ORIGINAL REPORT
High BRAF V600 Mutation Level Associated with Worse Outcome in Metastatic Melanoma Patients Receiving BRAF and MEK Inhibitors
Ariane FIZAZI1, Chris SERRAND2, Alexandre EVRARD3,4, Blanche BERGERET1, Pierre-Emmanuel STOEBNER1,4# and Myriam MARQUE1#
1Department of Dermatology, CHU Nîmes, University of Montpellier, Nîmes, 2Department of Biostatistics, Epidemiology, Public Health and Innovation in Methodology (BESPIM), CHU Nîmes, University of Montpellier, Nîmes, 3Laboratory of Biochemistry and Molecular Biology, CHU Nîmes, University od Montpellier, Nîmes, and 4Institut de Recherche en Cancérologie de Montpellier (IRCM), Institut régional du Cancer Montpellier (ICM), INSERM U1194, University Montpellier, Montpellier, France
#Equal senior contribution
The prognostic value of BRAF V600 mutation level on clinical outcomes in patients with BRAF V600-mutated metastatic melanoma treated with BRAF and MEK inhibitors remains uncertain. The association was retrospectively analysed between BRAF V600 mutation level (defined as the ratio of the quantification of the BRAF V600 allele to the percentage of tumoral cells in the sample analysed) and progression-free and overall survival (PFS and OS, respectively) and 3-month response rate in a cohort of 58 patients with metastatic melanoma who harboured BRAF V600E/K mutations and received dual targeted-therapy BRAF/MEK inhibitors. The BRAF mutation level cut-off determined by the area under the receiver operating characteristic curve after internal validation by bootstrap methods was 0.44. Risk of poor PFS and OS was associated with BRAF V600 mutation level > 0.44 on multivariate analysis (p = 0.02 and p = 0.02, respectively) after adjusting for major confounding factors (age, sex, lactate dehydrogenase level, brain metastasis, and treatment line). No association was found between BRAF mutation level and 3-month response rate. Our study shows that high BRAF V600 mutation level in melanoma tissue was associated with poor prognosis in patients with metastatic melanoma treated with BRAF and MEK inhibitors.
SIGNIFICANCE
We retrospectively evaluated whether the level of BRAF V600 mutation in tumor tissue could predict clinical response and outcome of BRAF V600 metastatic melanoma patients treated with the BRAF/MEK inhibitors. Our results suggest that high BRAF V600 mutation level is a significant poor prognostic factor and is not predictive of therapeutic response. Indeed, patients with high BRAF V600 mutation level may benefit from other treatment strategies and tumor BRAFV600 levels could be used as new biomarker in future trials evaluating BRAF/MEK inhibitors. Further prospective studies are needed to corroborate these data.
Key words: melanoma; BRAF V600; MAPKi; biomarker.
Citation: Acta Derm Venereol 2024; 104: adv40913. DOI: https://doi.org/10.2340/actadv.v104.40913.
Copyright: 2024 © The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: May 31, 2024. Accepted after revision: Oct 15, 2024. Published: Nov 7, 2024
Corr: Pierre-Emmanuel Stoebner, Department of Dermatology, CHU Nîmes, University Montpellier, FR-30029 Nîmes, France. E-mail: pierre.stoebner@chu-nimes.fr
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
BRAF- and MEK-targeted therapies and immune checkpoint inhibitors have significantly improved survival rates for patients with BRAF V600-mutant metastatic melanoma (1). The 4-year survival results of the randomized phase II SECOMBIT trial confirmed the status of dual anti-CTLA4 and anti-PD-1 immunotherapy with or without an 8-week course of BRAF/MEK inhibitors as the preferred first-line treatment for BRAF V600E/K-mutant metastatic melanoma (2). However, first-line treatment with BRAF/MEK inhibitors is still recommended in guidelines (3) and led to long-term benefit in approximately one-third of patients who had unresectable or metastatic melanoma with a BRAF V600E/K mutation (4). In the COMBI-d and COMBI-v trials, baseline lactate dehydrogenase (LDH) level was significantly associated with both progression-free survival (PFS) and overall survival (OS) (4). However, new predictive biomarkers are needed to guide which patients are more likely to have sustained response to BRAF/MEK inhibitors.
The BRAF V600E/K variant allele frequency (BRAF VAF) represents the proportion of BRAF molecules carrying the mutation in the sample analysed. VAF is now routinely determined by next-generation sequencing (NGS) after tissue macrodissection of formalin-fixed, paraffin-embedded primary and/or metastatic melanoma samples. Because the sample analysed may contain variable amounts of normal and tumoral cells, the BRAF VAF quantification can be normalized by the estimated tumour cell percentage (5, 6). This adjusted BRAF VAF was defined as the BRAF V600 mutation level (6). High BRAF VAF level in metastatic samples has been found to be significantly associated with worse progression-free survival (PFS) in melanoma patients receiving BRAF/MEK inhibitors in a multicentric retrospective study (7). Few studies have assessed the prognostic and predictive values of BRAF VAF and BRAF V600 mutation levels, and with conflicting results (8–13).
This retrospective study investigated the effect of BRAF mutation level determined by NGS on 3-month response rate, PFS, and OS in patients with metastatic or inoperable melanoma treated with BRAF/MEK inhibitors in our department from 2014 to 2022.
PATIENTS AND METHODS
Patient cohort
Institutional review board approval (no. 23.01.02) was obtained without conditions for this study. We collected data retrospectively for consecutive patients undergoing treatment at the University Hospital of Nîmes, France, from January 2014 to December 2022. Inclusion criteria were metastatic or inoperable melanoma (stage III or IV according to the American Joint Committee on Cancer classification), BRAF V600 mutation (49 V600E mutation, 9 V600K mutation), and treatment with anti-BRAF/anti-MEK dual targeted therapy. Response to therapy was assessed by clinical examination every 4 to 6 weeks and positron emission tomography-densitometry and MRI every 12 weeks (or less in case of clinically suspected progression).
The outcome measures were response after 3 months of treatment (according to Response Evaluation Criteria in Solid Tumours [RECIST] criteria): progression/stability/partial response/complete response), OS and PFS.
Tumour samples, DNA extraction, and determination of BRAF V600 mutation level
BRAF V600 alleles were quantified on formalin-fixed paraffin-embedded melanoma samples. A pathologist evaluated the percentage of tumour versus non-tumour cells in the tumour area on haematoxylin and eosin-stained slides. The tumour area was then macroscopically dissected and DNA was extracted by using the Maxwell 16 FFPE Plus LEV DNA Purification kit 48 (Promega Corp, Madison, WI, USA). After amplification of the target DNA region, a barcoded library was prepared and enriched via emulsion PCR and high throughput sequencing on the Genexus System (Thermo Fisher Scientific, Waltham, MA, USA). The obtained VAF for each patient was then normalized to the proportion of neoplastic cells in each specimen by the following formula: BRAF mutation level = BRAF VAF/estimated percentage of neoplastic cells.
Statistical analysis
NGS data from metastatic samples were used when available and were given priority over primary tumour NGS data, to maintain comparability with similar studies.
Quantitative variables are described with standard measures of central tendency and dispersion. Gaussian distribution was assessed graphically, and 2 groups were compared by Student’s t-test or Wilcoxon Mann–Whitney test. Qualitative variables are described with frequencies (proportions) and were compared by χ2 or Fisher’s exact test. Treatment response was classified as complete or partial response vs stability or progression, to study the link between the BRAF V600 mutation level and response at 3 months of treatment with a logistic regression model.
Time to death or disease progression by BRAF V600 mutation level was explored by Cox proportional-hazards regression. A multivariate analysis also adjusted for the potential confounders age, sex, LDH level, brain metastasis, and treatment line. Hazard ratios (HRs) and 5% confidence intervals (CIs) were estimated.
To determine a cut-off of BRAF V600 mutation level, we used logistic regression, explaining overall prognosis, to assess the cut-off that maximized sensitivity and specificity by the Youden Index. The area under the receiver operating characteristic curve (AUC) was also explored with its 95% CI. PFS and OS were then explored again by univariate and multivariate Cox proportional-hazards regression with the BRAF V600 mutation level dichotomized according to the determined cut-off. Graphical analysis involved Kaplan–Meier curves and the log-rank test.
To check for potential overfitting, the estimation of the cut-off of BRAF V600 mutation level underwent internal validation by the bootstrap method (14). Random draws with replacement were used with a 1:1 rate, and 1,000 samples were drawn. The 1,000 samples were then used with logistic regression to build 1,000 bootstrap models and their corresponding 1,000 optimum cut-off values by the same process as for the original data. We present the obtained median cut-off value and its 95% CI as well as the histogram of the cut-off distribution.
Two-tailed p < 0.05 was considered statistically significant. Statistical analyses were performed with SAS Enterprise Guide software, version 7.1 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics are summarized in Table I. We included 58 patients with unresectable melanoma (34 males; median age 66.3 years [range 24–100]). All patients received BRAF/MEK inhibitors as first-line (n = 50) or second-line therapy (n = 8) for a median of 9 months (interquartile range [IQR] 4.6–15.2). DNA samples were obtained from metastatic specimens for 41 patients and primary tumour specimens for 17 patients. Tumour cellularity ranged from 10% to 100% and BRAF VAF from 5% to 84%. The median BRAF V600E/K mutation level (defined as the ratio of the BRAF V600 allele quantification to the percentage of tumour cells in the sample) was 0.59 (IQR 0.43–0.80). The median follow-up was 13 months (IQR 7–30). Five patients died before the 3-month assessment. The 3-month response rate assessed in 53 patients (partial and complete response) was 64% (Table II). Overall, 46 patients (79%) experienced disease progression. The median PFS and OS for all patients was 7.36 months (IQR 3.18–13.80) and 13.2 months (6.49–30.07).
We found no significant association between BRAF V600 mutation level and 3-month response rate on univariate analysis (p = 0.57). We tested several cut-off values for BRAF V600 mutation level, from 0.4 to 0.7, to determine whether grouping patients would reveal a difference in treatment response but found no significant association in our population.
To find the BRAF V600 mutation level that best discriminated patients with good and poor prognosis, we estimated the cut-off value that maximized the sensitivity and specificity regarding overall progression. The overall AUC for BRAF V600 mutation level was 0.78 (95% CI 0.62–0.94), and the cut-off value with the maximum sensitivity and specificity was 0.44. Fig. SI presents the different cut-off values and their sensitivity and specificity values. After internal validation by bootstrap analysis, the optimal cut-off was still 0.44 but with some variation (95% CI 0.23–0.73).
Risk of poor PFS was not associated with BRAF V600 mutation level on univariate analysis (HR 1.21, 95% CI 0.99–1.48, p = 0.05) or multivariate analysis (HR 1.21, 95% CI 0.89–1.40, p = 0.32). However, it was associated with BRAF V600 mutation level > 0.44 on univariate analysis (HR 2.31, 95% CI 1.08–4.92, p = 0.02) and multivariate analysis (HR 2.90, 95% CI 1.15–7.31, p = 0.02) after adjusting for major confounding factors (age, sex, LDH level, brain metastasis, and treatment line) (Fig. 1A and Table III).
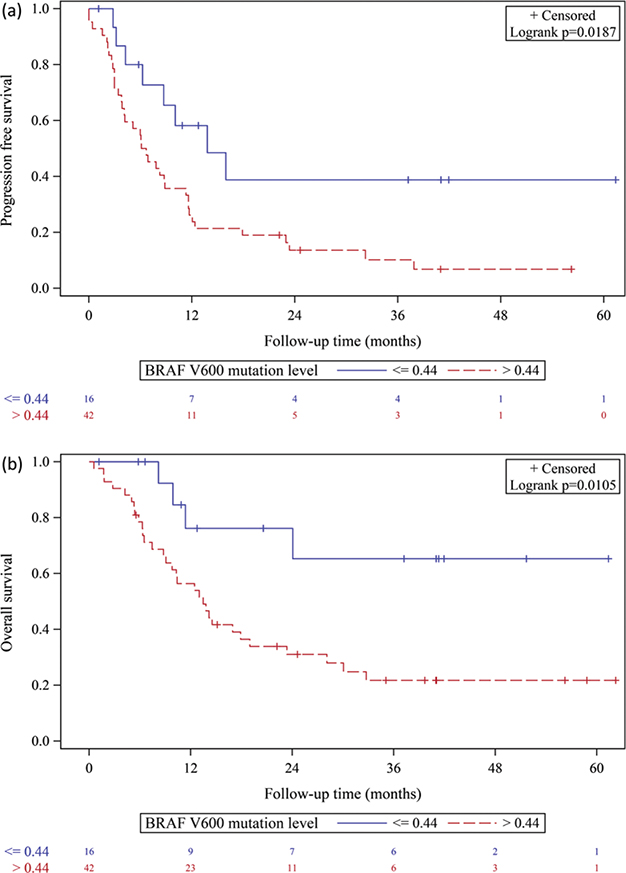
Fig. 1. Kaplan–Meier curves for (A) progression-free survival and (B) overall survival according to BRAF V600 mutation level in patients with melanoma treated with MAPKi.
Risk of poor OS was associated with BRAF V600 mutation level on univariate analysis (HR 1.45, 95% CI 1.16–1.81, p = 0.001) and multivariate analysis (HR 1.37, 95% CI 1.06–1.78, p = 0.02) after adjusting for major confounding factors (age, sex, LDH level, brain metastasis, and treatment line). It was also associated with BRAF V600 mutation level > 0.44 on univariate analysis (HR 3.23, 95% CI 1.18–8.83, p = 0.02) and after multivariate adjustment (HR 3.55, 95% CI 1.07–11.79, p = 0.02) (Fig. 1B and Table III).
DISCUSSION
In patients with BRAFV600 mutated metastatic melanoma treated with BRAF/MEK inhibitors, poor OS and PFS was associated with high BRAF V600 mutation level > 0.44 (determined by NGS). Of note, the 3-month response rate did not differ between patients with low mutation level and those with high mutation level.
BRAF and MEK inhibition has improved PFS and OS in patients with BRAF V600-mutant melanoma, although the degree of treatment benefit varies. The 5-year OS for patients receiving dabrafenib plus trametinib in the COMBI-d and COMBI-v trials was 34% but could reach 71% among patients with complete response (4). Several baseline factors (e.g., performance status, age, sex, number of organ sites with metastasis, and LDH level) have been found to be significantly associated with both PFS and OS. However, there is a need for new biomarkers that identify the optimal treatment strategy for individual patients with BRAF-mutated melanoma.
Our results agree with a recent multicentric retrospective study. Mandalà et al. (7) assessed BRAF VAF by NGS in melanoma samples from 107 patients with metastatic melanoma treated with first-line dual targeted therapy. PFS was significantly lower in patients with BRAF VAF > 41.3% in metastatic samples. A similar trend was found for OS, although was not statistically significant. In this report, the overall response rate was not associated with BRAF VAF level, which suggests, as in our study, that BRAF mutation level could affect the emergence of secondary resistance with no impact on primary resistance. In a previous study, Lebbé et al. (6) reported a time-dependent association between BRAF V600 mutation level (determined by pyrosequencing, which is less sensitive than NGS) (15) and best response rate to vemurafenib. These results agreed in part with our findings by showing high mutation level ≥ 0.5 associated with short PFS but only after the first 10 months of treatment.
Our results disagree with previous retrospective studies. Berrino et al. (13) reported high BRAF VAF significantly correlated with better outcome in 62 patients. In this study, BRAF VAF was assessed by pyrosequencing and patients received BRAF inhibitor monotherapy or dual targeted therapy. Stagni et al. (5) described a significantly longer PFS in patients with BRAF VAF > 35% (determined by quantitative PCR), finding no correlation with best response rate in 46 patients receiving BRAF inhibitor or combined BRAF+MEK inhibitor treatment. Conversely, Satzger et al. reported that BRAF VAF (determined by NGS, without considering tumoral cellularity) had no impact on PFS, OS, and objective response rates in 76 patients with metastatic melanoma treated with BRAF inhibitor monotherapy (8), and Mesbah et al. (9) described similar results in 33 patients receiving BRAF inhibitor monotherapy, with no difference in OS or PFS or degree of response when patients were classified into high or low BRAF VAF groups (determined by pyrosequencing without considering tumoral cellularity).
Many factors could explain such discrepancies: (i) BRAF VAF was not always assessed with the same technology (pyrosequencing, NGS, or quantitative PCR), (ii) most of the studies included melanoma samples with high tumour cellularity and did not normalize the BRAF VAF to the proportion of neoplastic cells, and (iii) the methods used to determine the BRAF VAF threshold differed, as did the BRAF VAF cut-off value. Moreover, because of the evolution of treatment options over the years, patients underwent variable treatment regimens (BRAF inhibitor alone or dual targeted therapy with BRAF and MEK inhibitors).
Our study has several strengths: all patients uniformly received dual targeted therapy and were homogeneously cared for in a unique onco-dermatology department. In routine clinical practice, melanoma samples analysed by NGS may contain few tumoral cells. Thus, we incorporated all melanoma samples, including those with a low percentage of tumour cells. Finally, to better estimate the BRAF VAF in tumoral cells, we adjusted the VAF to the estimated tumour cellularity.
We acknowledge that our results should be replicated in a larger cohort. A single pathologist determined the tumour cell percentage, which may suggest a bias in calculating BRAF mutation level, although this bias would have been systematic. Another limitation is that not all analysed samples were derived from metastatic sites. Among 58 patients, 14 were studied by analysing the primary tumour, and the small sample size did not allow for subgroup analyses.
In conclusion, our study shows high baseline BRAF V600 mutation level determined by NGS associated with poor OS and PFS in patients with metastatic melanoma treated with BRAF/MEK inhibitors. Patients with high BRAF V600 mutation level may benefit from other treatment strategies and tumour BRAFV600 levels could be used as stratification factor in future trials evaluating targeted therapies alone or combined with other types of treatment. Further prospective larger cohorts are warranted to corroborate these data.
REFERENCES
- Schummer P, Schilling B, Gesierich A. Long-term outcomes in BRAF-mutated melanoma treated with combined targeted therapy or immune checkpoint blockade: are we approaching a true cure? Am J Clin Dermatol 2020; 21: 493–504. https://doi.org/10.1007/s40257-020-00509-z
- Ascierto PA, Casula M, Bulgarelli J, Pisano M, Piccinini C, Piccin L, et al. Sequential immunotherapy and targeted therapy for metastatic BRAF V600 mutated melanoma: 4-year survival and biomarkers evaluation from the phase II SECOMBIT trial. Nat Commun 2024; 15: 146. https://doi.org/10.1038/s41467-023-44475-6
- Seth R, Agarwala SS, Messersmith H, Alluri KC, Ascierto PA, Atkins MB, et al. Systemic therapy for melanoma: ASCO Guideline Update. J Clin Oncol 2023; 41: 4794–4820. https://doi.org/10.1200/JCO.23.01136
- Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019; 381: 626–636. https://doi.org/10.1056/NEJMoa1904059
- Stagni C, Zamuner C, Elefanti L, Zanin T, Bianco PD, Sommariva A, et al. BRAF gene copy number and mutant allele frequency correlate with time to progression in metastatic melanoma patients treated with MAPK Inhibitors. Mol Cancer Ther 2018; 17: 1332–1340. https://doi.org/10.1158/1535-7163.MCT-17-1124
- Lebbé C, How-Kit A, Battistella M, Sadoux A, Podgorniak MP, Sidina I, et al. BRAFV600 mutation levels predict response to vemurafenib in metastatic melanoma. Melanoma Res 2014; 24: 415–418. https://doi.org/10.1097/CMR.0000000000000088
- Mandalà M, Palmieri G, Ludovini V, Baglivo S, Marasciulo F, Castiglione F, et al. BRAFV600 variant allele frequency predicts outcome in metastatic melanoma patients treated with BRAF and MEK inhibitors. J Eur Acad Dermatol Venereol 2023; 37: 1991–1998. https://doi.org/10.1111/jdv.19281
- Satzger I, Marks L, Kerick M, Klages S, Berking C, Herbst R, et al. Allele frequencies of BRAFV600 mutations in primary melanomas and matched metastases and their relevance for BRAF inhibitor therapy in metastatic melanoma. Oncotarget 2015; 6: 37895–37905. https://doi.org/10.18632/oncotarget.5634
- Mesbah Ardakani N, Leslie C, Grieu-Iacopetta F, Lam WS, Budgeon C, Millward M, et al. Clinical and therapeutic implications of BRAF mutation heterogeneity in metastatic melanoma. Pigment Cell Melanoma Res 2017; 30: 233–242. https://doi.org/10.1111/pcmr.12569
- Tonella L, Pala V, Ponti R, Rubatto M, Gallo G, Mastorino L, et al. Prognostic and predictive biomarkers in stage III melanoma: current insights and clinical implications. Int J Mol Sci 2021; 22: 4561. https://doi.org/10.3390/ijms22094561
- Soria X, Vilardell F, Maiques Ó, Barceló C, Sisó P, de la Rosa I, et al. BRAFV600E mutant allele frequency (MAF) influences melanoma clinicopathologic characteristics. Cancers (Basel) 2021; 13: 5073. https://doi.org/10.3390/cancers13205073
- Sevilla A, Morales MC, Ezkurra PA, Rasero J, Velasco V, Cancho-Galan G, et al. BRAF V600E mutational load as a prognosis biomarker in malignant melanoma. PLoS One 2020; 15: e0230136. https://doi.org/10.1371/journal.pone.0230136
- Berrino E, Balsamo A, Pisacane A, Gallo S, Becco P, Miglio U, et al. High BRAF variant allele frequencies are associated with distinct pathological features and responsiveness to target therapy in melanoma patients. ESMO Open 2021; 6: 100133. https://doi.org/10.1016/j.esmoop.2021.100133
- Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. 2nd ed. Cham, Switzerland: Springer; 2019: p. 104–111.
- Colombino M, Rozzo C, Paliogiannis P, Casula M, Manca A, Doneddu V, et al. Comparison of BRAF mutation screening strategies in a large real-life series of advanced melanoma patients. J Clin Med 2020; 9: 2430. https://doi.org/10.3390/jcm9082430