QUIZ SECTION
Repeated Annular Erythema on the Trunk: A Quiz
Yoshihito MIMA1,2*, Tsutomu OHTSUKA2, Ippei EBATO2, Yoshimasa NAKAZATO3 and Yuta NORIMATSU4
1Department of Dermatology, Tokyo Metropolitan Police Hospital, 4-22-1 Nakano, Nakano-ku, Tokyo 164-8541, 2Department of Dermatology and 3Department of Diagnostic Pathology, International University of Health and Welfare Hospital, Tochigi, and 4Department of Dermatology, International University of Health and Welfare, Narita Hospital, Chiba, Japan. *E-mail: yoshihito11.mima@gmail.com
Citation: Acta Derm Venereol 2024; 104: adv40867. DOI: https://doi.org/10.2340/actadv.v104.40867.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Published: Oct 10, 2024
A 76-year-old man presented with an itchy annular erythema on the trunk, which had been present for 5 years. His medical history included gastric ulcers and hypertension. He had been taking lansoprazole for 20 years and candesartan for 6 years.
Physical examination revealed annular erythema on his entire trunk, varying in size from that of a thumb to that of an egg, with raised edges and a tendency toward central regression (Fig. 1). The rash location differed at each time point. One year after initiating candesartan therapy, he experienced cyclic occurrences and resolution of annular erythema over the past 5 years, despite continuing topical corticosteroids such as betamethasone valerate and betamethasone butyrate propionate. No systemic symptoms were observed.
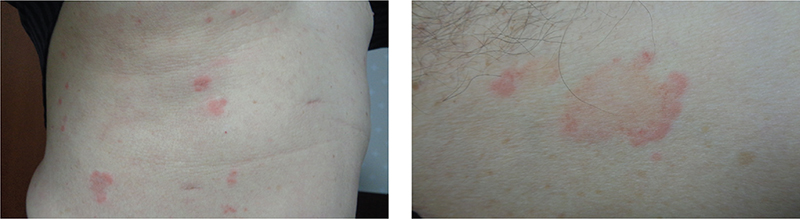
Fig. 1. Clinical photograph. (A) Erythema on patient’s entire trunk, ranging from the size of a thumb to the size of an egg. (B) Annular erythema with raised edges and tendency for central regression.
Laboratory examinations yielded negative results for inflammatory markers, immunoglobulins, antinuclear antibodies, specific antibodies indicative of connective tissue diseases such as Sjögren’s syndrome and systemic lupus erythematosus, and markers of infections such as syphilis and HIV.
Histopathological examination from the edge of the rash revealed sawtooth changes in the epidermis, liquefactive degeneration, lymphocyte and eosinophil infiltration around the capillaries in the dermis, and infiltration of lymphocytes into the basal epidermis (Fig. 2). Leukocytoclastic vasculitis and abnormal cell infiltration were not observed.
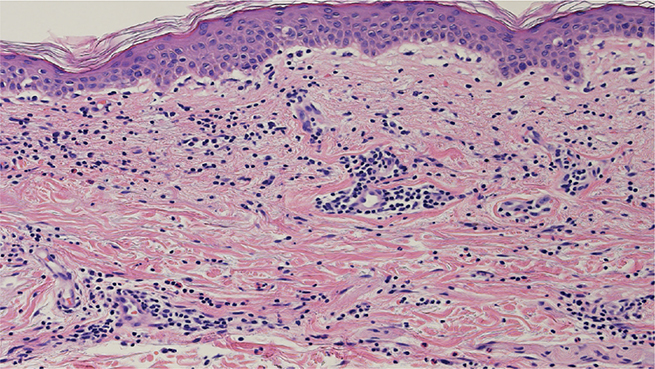
Fig. 2. Histopathological findings at the edges of annular erythema. Histopathological examination revealed sawtooth changes in the epidermis, liquefactive degeneration, lymphocyte and eosinophil infiltration around capillaries in the dermis, and infiltration of lymphocytes into the basal epidermis.
What is your diagnosis?
Differential diagnosis 1: Annular lichenoid drug eruption
Differential diagnosis 2: Erythema annulare centrifugum
Differential diagnosis 3: Annular erythema due to connective tissue disease
Differential diagnosis 4: Sweet disease
See next page for answer.
ANSWERS TO QUIZ
Repeated Annular Erythema on the Trunk: A Commentary
Diagnosis: Annular lichenoid drug eruption
Annular lichenoid drug eruption (ALDE) is a rare variant of lichenoid drug eruptions (LDE), characterized by circular lesions with elevated borders and central clearing (1). Apart from its shape, ALDE shares similarities with conventional LDE (1). LDE itself is a rare drug-induced eruption that closely resembles idiopathic lichen planus (LP) both clinically and histopathologically (2). On average, the patients are around 60 years old, with approximately 40% being women. The medications that induce LDE include antihypertensives, diuretics, antimalarials, nonsteroidal anti-inflammatory drugs, and antitubercular medications. Recently, there has been a notable increase in the number of LDE cases associated with the use of checkpoint inhibitors, tyrosine kinase inhibitors, and anti-TNF-α monoclonal antibodies (3). LDE has an extended latent period, ranging from 4 weeks to 3 years, whereas most drug eruptions exhibit a shorter latent period, typically 1–2 weeks or up to a month. Identifying the causative drug can be challenging because of the variability in the time between the period of drug intake and the onset of eruption. The duration of the latent period may vary depending on specific drugs, dosages, and patient factors (3).
Lichen planus is thought to be mediated by CD8+ T cells and natural killer cells, which induce apoptosis in keratinocytes through the Fas/FasL system or by releasing cytotoxic molecules such as perforin and granzyme B (4). However, the mechanisms underlying the development of LDE remain unknown. LDE development is primarily associated with granzyme B (CD8) cells, and their synthesis is linked to more pronounced apoptosis. Consequently, it has been suggested that LP and LDE exhibit distinct pathogenic mechanisms. It has been suggested that the annular shape of annular LP occurs because of increased expression of ICAM-1 and TNF-α at the periphery, which intensifies the infiltration of inflammatory cells, especially at the edges (5). However, the number of cases of annular LDE is limited; therefore, no studies have discussed why lesions form a ring shape.
The lichenoid tissue reaction pattern is typically characterized by basal cell damage, initiating a cascade of histobiological effects. These encompass basal keratinocyte apoptosis, vacuolar degeneration, inflammatory cell infiltration, and melanin incontinence, which delineate the histopathological features (6). Histopathological findings suggestive of LDE rather than LP include the presence of eosinophilic infiltrates, focal parakeratosis, focal disruption of the granular layer, cytoid bodies in the cornified and granular layers, deep involvement of lymphocytic infiltrate, and numerous individual necrotic keratinocytes in the epidermis (2, 7). In LDE, the infiltration of inflammatory cells into the basal layer of the epidermis is more pronounced compared with LP, while the band-like infiltration of inflammatory cells in the superficial dermis is sometimes milder (7).
Annular erythema with the characteristics of lichenoid eruptions presents with various differential diagnoses, including annular lichenoid dermatitis in youth, annular LP, annular LDE, erythema dyschromicum perstans, erythema multiforme, fixed drug eruption, lichen sclerosus, cutaneous lupus, porokeratosis, subacute cutaneous lupus erythematosus, and lichenoid syphilis.
It is essential to rule out these diseases to diagnose LDE. The most definitive method for diagnosing LDE and identifying the causative drug involves observing the disappearance of the eruption after discontinuing the suspected drug and reproducing the eruption by readministering it (8). However, re-exposure to the causative drug for diagnostic purposes can be hazardous; therefore, most patients hesitate to take this medication again (8). Although patch tests and drug lymphocyte stimulation tests (DLSTs) are safer alternatives, they have a higher rate of false negatives (2). Therefore, the diagnosis of LDE is often confirmed by the regression of the rash following discontinuation of the suspected medication (2).
The primary treatment strategy for LDE is the cessation of the offending medication. However, as continuation of medication poses a low risk of exacerbating symptoms, often the benefits of maintaining treatment surpass the associated risks. Consequently, it is common practice to continue medication in cases of LDE. Moreover, effective alternative treatment options include the use of topical steroids, topical calcineurin inhibitors, oral steroids, oral retinoids, oral cyclosporine, and oral methotrexate (3, 9). It has been documented that LDE typically resolves within approximately 14 weeks after the initiation of LDE treatment (3).
In the present case, on the basis of the clinical course and laboratory and histopathological findings, diseases other than LDE and LP were ruled out. Furthermore, in our case, the marked infiltration of eosinophils around the vessels in the dermis and the significant infiltration of lymphocytes into the epidermal basal layer were suggestive of LDE rather than LP. While on candesartan, the annular erythema continued to recur and resolve repeatedly, even with the ongoing topical corticosteroid treatments. However, after discontinuation of candesartan, the eruptions clearly regressed within 3 months, despite continuing the same topical application of betamethasone butyrate propionate. There have been no recurrences of the rash in the following 6 months, and the patient’s condition remains stable. This time course is consistent with matching the clinical course of LDE. The DLST was 140% for candesartan and 90% for lansoprazole, both negative. However, the value for candesartan was the highest. This value may have been due to the higher rate of false-negative results. We suggested patch and oral tests to the patient. However, further testing was refused due to concerns regarding the risk of a rash recurrence. The lack of additional tests is a limitation of this study. Although annular LDE is rare, its rash can persist for several years if the offending drug is administered indiscriminately. We need to keep in mind that an annular LDE should be considered as a possible differential diagnosis when an annular erythema is present.
REFERENCES
- Baumrin E, Mosam A, Dlova NC. Giant annular lichenoid drug eruption caused by efavirenz therapy. JAAD Case Rep 2018; 4: 256–258. https://doi.org/10.1016/j.jdcr.2017.09.033
- Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol 1993; 29: 249–255. https://doi.org/10.1016/0190-9622(93)70176-T
- Maul JT, Guillet C, Oschmann A, Maul LV, Meier-Schiesser B, Stadler PC, et al. Cutaneous lichenoid drug eruptions: a narrative review evaluating demographics, clinical features and culprit medications J Eur Acad Dermatol Venereol 2023; 37: 965–975. https://doi.org/10.1111/jdv.18879
- Lage D, Juliano PB, Metze K, de Souza EM, Cintra ML. Lichen planus and lichenoid drug-induced eruption: a histological and immunohistochemical study. Int J Dermatol 2012; 51: 1199–205. https://doi.org/10.1111/j.1365-4632.2011.05113.x
- Ohta Y, Yonemoto K, Asai T, Yaguchi A. Lichen planus annularis: an immunohistochemical study. J Dermatol 1992; 19: 414–419. https://doi.org/10.1111/j.1346-8138.1992.tb03251.x
- McNally MA, Farooq S, Brown AE, Rees A, Hsu S, Motaparthi K. Annular lichenoid diseases. Clin Dermatol 2022; 40: 466–479. https://doi.org/10.1016/j.clindermatol.2021.12.009
- Van den Haute V, Antoine JL, Lachapelle JM. Histopathological discriminant criteria between lichenoid drug eruption and idiopathic lichen planus: retrospective study on selected samples. Dermatologica 1989; 179: 10–13. https://doi.org/10.1159/000248091
- Muramatsu K, Ujiie H, Natsuga K, Nishie W, Shimizu H. Lichenoid drug eruption caused by clonazepam. J Eur Acad Dermatol Venereol. 2017; 31: e117–e118. https://doi.org/10.1111/jdv.13862
- Cohen PR, Erickson CP, Calame A. Terbinafine-induced lichenoid drug eruption: case report and review of terbinafine-associated cutaneous adverse events. Dermatol Online J 2020; 26: 13030/qt9jh9p0xp. https://doi.org/10.5070/D3267049554