ORIGINAL REPORT
Suppression of Epidermal Growth Factor Receptor by Erlotinib Attenuates Carvacrol-induced Skin Inflammation
Yujing WANG1#, Wenjie HUANG1#, Haidong JIA2, Qinglian TANG1, Qingfei YIN2, Yuanyuan CHEN2, Wumei WANG3 and Zhengyu CAO1
1Department of TCM Pharmacology, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China, 2R&D Center, Shanghai Jahwa United Co., Ltd., Shanghai, China, and 3Department of Neurosurgery, Renmin Hospital of Wuhan University, School of Pharmaceutical Sciences, Wuhan University, Wuhan, China
#These authors contributed equally, and should be considered as first authors.
Epidermal growth factor receptors (EGFRs) regulate the growth and repair process of epithelia, as well as carcinogenesis. Activation of TRPV3 by carvacrol stimulates skin inflammation and epidermal hyperplasia; the latter can be suppressed by EGFR inhibition. However, whether EGFR signalling is responsible for skin inflammation remains elusive. The current study investigated the effect of erlotinib, an EGFR inhibitor, on skin inflammation in a carvacrol-induced atopic dermatitis mouse model. It was observed that erlotinib significantly attenuated carvacrol-induced overexpression of proinflammatory cytokines and suppressed peripheral blood mononuclear cell recruitment in HaCaT keratinocytes. In addition, it was demonstrated that erlotinib suppressed carvacrol-induced Akt and NF-κB signalling pathways. Furthermore, inhibition of Akt and NF-κB signalling pathways also attenuated the carvacrol-induced keratinocyte proinflammatory response. Finally, it was demonstrated that erlotinib treatment alleviated carvacrol-induced dermatitis. These data demonstrate that erlotinib ameliorates skin inflammation by regulating Akt and NF-κB-mediated keratinocyte proinflammation, suggesting the therapeutic potential of erlotinib, a clinically used EGFR inhibitor, in skin inflammatory diseases.
SIGNIFICANCE
Epidermal growth factor receptors regulate epithelial growth and repair as well as carcinogenesis. Recent studies have shown that epidermal growth factor receptor inhibition by erlotinib is used to treat Olmsted syndrome, a severe skin disease caused by TRPV3 gain-of-function mutants. We demonstrate that suppression of epidermal growth factor receptor by erlotinib ameliorates skin inflammation through regulation of Akt and NF-κB-mediated keratinocyte proinflammation. Our results highlight the therapeutic potential of erlotinib, a clinically used agent, to treat skin inflammatory diseases.
Key words: carvacrol; dermatitis; EGFR; erlotinib; keratinocyte.
Citation: Acta Derm Venereol 2024; 104: adv40975. DOI: https://doi.org/10.2340/actadv.v104.40975.
Copyright: 2024 © The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Jun 10, 2024; Accepted after revision: Oct 15, 2024; Published: Dec 9, 2024.
Corr: Wumei Wang, PhD, Department of Neurosurgery, Renmin Hospital of Wuhan University, School of Pharmaceutical Sciences, Wuhan University, Wuhan, China, 430071, and Zhengyu Cao, PhD, Department of TCM Pharmacology, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China, 211198. E-mails: 2022103060039@whu.edu.cn; zycao@cpu.edu.cn
Competing interests and funding: The authors have no conflicts of interest to declare.
This work was supported by National Natural Science Foundation of China (82373929, 81972960, and 82100585), “Double First-Class” University Project (CPU2018GF06), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23_0868), and Tibet Autonomous Region Science and Technology Plan Project Key Project (XZ202301ZY0014G).
INTRODUCTION
Skin inflammation is a common condition that affects about 20% of the population and contributes to ~1.79% of the disease burden worldwide (1). Skin inflammation can be derived from immune system dysfunction, allergic reaction, or infection by bacteria, virus, or fungi (2). Common skin inflammatory diseases include atopic dermatitis, psoriasis, and cellulitis etc. (3). Among the most common type of skin inflammatory diseases, atopic dermatitis affects up to 25% of children and 2–3% of adults with symptoms that include dry, cracked, and thickened skin accompanied by severe itching (4). Despite the availability of glucocorticoids, calcineurin inhibitors, and biologics, therapeutic efficacy or safety remains unsatisfactory in clinical practice (5). Thus, novel target-engaged therapy may provide the potential to treat skin inflammatory diseases.
Carvacrol, a type of monoterpenoid, exists widely in the essential oil of oregano, thyme, pepperwort, and wild bergamot (6). Carvacrol has been shown to display a variety of beneficial effects such as anti-cancer, antimicrobial, anti-inflammatory, and pain-relieving activities (6, 7). Carvacrol has been shown to directly activate transient receptor potential ankyrin 1 (TRPA1) and transient receptor potential vanilloid 3 (TRPV3) channels as well as directly inhibiting the transient receptor potential melastatin subfamily member 7 (TRPM7) (8,9). We and others have demonstrated that topical application of 2% carvacrol for 5 consecutive days triggered skin inflammation as reflected by erythema, scaly skin lesions, pruritus, and acanthosis, as well as increased expression of inflammatory mediators (10, 11), resembling the features of atopic dermatitis (12). Such an effect was mitigated in TRPV3 knockout mice and also can be largely suppressed by the specific TRPV3 inhibitor, suggesting that carvacrol-induced skin inflammation was dependent on TRPV3 activity (10–12).
Epidermal growth factor receptor (EGFR) is a transmembrane receptor with signal-transducing tyrosine kinase activity, involved in the growth and repair process of epithelia, as well as carcinogenesis (13). TRPV3 and EGFR have been proven to form a signalling complex to regulate hair morphogenesis and skin barrier formation (14). It has been demonstrated that activation of TRPV3 by carvacrol causes chemokine overexpression in human HaCaT keratinocytes (15). Our previous study has also demonstrated that TRPV3 enhances keratinocyte proliferation by transforming growth factor-α (TGF-α) autocrine activation of the EGFR signalling pathway (16). However, whether EGFR signalling is responsible for a carvacrol-induced proinflammatory response in keratinocytes remains elusive.
Although chemotherapy with the use of EGFR inhibitors frequently causes cutaneous adverse reactions, including papulopustular rash, xerosis, paronychia, and pruritus (17), recent case reports show that patients with Olmsted syndrome caused by TRPV3 gain-of-function mutants had substantial improvement in hyperkeratosis and pain on treatment with erlotinib (18–20). This discrepancy suggests that there is a need to re-evaluate the role of EGFR in cutaneous inflammation. In the current study, we aimed to investigate the effects of erlotinib on carvacrol-induced proinflammation in HaCaT cells and dermatitis in mice.
MATERIALS AND METHODS
Reagents
Carvacrol was purchased from Sigma-Aldrich (St. Louis, MO, USA). Erlotinib (ERT), gefitinib (GEF), afatinib (AFA), MK-2206, BAY11-7085, and LY294002 were obtained from MedChemExpress LLC (Shanghai, China). Protease inhibitor cocktail was from ApexBio Technology (Houston, TX, USA). Primary antibodies against phospho (p)-EGFR, EGFR, p-Akt, Akt, p-NF-κB (p65 subunit), NF-κB (p65 subunit), inhibitor of NF-κB (I-κB), p-I-κB , β-tubulin and GAPDH were purchased from Cell Signaling Technology (Danvers, USA). IRDye®800CW and 680RD secondary antibodies were purchased from LI-COR (Lincoln, NE, USA).
Animal testing
All animal protocols were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and approved by the laboratory animal care and use committee of China Pharmaceutical University. WT C57BL/6 mice (6–7 weeks old) were purchased from the animal core facility of Nanjing Medical University (Nanjing, Jiangsu, China). After shaving the dorsal hair for 1 day, 100 μL of 1% carvacrol (dissolved in 40% ethanol solution) was topically applied to the back skin of mice once daily for 4 consecutive days. To study the effect of erlotinib on skin dermatitis induced by carvacrol, a 100 μL erlotinib solution (0.4 mg/mL) was injected subcutaneously 1 h prior to each topical application of carvacrol. The severity of dermatitis was assessed based on 4 symptoms: (1) erythema/haemorrhage, (2) scarring/dryness, (3) oedema, and (4) exfoliation/erosion. Each symptom was scored from 0 to 3 points (none, 0; mild, 1; moderate, 2; severe, 3). The sum of the scores from the 4 symptoms was used to quantify the severity of dermatitis. On the 4th day, a Canon EOS 60D camera was used to photograph the gross appearance of the dorsal skin. The degree of pruritis was calculated by counting scratching bouts in a period of 10 min after carvacrol application. A scratching bout consisted of a series of movements when a mouse lifted its hind limb towards the dorsal skin and scratched until the limb returned to the floor or mouth. Mice were sacrificed, and the dorsal skin was dissected and fixed with 4% paraformaldehyde for 48 h at room temperature (RT) or stored in liquid nitrogen.
Histopathological examination
Histopathological examination was performed as described previously (10). In brief, fixed tissue samples were embedded in paraffin, sectioned at 2 μm, and stained with haematoxylin and eosin (H&E). The slides were photographed using an Eclipse Ti inverted microscope (Nikon, Tokyo, Japan). Epidermal thickness was quantified using NIS-Elements (V4.3, Nikon, Tokyo, Japan).
Quantitative real-time PCR
Total RNA was extracted using TRIzol (Vazyme, Nanjing, China), cDNA was generated with the HiScript II Q RT SuperMix kit for qPCR (Vazyme, Nanjing, China), and quantitative real-time PCR was performed using the SYBR green protocol. The mouse and human primers for GAPDH, interleukin-6 (IL), and IL-8 can be found from PrimerBank (10). The mRNA expression levels were quantified using the 2−ΔΔCt method, and normalized to GAPDH.
Cell culture
The human epidermal keratinocyte (HaCaT) cells were obtained from iCell Bioscience Inc (Shanghai, China). HaCaT keratinocytes were cultured in DMEM containing 100 U/mL penicillin and streptomycin and 10% foetal bovine serum (FBS). Cells were incubated at 37°C in an environment of 5% CO2 and 95% humidity.
Western blot analysis
Western blotting was performed as described by Li et al. (21). Briefly, HaCaT cells were lysed using the RIPA buffer (in millimoles: 150 NaCl, 20 Tris-HCl, 2.5 sodium pyrophosphate, 1 EGTA, 2 Na2EDTA, 1 sodium orthovanadate, 1% Nonidet P40, 1% sodium deoxycholate, 1 β-glycerophosphate, pH 7.4) supplemented with a cocktail of protease inhibitors. After quantification of the protein concentration using a BCA kit (Beyotime, Shanghai, China), a 30 μg of protein from each sample was loaded to SDS-PAGE for separation. Separated proteins were then transferred onto nitrocellulose membranes. After blocking with skimmed milk, primary antibodies against p-EGFR (1:500), EGFR (1:500), p-Akt (1:500), Akt (1:500), p-NF-κB (1:1000), NF-κB (1:1000), p-I-κB (1:1000), I-κB (1:1000), and β-tubulin (1:10,000) were added. After incubation overnight at 4°C, the membranes were incubated with the secondary antibodies for 1 h at RT. The blot images were acquired and the densitometry was quantified using the LI−COR Odyssey Infrared Imaging System (LI−COR Biotechnology).
Peripheral blood mononuclear cells (PBMC) recruitment assay
Isolation and purification of PBMCs from healthy donors were performed as described previously (10). The study was approved (protocol code: 2019-ky013) by the research ethics board of the Institute of Dermatology and Hospital for Skin Diseases, Chinese Academy of Medical Sciences, and informed consent of donors was obtained. To evaluate PBMC recruitment by HaCaT cells, 200 μL PBMC suspension (5×105 cells/mL in RPMI-1640 was loaded onto the upper chamber of a Transwell. A volume of 600 μL supernatant from HaCaT cells treated with the respective drug for 6 h was added to the lower chamber. After incubating in 5% CO2 at 37°C for another 3 h, cells on the membrane of the inlet were fixed with 10% methanol for 10 min and stained with crystal violet for 30 min. Cell numbers were counted blindly from 5 random fields photographed at 200× magnification using a digital colour camera (Nikon, Tokyo, Japan) attached to an inverted microscope.
Statistical analysis
GraphPad Prism version 6.0 (San Diego, CA, USA) was used to generate graphs. Data were presented as mean ± SEM. The statistical significance was analysed using an unpaired Student’s t-test or one-way ANOVA followed by Dunnett’s multiple comparisons test. A p-value less than 0.05 is considered to be statistically significant.
RESULTS
Erlotinib attenuated carvacrol-induced proinflammatory response in keratinocytes
We previously have demonstrated that in human HaCaT keratinocytes, carvacrol induced a proinflammatory response that was nearly abolished by a selective TRPV3 inhibitor, scutellarein (10). We have also demonstrated that carvacrol promoted proliferation of HaCaT cells through TRPV3-mediated EGFR activity (16). We therefore examined whether EGFR activity was also involved in the regulation of carvacrol-induced proinflammatory response. Consistent with previous demonstration, carvacrol (100 μM) treatment for 3 h increased mRNA expression levels of IL-6 and IL-8 to 3.61 ± 0.40 (p < 0.01) and 10.48 ± 1.29-fold (p < 0.01) of vehicle-treated HaCaT cells, respectively (Fig. 1A, B). The carvacrol-enhanced IL-6 and IL-8 expression were reduced by erlotinib in a dose-dependent manner (10–100 nM). The reductions were 14.3 ± 6.1% (10 nM), 80.2 ± 5.6% (30 nM, p < 0.01), and 99.0 ± 1.5% (100 nM, p < 0.01), and 47.1 ± 3.4% (10 nM, p < 0.01), 70.8 ± 1.5% (30 nM, p < 0.01), and 69.0 ± 0.3% (100 nM, p < 0.01) for IL-6 and IL-8, respectively (Fig. 1A, B). Similarly, treatment with gefitinib dose-dependently reduced carvacrol-enhanced IL-6 and IL-8 expression by 16.7 ± 15.7% (10 nM), 71.5 ± 9.5% (30 nM, p < 0.01), and 93.4 ± 4.0% (100 nM, p < 0.01), and 85.5 ± 2.0% (10 nM, p < 0.01), 79.4 ± 0.2% (30 nM, p < 0.01), and 91.4 ± 0.4% (100 nM, p < 0.01), respectively (Fig. S1A, B). However, afatinib caused a more robust inhibition of carvacrol-induced IL-8 expression but a remarkable promotion of carvacrol-induced IL-6 expression, which would contribute to adverse drug reactions in the clinic (Fig. S1C, D). We next investigated the chemotactic effect of the conditional medium collected from the HaCaT cell cultures. The culture medium collected from carvacrol (100 μM)-treated HaCaT cells for 6 h significantly increased the number of chemotactic PBMCs from 50.0 ± 0.57 cells/field to 129.0 ± 15.5 cells/field (p < 0.01) (Fig. 1C, D). The medium from erlotinib and carvacrol-treated HaCaT cells recruited significantly fewer PBMCs (74.7 ± 5.7 cells/field) (p < 0.05).
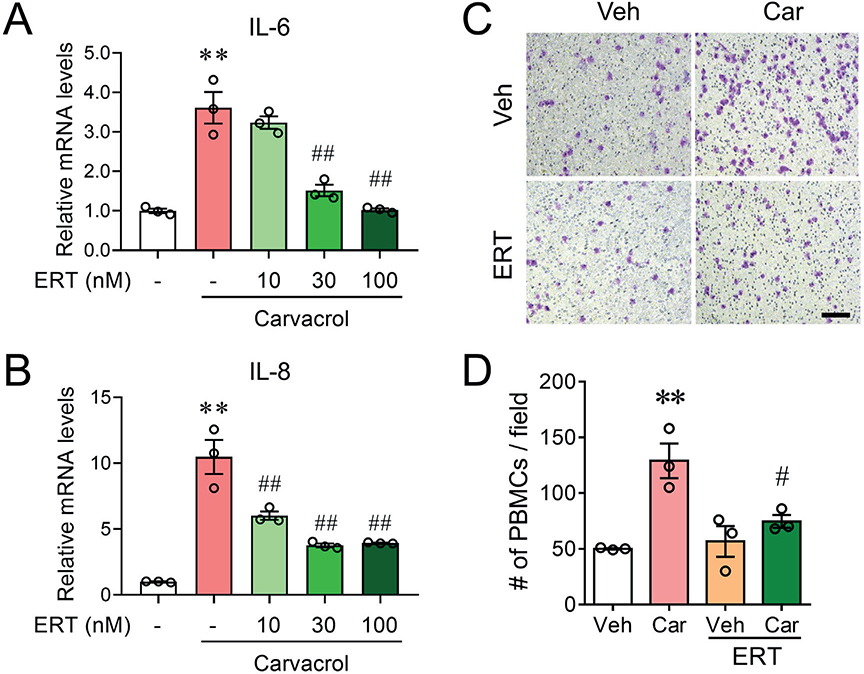
Fig. 1. Erlotinib attenuates carvacrol-induced proinflammatory responses in keratinocytes. (A–B) Relative mRNA levels of IL-6 (A) and IL-8 (B) in HaCaT cells treated with 100 μM carvacrol (Car) in the absence and presence of erlotinib (ERT) at different concentrations for 3 h. Data were normalized to the vehicle (Veh) control. (C) Representative microphotographs showing fixed and stained PBMCs on the bottom side of the invasion membrane. Scale bar, 50 μm. (D) Quantification of PBMC chemotaxis induced by media harvested from cultured HaCaT cells treated with Car in the absence and presence of ERT for 6 h. Data in (A), (B), and (D) are mean ± SEM from 3 independent experiments. **p < 0.01, vs vehicle (Veh); #p < 0.05, ##p < 0.01 vs carvacrol (Car) by one-way ANOVA followed by Dunnett’s multiple comparison tests.
Erlotinib suppressed carvacrol-evoked Akt and NF-κB signalling in HaCaT keratinocytes
Given that erlotinib robustly suppressed the carvacrol-induced chemokine expression and chemotactic effect to PBMC, we evaluated the signalling pathways responsible for erlotinib effect. Carvacrol treatment (30 min) increased EGFR phosphorylation to 2.28 ± 0.67-fold of vehicle control (p < 0.01) in HaCaT cells (Fig. 2). Erlotinib (100 nM), which did not affect the basal level of EGFR phosphorylation, but abolished carvacrol-induced EGFR phosphorylation (Fig. 2). Similarly, carvacrol treatment also increased the phosphorylation levels of Akt, I-κB, and NF-κB to 1.98 ± 0.07 (p < 0.05), 4.07 ± 0.55 (p < 0.01), and 1.94 ± 0.27-fold (p < 0.05) of respective vehicle control (Fig. 2). Erlotinib treatment abolished carvacrol-induced Akt, I-κB, and NF-κB phosphorylation by 96.1 ± 13.1% (p < 0.05), 99.5 ± 0.6% (p < 0.01), and 76.9 ± 7.3% (p < 0.05), respectively (Fig. 2).
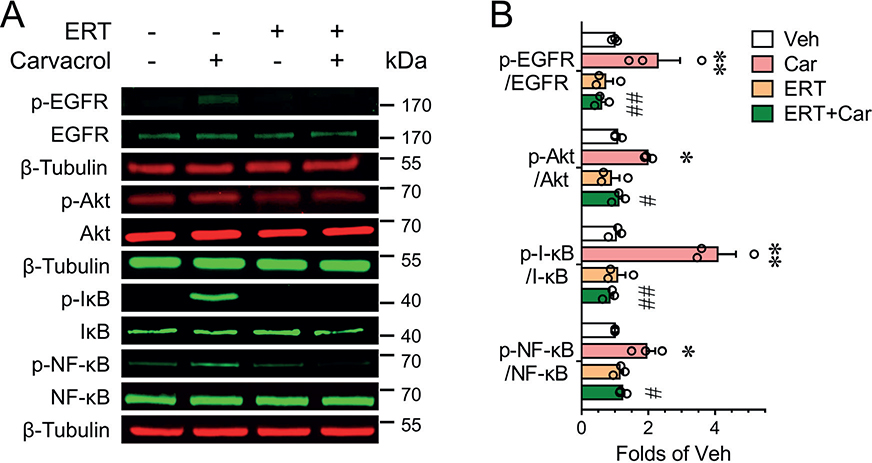
Fig. 2. Erlotinib suppressed carvacrol-evoked EGFR signalling in keratinocytes. (A–B) Representative western blots (A) and quantification (B) for phosphorylation of EGFR, Akt, I-κB, and NF-κB in HaCaT cells treated with 100 μM carvacrol (Car) in the absence or presence of 100 nM erlotinib (ERT) for 30 min. *p < 0.05, **p < 0.01, vs vehicle (Veh); #p < 0.05, ##p < 0.01 vs carvacrol (Car) by one-way ANOVA followed by Dunnett’s multiple comparison tests.
Inhibition of PI3K/Akt and I-κB/NF-κB suppressed carvacrol-induced proinflammatory response in keratinocytes
Given that erlotinib suppressed Akt and NF-κB phosphorylation, we examined the role of Akt and NF-κB on carvacrol-induced inflammatory response. The NF-κB inhibitor, BAY11-7085 (10 μM), which had no effect on basal IL-6 and IL-8 mRNA expression, decreased carvacrol-enhanced IL-6 and IL-8 expression by 80.0 ± 2.2% (p < 0.01), and 84.3 ± 0.9% (p < 0.01), respectively (Fig. 3A, B). Interestingly, inhibition of PI3K appeared to be less effective on carvacrol-enhanced IL-6 and IL-8 expression. LY294002 (10 μM) treatment inhibited carvacrol-enhanced IL-6 and IL-8 expression by 60.2 ± 3.5% (p < 0.01) and 25.8 ± 1.2% (p < 0.01), respectively (Fig. 3A, B). We further investigated the effects of Akt inhibition on carvacrol-enhanced cytokines expression. MK-2206 (100 nM) abolished carvacrol-enhanced IL-6 and IL-8 expression (Fig. S2). Moreover, PBMC recruitment assay showed that BAY11-7085 abolished the PBMC recruitment and LY294002 decreased the PBMC recruitment by 71.6 ± 7.7% (p < 0.01) (Fig. 3C, D).
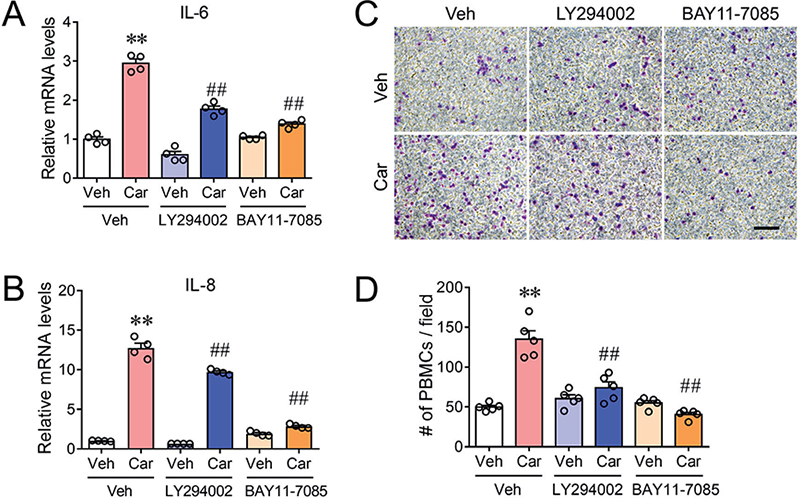
Fig. 3. Inhibition of PI3K/Akt and I-κB/NF-κB signalling suppressed carvacrol-induced proinflammatory responses in keratinocytes. (A–B) Relative mRNA levels of IL-6 (A) and IL-8 (B) in HaCaT cells treated with 100 μM carvacrol (Car) in the absence and presence of 1 μM BAY11-7085 or 10 μM LY294002 for 3 h. Data were normalized to the vehicle (Veh) control. (C) Representative microphotographs showing fixed and stained PBMCs on the bottom side of the invasion membrane. Scale bar, 50 μm. (D) Quantification of PBMC chemotaxis induced by media harvested from cultured HaCaT cells treated with Car in the absence and presence of BAY11-7085 or LY294002 for 6 h. Data in (A), (B), and (D) are mean ± SEM from 3 independent experiments. **p < 0.01, vs vehicle (Veh); ##p < 0.01 vs carvacrol (Car) by one-way ANOVA followed by Dunnett’s multiple comparison tests.
Erlotinib ameliorates carvacrol-induced skin inflammation in C57BL/6 mice
Given that erlotinib suppressed the proinflammation in HaCaT keratinocytes and inhibited the chemotaxis of PBMCs, we therefore examined the potential therapeutic effect of erlotinib against carvacrol-induced dermatitis in C57BL/6 mice. Consistent with our previous finding, topical application of 2% carvacrol once daily for 4 consecutive days evoked inflamed and scaly lesions. Subcutaneous injection of erlotinib alleviated atopic dermatitis-like symptoms including redness, oedema, and thickening (Fig. 4A, upper panel). Erlotinib treatment reduced the dermatitis score to 43.6 ± 4.7% (p < 0.01) (Fig. 4B). Carvacrol treatment induced remarkable acanth-osis, as indicated by the increased epidermal thickness (Fig. 4A, bottom panel and C). Erlotinib decreased the carvacrol-induced acanthosis by 45.0 ± 7.6% (p < 0.01) (Fig. 4C) without affecting the gross appearance and the skin thickness in vehicle-treated mice (Fig. S3). Carvacrol also triggered scratching behaviour, quantified by the increased number of scratching bouts from 3.20 ± 1.24 to 29.40 ± 3.98 per 10 min (p < 0.01). Erlotinib did not affect carvacrol-induced pruritus (27.00 ± 5.86 vs 29.40 ± 3.98 per 10 min, p > 0.05) (Fig. 4D). The serum IgE level in carvacrol-treated mice was increased from 2.37 ± 0.04 μg/mL to 7.44 ± 1.20 μg/mL (p < 0.01), which was decreased by 70.9 ± 1.4% with erlotinib treatment (p < 0.01) (Fig. 4E). The mRNA levels of inflammatory cytokines including Il-1β, Il-4, Tnf-α, Il-6, and Cxcl15 were increased by carvacrol treatment to 7.22 ± 1.47 (p < 0.01), 2.96 ± 0.19 (p < 0.01), 5.51 ± 0.83 (p < 0.01), 25.52 ± 5.18 (p < 0.01), and 5.03 ± 0.35-fold (p < 0.01) of respective control of the vehicle-treated group. Erlotinib caused reductions on the carvacrol-induced mRNA levels by 56.0 ± 1.9% for Il-1β (p < 0.01), 57.2 ± 15.2% for Il-4 (p < 0.05), 39.0 ± 7.7% for Tnf-α (p < 0.01), 77.3 ± 4.1% for Il-6 (p < 0.01), and 58.4 ± 9.9% for Cxcl15 (p < 0.01) (Fig. 4F), respectively. These results suggest that erlotinib can alleviated the pathological hallmarks in the carvacrol-induced dermatitis.
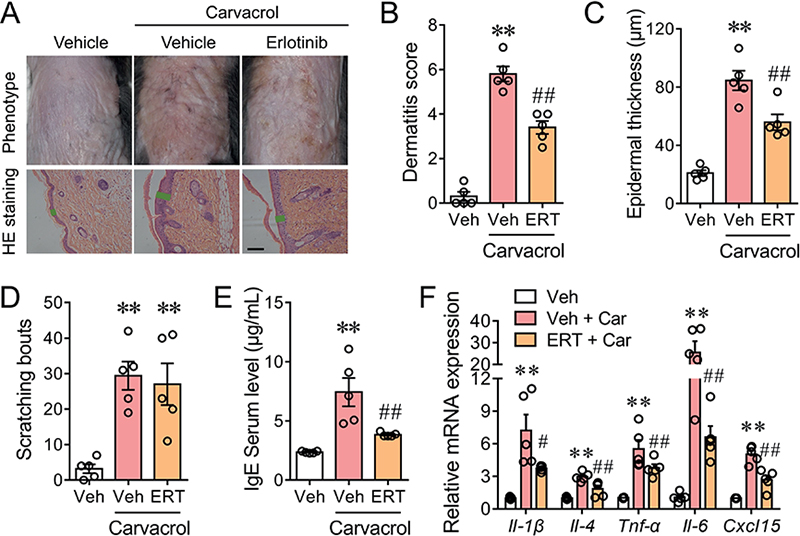
Fig. 4. Erlotinib ameliorates carvacrol-induced skin inflammation in C57BL/6 mice. (A) Representative images of the gross appearance (upper panel) and H&E-stained sections (bottom panel) of the back skins of WT C57BL/6J mice treated with vehicle (Veh), 2% carvacrol (Car), and Car plus 2.0 mg/kg erlotinib (ERT). Black scale bar, 50 μm. Green lines indicate the thickness of stratum epidermis. (B–E) Quantification of dermatitis score (B), epidermal thickness (C), scratching bouts (D), and serum IgE level (E) in mice treated with Veh, Car, and Car plus ERT. (F) Relative mRNA levels of Il-1β, Il-4, Tnf-α, Il-6, and Cxcl15 in the back skins of the indicated groups, normalized to that of the Veh group. All bar graphs represent mean ± SEM (n = 5 mice). **p < 0.01, vs Veh; #p < 0.05, ##p < 0.01 vs Car, by one-way ANOVA followed by Dunnett’s multiple comparison tests.
DISCUSSION
EGFRs are prominently expressed in the epithelial cells, where they regulate the growth and repair process of epithelia (22). Mutation and/or overexpression of EGFR leads to carcinoma such as non-small-cell lung cancer (NSCLC), squamous cell carcinoma, and breast cancer (23). Previous studies have shown that activation of TRPV3 by carvacrol triggers cell proliferation and chemokine release in HaCaT cells (15, 16). Such cell proliferative effect is dependent on the EGFR activation through Ca2+-mediated TGFα release (16). A study has also demonstrated that EGFR activation in keratinocytes induces expression of granulocyte/macrophage colony-stimulating factor (GM-CSF), a cytokine associated with psoriasis or allergic contact dermatitis progression (24). TNF-α-induced EGFR transactivation results in increased expression of keratinocyte-derived thymic stromal lymphopoietin (TSLP) (25). In the current study, we showed that erlotinib, an EGFR inhibitor, suppressed the expression of chemokines in human HaCaT keratinocytes and inhibited the chemotaxis of PBMCs, unambiguously demonstrating that in addition to cell proliferation EGFR also regulated the inflammatory response in keratinocytes. Moreover, we demonstrated that erlotinib suppressed carvacrol-induced dermatitis score, acanthosis, and inflammatory mediator expression in the mouse. These data demonstrated that erlotinib, a clinically used drug compound for non-small-cell lung carcinoma therapy may have the potential to treat skin inflammatory diseases. To support our finding, erlotinib has been used to treat Olmsted syndrome, a severe skin disease resembling the features of psoriasis, with satisfactory efficacy (18). Interestingly, erlotinib has no effect on carvacrol-induced purpurites, suggesting that erlotinib does not affect the neuronal firing.
Our study also demonstrated that erlotinib suppressed proinflammation in HaCaT keratinocytes through decreased activity of EGFR with subsequent attenuated activities of PI3K/Akt and I-κB/NF-κB. As a downstream signalling effector of EGFR receptor tyrosine kinase activity, PI3K/Akt is generally responsible for the proliferative effect by upregulation of a variety of cell growth and proliferative genes (26). Interestingly, we found that pharmacological suppression of PI3K/Akt robustly suppressed the carvacrol-induced chemokine expression, and chemotaxis of PBMCs suggesting that, in addition to its role in the proliferation, the PI3K/Akt signalling pathway was also involved in keratinocyte proinflammation. Erlotinib suppressed the carvacrol-induced phosphorylation level of Akt, suggesting that erlotinib-suppressed proinflammation in HaCaT keratinocytes was through decreased PI3K/Akt activity. We also demonstrated that pharmacological suppression of I-κB/NF-κB suppressed the carvacrol-induced chemokine expression and chemotaxis of PBMCs. Moreover, erlotinib suppressed carvacrol-induced phosphorylation level of I-κB and NF-κB. Together, these data suggested the involvement of I-κB/NF-κB in the erlotinib suppression of proinflammation in keratinocytes. Although it has been widely reported that NF-κB regulates the expression of an array of inflammatory mediators, how EGFR signalling to NF-κB occurs has not been fully clarified. Whether I-κB/NF-κB depends on PI3K/Akt activity needs further elucidation.
In conclusion, we demonstrate that erlotinib alleviates carvacrol-induced proinflammation responses in HaCaT keratinocytes through inhibition of the EGFR-mediated PI3K/Akt and I-κB/NF-κB signalling pathway. We also demonstrated that erlotinib ameliorates carvacrol-induced atopic dermatitis-like skin inflammation. Erlotinib, a clinically used agent, has the potential to treat skin inflammatory diseases.
ACKNOWLEDGEMENTS
IRB approval status: All animal protocols were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and approved by the laboratory animal care and use committee of China Pharmaceutical University.
REFERENCES
- Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol 2017; 153: 406–412. https://doi.org/10.1001/jamadermatol.2016.5538
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014; 134: 1527–1534. https://doi.org/10.1038/jid.2013.446
- Peng D, Sun J, Wang J, Qi X, Li G. Burden of skin disease – China, 1990–2019. China CDC Weekly 2021; 3: 472–475. https://doi.org/10.46234/ccdcw2021.123
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers 2018; 4: 1. https://doi.org/10.1038/s41572-018-0001-z
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. https://doi.org/10.1016/j.jaad.2014.03.023
- Suntres ZE, Coccimiglio J, Alipour M. The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr 2015; 55: 304–318. https://doi.org/10.1080/10408398.2011.653458
- Arruri VK, Gundu C, Kalvala AK, Sherkhane B, Khatri DK, Singh SB. Carvacrol abates NLRP3 inflammasome activation by augmenting Keap1/Nrf-2/p62 directed autophagy and mitochondrial quality control in neuropathic pain. Nutr Neurosci 2022; 25: 1731–1746. https://doi.org/10.1080/1028415X.2021.1892985
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006; 9: 628–635. https://doi.org/10.1038/nn1692
- Khalil A, Shekh-Ahmad T, Kovac S, Wykes RC, Horgen FD, Fleig A, et al. Drugs acting at TRPM7 channels inhibit seizure-like activity. Epilepsia Open 2023; 10.1002/epi4.12773. https://doi.org/10.1002/epi4.12773
- Wang Y, Tan L, Jiao K, Xue C, Tang Q, Jiang S, et al. Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br J Pharmacol 2022; 179: 4792–4808. https://doi.org/10.1111/bph.15913
- Qi H, Shi Y, Wu H, Niu C, Sun X, Wang K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm Sin B 2022; 12: 723–734. https://doi.org/10.1016/j.apsb.2021.08.002
- Qu Y, Wang G, Sun X, Wang K. Inhibition of the warm temperature-activated Ca2+-permeable transient receptor potential vanilloid TRPV3 channel attenuates atopic dermatitis. Mol Pharmacol 2019; 96: 393–400. https://doi.org/10.1124/mol.119.116962
- Sabbah DA, Hajjo R, Sweidan K. Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr Top Med Chem 2020; 20: 815–834. https://doi.org/10.2174/1568026620666200303123102
- Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010; 141: 331–343. https://doi.org/10.1016/j.cell.2010.03.013
- Szöllősi AG, Vasas N, Angyal Á, Kistamás K, Nánási PP, Mihály J, et al. Activation of TRPV3 regulates inflammatory actions of human epidermal keratinocytes. J Invest Dermatol 2018; 138: 365–374. https://doi.org/10.1016/j.jid.2017.07.852
- Wang Y, Li H, Xue C, Chen H, Xue Y, Zhao F, et al. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol Toxicol 2021; 37: 313–330. https://doi.org/10.1007/s10565-020-09536-2
- Lee JS, Woo J, Kim TM, Kim N, Keam B, Jo SJ. Skin toxicities induced by epidermal growth factor receptor tyrosine kinase inhibitors and their influence on treatment adjustments in lung cancer patients. Acta Derm Venereol 2024; 104: adv40555. https://doi.org/10.2340/actadv.v104.40555
- Greco C, Leclerc-Mercier S, Chaumon S, Doz F, Hadj-Rabia S, Molina T, et al. Use of epidermal growth factor receptor inhibitor erlotinib to treat palmoplantar keratoderma in patients with Olmsted syndrome caused by TRPV3 mutations. JAMA Dermatol 2020; 156: 191–195. https://doi.org/10.1001/jamadermatol.2019.4126
- Zhang A, Duchatelet S, Lakdawala N, Tower RL, Diamond C, Marathe K, et al. Targeted inhibition of the epidermal growth factor receptor and mammalian target of rapamycin signaling pathways in Olmsted syndrome. JAMA Dermatol 2020; 156: 196–200. https://doi.org/10.1001/jamadermatol.2019.4141
- Butala S, Phan S, Siegel DH, Carlberg V, Paller AS. Two for two: dual therapy with erlotinib and acitretin for twins with severe keratoderma in Olmsted syndrome. Pediatr Dermatol 2023; 40: 735–737. https://doi.org/10.1111/pde.15264
- Li S, Zhao F, Tang Q, Xi C, He J, Wang Y, et al. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2b) mediates oxidation-induced endoplasmic reticulum stress to regulate neuropathic pain. Br J Pharmacol 2022; 179: 2016–2036. https://doi.org/10.1111/bph.15744
- Schlessinger J. Receptor tyrosine kinases: legacy of the first two decades. CSH Perspect Biol 2014; 6. https://doi.org/10.1101/cshperspect.a008912
- Liu X, Wang P, Zhang C, Ma Z. Epidermal growth factor receptor (EGFR): a rising star in the era of precision medicine of lung cancer. Oncotarget 2017; 8: 50209–50220. https://doi.org/10.18632/oncotarget.16854
- Mascia F, Cataisson C, Lee TC, Threadgill D, Mariani V, Amerio P, et al. EGFR regulates the expression of keratinocyte-derived granulocyte/macrophage colony-stimulating factor in vitro and in vivo. J Invest Dermatol 2010; 130: 682–693. https://doi.org/10.1038/jid.2009.336
- Segawa R, Shigeeda K, Hatayama T, Dong J, Mizuno N, Moriya T, et al. EGFR transactivation is involved in TNF-α-induced expression of thymic stromal lymphopoietin in human keratinocyte cell line. J Dermatol Sci 2018; 89: 290–298. https://doi.org/10.1016/j.jdermsci.2017.12.008
- Padfield E, Ellis HP, Kurian KM. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol 2015; 5: 5. https://doi.org/10.3389/fonc.2015.00005