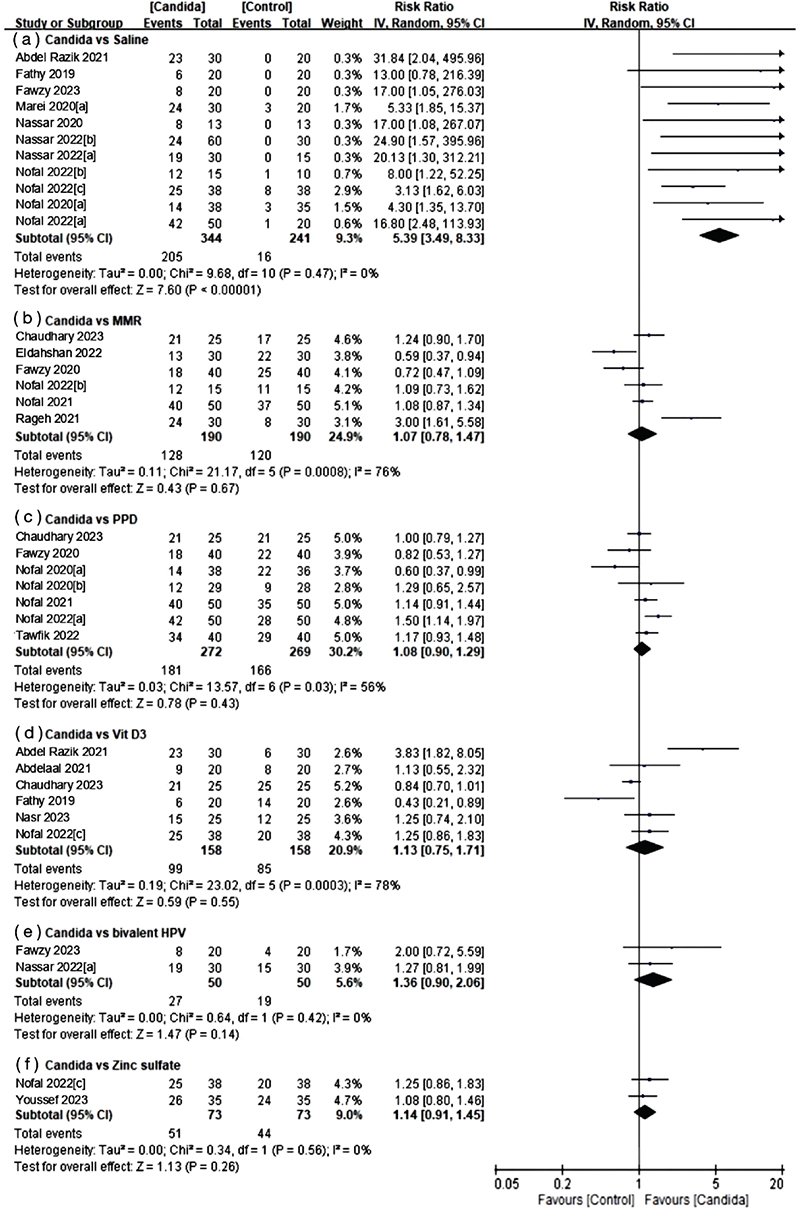
Fig. 3. Forest plots of meta-analysis for complete response rate.
Regarding partial clearance (Fig. 4), 6 subgroups were also established, with the control groups being identical to those of the complete clearance group. Intralesional Candida injection therapy demonstrated some improvement compared with saline (RR 1.71, 95% CI 0.86–3.41; I2=54%) and PPD (RR 1.24, 95% CI 0.59–2.59; I2=72%), although these differences were not statistically significant. Conversely, other therapies showed better partial response rates when compared with Candida injection, including MMR (RR 0.88, 95% CI 0.55–1.39; I2=0%), vitamin D3 (RR 0.75, 95% CI 0.48–1.16; I2=0%), bivalent HPV vaccine (RR 0.57, 95% CI 0.32–1.01; I2=0%), and zinc sulphate (RR 0.85, 95% CI 0.48–1.48; I2=0%).
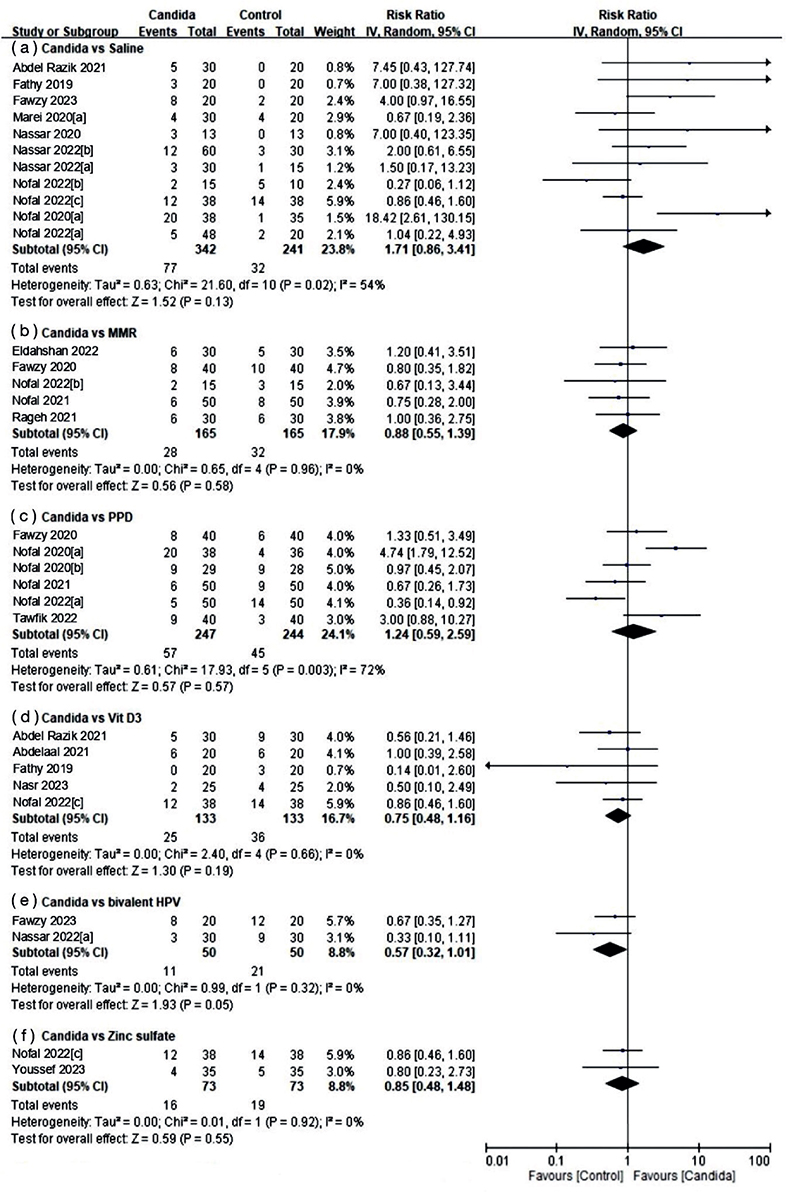
Fig. 4. Forest plots of meta-analysis for partial response rate.
Of the studies included in this meta-analysis, 11 provided comprehensive treatment response data for distant warts situated in anatomically different body parts (Fig. 5). Intralesional Candida injection therapy demonstrated a significant improvement in the rate of distant complete response for wart treatment when compared with both saline (RR 10.54, 95% CI 3.81–29.17; I2=0%) and bivalent HPV vaccine (RR 1.75, 95% CI 1.06–2.88). Conversely, when compared with MMR (RR 1.06, 95% CI 0.74 –1.50; I2=47%), PPD (RR 1.20, 95% CI 0.95–1.53; I2=0%), vitamin D3 (RR 1.98, 95% CI 0.78–5.00; I2=60%), and zinc sulphate (RR 2.78, 95% CI 0.33–23.63; I2=65%), no statistically significant differences were found from that of the Candida group.
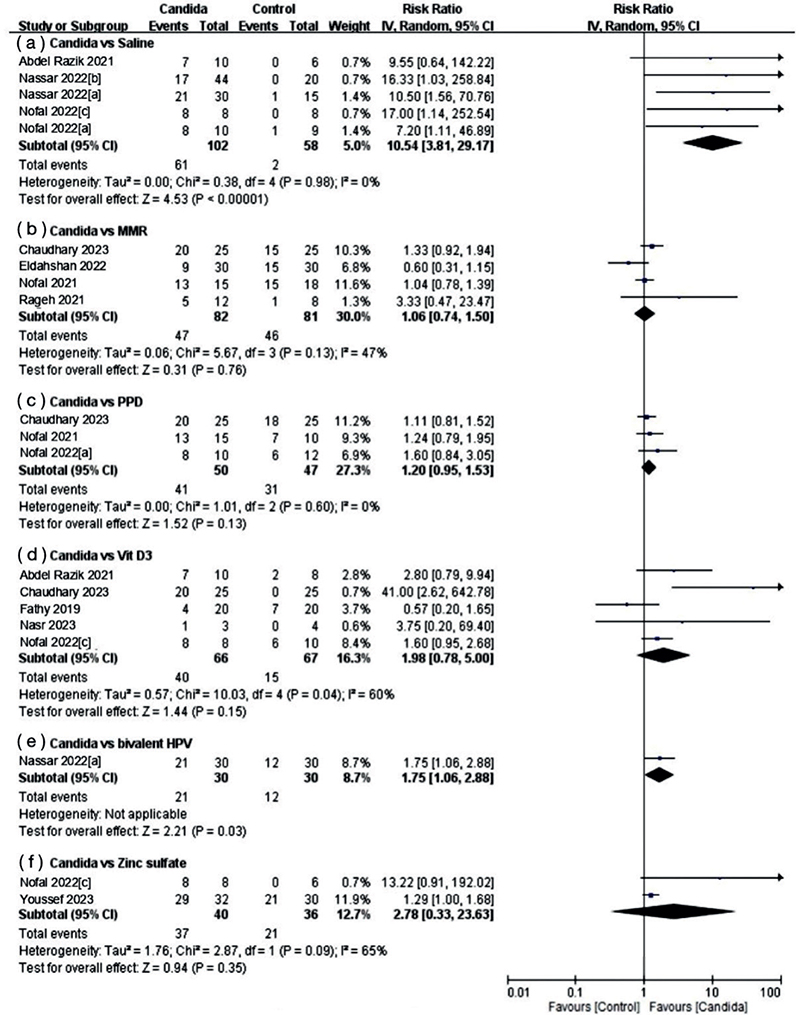
Fig. 5. Forest plots of meta-analysis for distant response rate.
Adverse effects
This analysis encompassed 24 clinical studies, wherein general reported adverse effects of intra-lesional injections were documented. Among these studies, 19 provided specific frequency data for these events. The most commonly observed adverse event was injection-related pain, which was reported in 20 of 24 studies regardless of the intralesional injection agent used. Additionally, flu-like symptoms were noted in 16 of the 24 studies (frequency of 0% to 60%). Other adverse events, such as erythema and swelling, were frequently reported. Rare adverse events included hypopigmentation, injection site blisters, desquamation, vomiting, and severe headache. Although the aforementioned side effects specifically refer to Candida, similar symptoms are also observed with other intralesional injections (see Table SIV).
Publication bias
Funnel plots of complete and partial response of Candida compared with saline were conducted, respectively. The funnel plot for Candida’s partial response versus saline showed symmetry (Fig. S2B). Conversely, the result of complete response of Candida compared with saline appeared to be asymmetric. Egger’s test for a regression intercept had a p-value of < 0.001, indicating possible publication bias. Owing to the presence of publication bias, trim and fill analysis using a random-effects model was performed to correct for funnel plot asymmetry and adjust for the final pooled estimate (Fig. S2A). Six studies were missing on the left side of the mean effect in complete response to Candida compared with the saline group. After inserting 6 imputed studies, the results remained significant with an RR of 4.355 (95% CI 2.582–7.345). Accordingly, potential publication bias does not have a considerable effect on the estimated risk.
Quality of evidence
This meta-analysis explored 3 types of outcomes related to the treatment efficacy of Candida antigen. The quality of evidence for outcome measures according to the GRADE system is presented in Tables SV5–SVII. In this review, there were 6 trials with moderate quality of evidence, 9 with low quality of evidence, and 3 with very low evidence quality.
DISCUSSION
The analysis of RCTs revealed that intralesional Candida injection was significantly more effective than placebo in treating warts. Furthermore, we analysed the therapeutic efficacy of intralesional injection agents by reviewing all known RCTs involving Candida. The complete response rate for the Candida immunotherapeutic agent was slightly higher than that for MMR, PPD, vitamin D3, bivalent HPV vaccine, and zinc sulphate; however, the difference was not statistically significant. Conversely, intralesional Candida injection therapy significantly improved distant complete response rates for wart treatment compared with saline and bivalent HPV vaccine.
Immunotherapy is a treatment approach for warts that stimulates a systemic immune response. The precise mechanism underlying the effectiveness of intralesional immunotherapy, including Candida immunotherapeutic agents, remains unclear but it has been hypothesized that type 1 T helper (Th1) cytokine, tumour necrosis factor alpha (TNF-α), and interferon-γ (INF-γ) production may suppress HPV gene transcription, leading to cytotoxic T cell and natural killer cell activation, ultimately eliminating HPV-infected cells (26, 36). Although intralesional immunotherapy methods may share some common mechanisms of action, immune response between them may be variable. Recently, Nassar et al. (26) reported the roles of Interleukin 17A (IL17A) and migration inhibitory factor (MIF) in the mechanism of action of Candida antigen for treating common warts. In another study conducted by Sorour et al. (37), a significant increase in the intensity of cathelicidin (LL37) expression was noted following an intralesional vitamin D3 injection for verruca vulgaris, to suggest immune action mechanisms. Although no statistically significant differences were noted in the therapeutic effectiveness among these intralesional immunotherapy agents in our analysis, further research into the specific mechanistic variations of each agent is warranted.
It is important to note that Candida antigen comes from different commercial sources, each with varying compositions and manufacturing processes. However, few studies directly compare these sources. Most of the studies we reviewed did not specify how the Candida antigen was prepared. Three studies did mention using Candida antigen from Allergy Laboratories, Inc. (17, 22, 25), at a concentration of 1:1,000 and a dose of 0.2 mL. This product, made by an FDA-licensed manufacturer, is subject to strict quality control. Differences in antigen formulation could affect immune response and treatment efficacy, highlighting the need for consistent testing procedures to ensure reliable clinical results.
Adverse effects associated with intralesional Candida therapy included pain, flu-like symptoms, erythema, and localized oedema at the injection site. The most common reaction is pain at the injection site, which is typically of short duration. Although no severe adverse events were reported in the included studies, a single case report documented a severe adverse event involving a painful purple digit after injection of Candida antigen for periungual wart treatment (38). In summary, the side effects observed did not raise significant safety concerns. Thus, intralesional Candida immunotherapy is widely considered as a safe and well-tolerated option, and patients have consistently reported satisfaction with this treatment (35).
The strengths of the review include a rigorous approach of minimizing bias in the study selection and data analysis. We employed systematic search strategies to identify relevant studies, thereby reducing the likelihood of missing critical evidence. Moreover, the review assessed the risk of bias within the included studies, providing transparency in evaluating the quality of the evidence.
This review has several limitations. First, the exclusion of unpublished or non-English studies may affect the overall comprehensiveness of the findings. Second, most of the included studies were done in Egypt and 1 in India, which raises questions regarding the generalizability of treatment efficiency across diverse racial populations. Third, the treatment efficacy of warts is associated with age and sex; however, most of the included studies failed to categorize patients based on these factors, preventing a comprehensive discussion on their potential influence. Fourth, more than half of the studies featured a relatively small sample size, with fewer than 30 participants per treatment group. The limited number of participants may have affected the generalizability of the results. Fifth, the included studies generally lacked assessments of the long-term effects and recurrence rate of intralesional Candida injection therapy, typically following patients for up to 6 months. This limited duration suggests that the long-term efficiency is not well known. Lastly, 8 of the 24 included studies showed a high risk of bias overall. These studies had flaws in allocation concealment, random sequence, and blinding of patients and professionals in treatments. A “high risk” rating indicates a significant bias that may invalidate the results. To address these concerns, initiating more robust multicentre RCTs on intralesional Candida injection therapy for warts is crucial to prevent bias. Future research should aim for larger and more diverse studies to provide more conclusive evidence.
In summary, our systematic review and meta-analysis underscore the efficacy of intralesional Candida injection therapy for cutaneous warts, showing significant advantages over placebo in achieving complete primary and distant warts recovery. Mild and manageable adverse effects support the safety profile of this immunotherapeutic approach. In conclusion, intralesional Candida immunotherapy holds promise for warts treatment; however, ongoing research is essential to enhance the understanding of the most appropriate form of Candida antigen, and its long-term efficacy and safety.
REFERENCES
- Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012; 30 Suppl 5: F12–23. https://doi.org/10.1016/j.vaccine.2012.07.055
- Friedman PC. Management of difficult-to-treat warts: traditional and new approaches. Am J Clin Dermatol 2021; 22: 379–394. https://doi.org/10.1007/s40257-020-00582-4
- Khozeimeh F, Jabbari Azad F, Mahboubi Oskouei Y, Jafari M, Tehranian S, Alizadehsani R, et al. Intralesional immunotherapy compared to cryotherapy in the treatment of warts. Int J Dermatol 2017; 56: 474–478. https://doi.org/10.1111/ijd.13535
- Majid I, Imran S. Immunotherapy with intralesional Candida albicans antigen in resistant or recurrent warts: a study. Indian J Dermatol 2013; 58: 360–365. https://doi.org/10.4103/0019-5154.117301
- Fawzy MM, Nofal A, Alakad R. Intralesional antigen immunotherapy for the treatment of plane warts: a comparative study. Dermatol Ther 2020; 33: e13807. https://doi.org/10.1111/dth.13807
- Salman S, Ahmed MS, Ibrahim AM, Mattar OM, El-Shirbiny H, Sarsik S, et al. Intralesional immunotherapy for the treatment of warts: a network meta-analysis. J Am Acad Dermatol 2019; 80: 922–930.e924. https://doi.org/10.1016/j.jaad.2018.07.003
- Ju HJ, Park HR, Kim JY, Kim GM, Bae JM, Lee JH. Intralesional immunotherapy for non-genital warts: a systematic review and meta-analysis. Indian J Dermatol Venereol Leprol 2022; 88: 724–737. https://doi.org/10.25259/IJDVL_1369_20
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. https://doi.org/10.1371/journal.pmed.1000097
- Furlan JC, Singh J, Hsieh J, Fehlings MG. Methodology of systematic reviews and recommendations. J Neurotrauma 2011; 28: 1335–1339. https://doi.org/10.1089/neu.2009.1146
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. https://doi.org/10.1136/bmj.327.7414.557
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–463. https://doi.org/10.1111/j.0006-341X.2000.00455.x
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. https://doi.org/10.1136/bmj.315.7109.629
- Abdel Razik LH, Obaid ZM, Fouda I. Intralesional Candida antigen versus intralesional vitamin D3 in the treatment of recalcitrant multiple common warts. J Cosmet Dermatol 2021; 20: 3341–3346. https://doi.org/10.1111/jocd.14335
- Abdelaal MA, Abdelaziz HM, Ahmed KA, Elsaie ML. Comparative study of intralesional vitamin D3 injection and candida albicans antigen in treating plantar warts. J Drug Dermatol 2021; 20: 546–549.
- Amer A, Nassar A, Gamal D, Marei A. Effect of varicella zoster vaccine vs candida antigen injection in treatment of warts. Dermatol Ther 2021; 34: e14667. https://doi.org/10.1111/dth.14667
- Chaudhary M, Brar A, Agarwal P, Chavda V, Jagati A, Rathod SP. A study of comparison and evaluation of various intralesional therapies in cutaneous warts. Indian Dermatol Online J 2023; 14: 487–492. https://doi.org/10.4103/idoj.idoj_492_22
- Eldahshan RM, Ashry WMO, Elsaie ML. Comparative study between intralesional injection of MMR, BCG, and candida albicans antigen in treatment of multiple recalcitrant warts. J Cosmet Dermatol 2022; 21: 1120–1126. https://doi.org/10.1111/jocd.14737
- Fathy G, Sharara MA, Khafagy AH. Intralesional vitamin D3 versus Candida antigen immunotherapy in the treatment of multiple recalcitrant plantar warts: a comparative case-control study. Dermatol Ther 2019; 32: e12997. https://doi.org/10.1111/dth.12997
- Fawzy M, Nofal E, Abdelkhalek N, Ehab R. Intralesional bivalent and quadrivalent human papillomavirus vaccines didn’t significantly enhance the response of multiple anogenital warts when co-administered with intralesional Candida antigen immunotherapy: a randomized controlled trial. Arch Dermatol Res 2023; 315: 2813–2823. https://doi.org/10.1007/s00403-023-02698-z
- Hodeib AAE, Al-Sharkawy BG, Hegab DS, Talaat RAZ. A comparative study of intralesional injection of Candida albicans antigen, bleomycin and 5-fluorouracil for treatment of plane warts. J Dermatolog Treat 2021; 32: 663–668. https://doi.org/10.1080/09546634.2019.1688236
- Marei A, Alakad R, Wahid RM. Evaluation of intralesional Candida antigen in diabetic patients with multiple warts. J Cosmet Dermatol 2021; 20: 1248–1253. https://doi.org/10.1111/jocd.13718
- Marei A, Nofal A, Alakad R, Abdel-Hady A. Combined bivalent human papillomavirus vaccine and Candida antigen versus Candida antigen alone in the treatment of recalcitrant warts. J Cosmet Dermatol 2020; 19: 758–762. https://doi.org/10.1111/jocd.13077
- Nasr M, Abdelaty S, Elkholy BM. A comparative clinico-dermoscopic study of intralesional injection of combined digoxin and furosemide, Candida antigen, and vitamin D3 for multiple warts. J Cosmet Dermatol 2023; 22: 1344–1353. https://doi.org/10.1111/jocd.15581
- Nassar A, Alakad R, Essam R, Bakr NM, Nofal A. Comparative efficacy of intralesional Candida antigen, intralesional bivalent human papilloma virus vaccine, and cryotherapy in the treatment of common warts. J AM Acad Dermatol 2022; 87: 419–421. https://doi.org/10.1016/j.jaad.2021.08.040
- Nassar A, Mostafa M, Khashaba SA. Photodynamic therapy versus candida antigen immunotherapy in plane wart treatment: a comparative controlled study. Photodiagnosis Photodyn Ther 2020; 32: 101973. https://doi.org/10.1016/j.pdpdt.2020.101973
- Nassar A, Nofal A, Bakr NM, Essam R, Alakad R. Correlation of serum interleukin 17 and macrophage migration inhibitory factor levels with clinical response to intralesional Candida antigen and their potential use as predictors of clinical outcome in patients with multiple common warts. J Cosmet Dermatol 2022; 21: 3970–3978. https://doi.org/10.1111/jocd.14688
- Nofal A, Adel L, Fawzy M, Elkholy BM. Intralesional immunotherapy for multiple recalcitrant plantar warts: Candida antigen is superior to intralesional purified protein derivative. Dermatol Ther 2022; 35: e15440. https://doi.org/10.1111/dth.15440
- Nofal A, Alakad R. Intralesional immunotherapy for the treatment of anogenital warts in pediatric population. J Dermatolog Treat 2022; 33: 1042–1046. https://doi.org/10.1080/09546634.2020.1800573
- Nofal A, Alakad R, Fouda I, Fawzy MM. Intralesional antigen immunotherapy in the treatment of periungual warts. J Cutan Med Surg 2021; 25: 286–292. https://doi.org/10.1177/1203475420988859
- Nofal A, Albalat W, Ismail A, Khattab FM. Immunotherapeutic modalities for the treatment of recalcitrant plantar warts: a comparative study. J Dermatolog Treatt 2022; 33: 922–927. https://doi.org/10.1080/09546634.2020.1789540
- Nofal A, Soliman M, Hamdy F, Alakad R. Intralesional Candida antigen versus intralesional tuberculin in the treatment of recalcitrant genital warts: a comparative study. J Am Acad Dermatol 2020; 82: 1512–1514. https://doi.org/10.1016/j.jaad.2019.12.050
- Nofal A, Yehia E, Khater E, Bessar H. Alternating intralesional purified protein derivative and Candida antigen versus either agent alone in the treatment of multiple common warts. J Am Acad Dermatol 2020; 83: 208–210. https://doi.org/10.1016/j.jaad.2020.01.054
- Rageh RM, Hewedy ES, Hegab DS. Intralesional injection of Candida albicans antigen versus measles, mumps, and rubella vaccine for treatment of plantar warts. Acta Dermatovenerol Alp Pannonica Adriat 2021; 30: 1–5. https://doi.org/10.15570/actaapa.2021.1
- Tawfik NZ, Eyada MMK, Abdel El Hamid RE, Halim HM. Intralesional injection of purified protein derivative versus Candida antigen in treatment of genital warts. Dermatolog Ther 2022; 35: e15762. https://doi.org/10.1111/dth.15762
- Youssef EMK, Eissa MAA, Bakr RM. Intralesional Candida albicans antigen versus intralesional zinc sulfate in treatment of cutaneous warts. Arch Dermatol Res 2023; 315: 1305–1314. https://doi.org/10.1007/s00403-022-02499-w
- Nofal A, Salah E, Nofal E, Yosef A. Intralesional antigen immunotherapy for the treatment of warts: current concepts and future prospects. Am J Clin Dermatol 2013; 14: 253–260. https://doi.org/10.1007/s40257-013-0018-8
- Sorour NE, Elesawy FM, Abdou AG, Abdelazeem SE, Akl EM. Intralesional injection of vitamin D in verruca vulgaris increases cathelicidin (LL37) expression: therapeutic and immunohistochemical study. J Dermatol Treat 2022; 33: 291–296. https://doi.org/10.1080/09546634.2020.1750554
- Perman M, Sterling JB, Gaspari A. The painful purple digit: an alarming complication of Candida albicans antigen treatment of recalcitrant warts. Dermatitis 2005; 16: 38–40. https://doi.org/10.1097/01206501-200503000-00008