REVIEW ARTICLE
A Systematic Review of 207 Studies Describing Validation Aspects of the Dermatology Life Quality Index
Jui VYAS1, Jeffrey R. JOHNS2, Faraz M. ALI2, John R. INGRAM2, Sam SALEK3 and Andrew Y. FINLAY2
1Centre for Medical Education, School of Medicine, Cardiff University, Cardiff, 2Division of Infection and Immunity, School of Medicine, Cardiff University, Cardiff, and 3School of Life and Medical Sciences, University of Hertfordshire, Hatfield, UK
This study systematically analysed peer-reviewed publications describing validation aspects of the Dermatology Life Quality Index (DLQI) and used Naicker’s Critically Appraising for Antiracism Tool to assess risk of racial bias. Seven online databases were searched from 1994 until 2022 for articles containing DLQI validation data. Methodology followed PRISMA guidelines, the protocol was registered in PROSPERO, and articles reviewed independently by two assessors. Of 1,717 screened publications, 207 articles including 58,828 patients from > 49 different countries and 41 diseases met the inclusion criteria. The DLQI demonstrated strong test–retest reliability; 43 studies confirmed good internal consistency. Twelve studies were performed using anchors to assess change responsiveness with effect sizes from small to large, giving confidence that the DLQI responds appropriately to change. Forty-two studies tested known-groups validity, providing confidence in construct and use of the DLQI over many parameters, including disease severity, anxiety, depression, stigma, scarring, well-being, sexual function, disease location and duration. DLQI correlation was demonstrated with 119 Patient Reported Outcomes/Quality of Life measures in 207 studies. Only 15% of studies explicitly recruited minority ethnic participants; 3.9% stratified results by race/ethnicity. This review summarizes knowledge concerning DLQI validation, confirms many strengths of the DLQI and identifies areas for further validation.
SIGNIFICANCE
The Dermatology Life Quality Index is a questionnaire that measures how skin disease affects people’s lives. It is commonly used because it is simple and easy to use, and the scores have meaning. This study looked at 207 published medical articles to find out about how appropriate and accurate the Dermatology Life Quality Index is to use. This confirmed the many strengths of the Dermatology Life Quality Index, supporting its very wide acceptance and use. This study provides valuable information for researchers and doctors who may want to use it in the future and continue its use in routine clinical practice as well as in clinical trials of new treatments.
Key words: Dermatology Life Quality Index (DLQI); validation; quality of life; patient-reported outcome measures.
Citation: Acta Derm Venereol 2024; 104: adv41120. DOI https://doi.org/10.2340/actadv.v104.41120.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Jul 16, 2024; Accepted after revision: Sep 12, 2024; Published: Nov 7, 2024
Corr: Jui Vyas, Centre for Medical Education, School of Medicine, Cardiff University, Cardiff, CF14 4XN, UK. E-mail: vyasjj@cardiff.ac.uk
Competing interests and funding: AYF is joint copyright owner of the DLQI. Cardiff University receives royalties from some use of the DLQI: AYF receives a proportion of these under standard university policy. JI receives a stipend as Editor-in-Chief of the British Journal of Dermatology and an authorship honorarium from UpToDate. He is a consultant for Abbvie, Boehringer Ingelheim, ChemoCentryx, Novartis and UCB Pharma and has served on advisory boards for Insmed, Kymera Therapeutics and Viela Bio. He is co-copyright holder of HiSQOL, Investigator Global Assessment and Patient Global Assessment instruments for HS. His department receives income from royalties from the Dermatology Life Quality Index (DLQI) and related instruments. SS has received an unrestricted educational grant from GSK, is a consultant for Novo Nordisk and produces educational materials for Abbvie. JV participated in an Advisory Board for Amgen, has received payment or honoraria from L’Oreal and support from UCB pharma for attending meetings. FA has received honorariums from Abbvie, Janssen, LEO pharmaceuticals, Lilly pharmaceuticals, L’Oreal, Novartis and UCB. His department receives income from royalties from the Dermatology Life Quality Index (DLQI) and related instruments. JJ has no conflicts of interest to report. His department receives income from royalties from the Dermatology Life Quality Index (DLQI) and related instruments.
Funding was provided by the Division of Infection and Immunity, School of Medicine, Cardiff University, Cardiff, UK.
INTRODUCTION
The Dermatology Life Quality Index (DLQI) (1) is the most widely used tool for clinicians and researchers to understand the burden of skin diseases on patients and to assess the effectiveness of interventions. The DLQI was created in order to measure the impact over the last seven days of skin disease on the quality of life of patients. A systematic review has identified the use of the DLQI in 454 randomised controlled trials encompassing 68 diseases and 42 countries (2). The extensive world-wide clinical use of the DLQI includes being incorporated in guidelines or registries in at least 45 countries (3).
It is important therefore that users have access to what has been published concerning the validation of this instrument. Validating quality of life questionnaires is critical to ensure they accurately and reliably measure what they intend to measure (4). However, often information is published alongside the reporting of other aspects of the use of the DLQI, resulting in much validation being difficult to identify and access. There have been systematic reviews of scoring methods applied to DLQI data (5) and of the correlation of the DLQI with psychiatric measures(6), but to date no comprehensive systematic reviews of DLQI validation has been carried out.
There have been many relevant studies published since two previous reviews (7,8) of DLQI validation. The aim of this systematic review was to identify all published aspects of DLQI validation since the DLQI was published in 1994 (1).
MATERIALS AND METHODS
Scope of the study
We defined validation as the collection and analysis of data to assess the validity and reliability of a Quality of Life (QoL) instrument to determine the extent to which an instrument measures what it purports to measure (4, 9). We defined patient-reported outcome (PRO) measures as those completed directly by the patient based on their own perception including: quality of life; patient satisfaction; and/or signs and symptoms.
Our eligibility criteria for validation included:
- Studies that presented data and analysis that supported validation of the DLQI e.g. factor structure, test–retest, internal consistency, responsiveness, differential item functioning (DIF), clinical meaning (Minimal Important Difference MID, Minimally Clinically Important Difference MCID), translation, cross-cultural adaptation, mapping and score banding.
- Translations and cross-cultural adaptations.
- Correlation of DLQI with other QoL/PRO measures (but not correlations with disease severity scales or non-QoL measures e.g. willingness to pay, patient satisfaction, cost-benefit.
Ineligible criteria for validation:
- Where the DLQI was used to validate another measure.
- Correlations with non-patient (physician) reported measures (mostly severity indices) e.g. PASI (Psoriasis Area and Severity Index), or correlations with clinical (laboratory) parameters e.g. T-cell counts or PROs that were not QoL measures.
Data sources
This study follows 2020 PRISMA guidelines for reporting systematic reviews (10). The study protocol and detailed search strategy was published on PROSPERO Prospective Register of Systematic Reviews (CRD42022308453) (11) and details are also given in the Appendix S1 DLQI Validation Studies Search strategy. Medline (Ovid), Cochrane Library, EMBASE, Web of Science, SCOPUS, CINAHL(EBSCO) and PsycINFO online databases from January 1, 1994 (DLQI creation) to December 31, 2022 were searched independently by two authors (JJ, JV), and results corroborated. Search terms included ‘DLQI’ and ‘dermatology life quality index’. As complete a list as possible of validation search terms was used to ensure comprehensive coverage without creating excessive non-relevant data. Database specific “article type/study type” keywords, language keywords (English) keywords were also used to search the required types of study to be included. Because of the difficulty of age selection (16 years old and over) using database search terms, all ages were included in the search, and those below the inclusion age were filtered manually in EndNote. Duplicate records were excluded.
Search strategy/Selection
A set of eligibility criteria were applied for selection of the included studies (Table I). Search results were imported into EndNote20® (12). Two authors (JJ, JV) independently compared study titles and abstracts retrieved by searches against the inclusion and exclusion criteria and examined full study texts. Rejected studies were recorded with reasoning. A third author (FA) resolved and recorded any study selection disagreements (10) (Fig. 1).
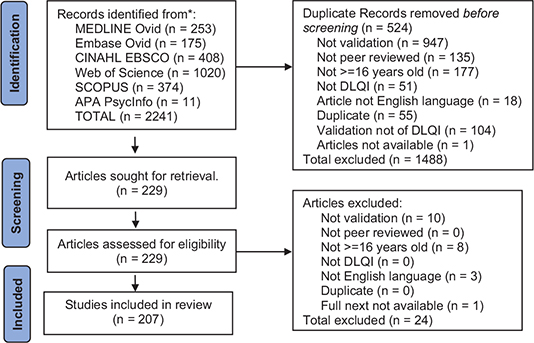
Fig. 1. PRISMA flow diagram reporting the number of records identified from each database. Inclusion criteria applied by search engines where applicable, i.e., English language, journal articles, peer reviewed.
Data extracted
The recorded Information included the study aim, disease studied, disease severity, research setting, e.g. trial, hospital, clinic, community, single or multi-centred, number of sites, study countries, the number of subjects for which DLQI data was collected, the study type and design of the original data collected, DLQI mean scores at baseline and DLQI endpoints, and details of validation methods used including type, statistical test or specific analysis methods e.g. exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) for factor structure, test–retest, internal consistency, responsiveness, clinical meaning (MCID) and validity. Data on cross-cultural adaptations and DIF were also collected. For convergent validity, only correlations with other PRO QoL measures were included (Appendix S1). Known group analysis was captured when statistical testing was applied to defined groups where there would be an expected difference e.g. disease severity as the anchor. However, when there was no indication from the author of the expectancy of a difference (a priori hypothesis or reference to a previously published study) by age and gender for example, the data was not extracted.
Data extraction and synthesis
For data extraction, guidance of the Cochrane Handbook for Systematic Reviews of Interventions was followed (13). A REDCap database (14–16) (a secure web application for building/managing online surveys and databases) was created. The authors JJ and JV independently extracted data from the included publications to parallel REDCap database tables, and an adjudicator (FA) resolved any disagreements in data extraction. Missing data were noted in the data templates, but none was sufficiently important to contact original authors. The two reviewers independently assessed the risk of bias (quality) of included studies using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines (17).
Racial bias in research can also impact a study’s validity, reliability and relevancy (18–20). Minoritised populations have different outcomes, in part due to genetic ancestry (21), and thus recruiting for diversity is essential and results should be stratified by race/ethnicity if relevant to the study (22). This aspect is currently rarely addressed in systematic reviews of validation. To raise awareness of this issue, appraisal of representation of minorities ethnic participants in the studies was conducted using Naicker’s Critically Appraising for Antiracism Tool (23).
We considered disease severity as a clinical outcome, not a patient reported outcome. Good correlations would only be expected between closely related QOL measures (convergent validity) and therefore correlations between the DLQI and disease severity/burden measures (objective parameters rated by clinicians e.g. PASI) were not extracted, only correlations with other PRO/QOL measures were considered appropriate as they are different constructs.
Good correlations would only be expected between closely related QOL measures (convergent validity) and therefore correlations between the DLQI and disease severity/burden measures (objective parameters rated by clinicians e.g. PASI) were not extracted, only correlations with other PRO-QOL measures.
As this is a systematic review, all methods used were reported, not just those that are considered good evidence or good measurement properties (24). Thus intra-class correlation coefficient (ICC), interclass relationship (ICR), Spearman’s, Pearson’s, Wilcoxon and interrater reliability kappa statistics were all reported, although only ICC and kappa measures (with their associated rating and criteria) are considered “good” methods by the COSMIN guidelines.
RESULTS
A total of 1661 studies were provided by database searching after removing 679 duplicates. After filtering these in an EndNote database for inclusion/exclusion criteria, 231 full text articles were assessed, of which 207 described research on 58,828 patients meeting the inclusion eligibility criteria (Fig. 1). Publications of validation of the DLQI are increasing, with 15 new studies reported in 2022 (Fig. S1).
Study sites and settings
136 (65.7%) of the studies were conduced at a single site, 40 were multicentre (19.3%) and 27 (8.7%) did not specify, for three (1.45%) a site was not applicable, and one (0.48%) was a postal survey. Of the multicentre studies, 14 (35.0%) were conducted at two sites, 15 (37.5% at 3–10 sites, 5 (12.5%) at 11–20 sites, and 6 (15.0%) at > 20 sites. Most studies (173, 83.6%) did not involve any intervention, and were not part of a clinical trial.
The original study designs comprised one randomised control trial (RCT, blinding not specified), five RCT double blinded, four RCT single blinded, four open-label, one cohort study, 15 case controlled, 173 with no specific intervention, and two not determinable.
The design used for validation analysis comprised 28 multiple arm, 165 single arm, 172 cross sectional, 25 longitudinal, 5 placebo controlled, 1 parallel group, 2 Phase II RCT, 3 Phase III RCT, 1 Phase IV RCT and one cross-over study (some in multiple categories).
Trials were conducted in at least 49 different countries, although two reported multiple countries without listing details (Table SI). Most studies were conducted in single countries: USA (n = 21, 9.7%), Turkey (17, 7.9%), UK (15, 6.9%), China (14, 6.5), Brazil (13, 6.0%), Germany (13, 6.0%), Iran (11, 5,1%), Italy (5,1%) with 101 (46.8%) countries having <5 studies, and 21 (9.7%) countries only having a single study.
At least 33 different language variants (including specific adaptations e.g. Arabic Egypt, Arabic Lebanon, Arabic Morocco, Arabic Saudi Arabia, Arabic Tunisia) were used in the studies. 53 (37.6%) of studies did not specify explicitly which language version of the DLQI they used, while 12 studies (8.5%) used multiple language versions. The English version was the most used (39 studies, 27.7%), followed by Turkish (13, 9.2%), Portuguese (12, 8.5%), Chinese Mandarin (10, 7.1%), and Farsi (10, 7.1%).
Disease profile
Forty-one different diseases were studied. Most studies were of psoriasis (n = 52, 25.5%), followed by atopic dermatitis (n = 18, 8.8%), vitiligo (n = 14, 1.9%), acne (n = 11, 5.4%), eczema (n = 10, 4.9%), and urticaria (n = 8, 3.9%). A complete list is given in Appendix S1. Overall, studies recruited patients with mild (n = 64, 18.9%), moderate (n = 82, 24.3%) and severe (n = 81, 24.0%) disease, with 111 (32.8%) unspecified.
Content validity
Validity measures included 43 known group, 10 construct, 21 convergent, 4 concurrent, 2 divergent/discriminant, 8 content, 4 criterion, 2 face and 2 predictive validity tests using Mann-Whitney (18), Spearman’s correlation (11), Pearson’s correlation (6) and Student’s t-test (6), EFA (1), CFA (1) and 11 with other tests. DLQI responsiveness analysis was performed in 12 studies, using paired t-test (1), effect size (5), correlation of DLQI with another measures (7), Analysis of Variance test (ANOVA) (1), Wilcoxon two-sample (2) and bivariate models (1) (Table SI(A)).
Dimensionality and factor structure
A total of 28 studies applied either factor analysis or item response theory to examine the dimensionality of the DLQI. A variable number of factors (one–four) underlying the DLQI structure was demonstrated in these studies (Table SI(B)).
Test–retest reliability and internal consistency reliability
Test–retest reliability of the DLQI was assessed in 13 studies (Table II), reporting Spearman’s rank correlations between 0.97 and 0.99, a Pearson’s correlation of 0.96, ICC between 0.77 and 0.983 with 7 of 9 above 0.90, ICR of 0.96, and a Kappa of 0.83. All of these were above acceptance values (ICC very high (ICC > 0.9), high (ICC > 0.75), moderate (ICC between 0.5–0.75) (25) or Kappa 0.81–1.00 as almost perfect agreement (26), and Spearman’s between 0.7 and 0.9 indicating strong correlations (27). Test–retest intervals reported were between 5 to 10 days (to minimise under or over-estimation) in line with published recommendations (9). The internal consistency of the DLQI was assessed in 43 studies (Table II) and ranged from 0.673 to 0.997 with a mean value of 0.834 and standard deviation of 0.069. 41 out of 42 (95.3%) of Cronbach’s alpha values reported were above the acceptance value of ≥ 0.70 (17).
| Reference | Country | DLQI completed | Disease | Method used | Results | COSMIN |
| Test–retest reliability | ||||||
| Finlay 1994(1) | United Kingdom | 100 | Any skin disease | Spearman’s rank correlation | Test–retest reliability correlation coefficients Spearman rank gamma = 0-99, p < 0.0001); test–retest reliability of individual question scores (gamma =0.95–0.98, p <0.001) | ? |
| Badia 1999(34) | Spain | 246 | Eczema and psoriasis | ICC | ICC eczema 0.77, psoriasis 0.90 | + |
| Jobanputra 2000(35) | South Africa | 660 | 84 different diagnoses were made during the study. Dermatitis, including atopic and contact dermatitis (26%), psoriasis (18%), and acne (10%) were the most common disorders | Spearman’s rank correlation | r=0.97; p < 0.0001 (n = 65) | |
| Ferraz 2006(36) | Brazil | 115 | Multiple for reliability incl. onychomycosis and psoriasis (6 patients each), Contact dermatitis 4, and solar keratosis, viral warts, vitiligo (3 patients each). Lupus Erthematous for validity | ICR,Pearson’s | Pearson correlation coefficient for inter-observer reliability was 0.96 (p <0.001), n = 44 | ? |
| Takahashi 2006(37) | Japan | 197 | Acne | ICC | Test–retest reliability of the DLQI-J was slightly less than that of the original English version. n = 44 ICC=0.90) | + |
| Baranzoni 2007(38) | Italy | 22 | Any skin disease | ICC, Wilcoxon’s signed rank test | Retest at 1–2 weeks. n = 19 ICC = 0.983, p <0.001. Weighted kappa between 0.644 and 0.984 for items. No statistically significant difference found in total score between 1st and 2nd assessments (p = 0.016). p > 0.15 for all but 2 questions: symptoms p = 0.083 and clothes p = 0.096. mean DLQI 1st assessment = 9.14 ± 5.50, and 2nd assessment = 9.45 ± 5.86 | + |
| Mackenzie, 2011(39) | Canada | 60 | Psoriasis and Psoriatic arthritis | ICC | 0.96 (0.93, 0.97) (n = 60) | + |
| Madarasingha 2011(40) | Sri Lanka | 200 | Eczema (24.5%), Psoriasis (23.0%), Acne (10.0%), Vitiligo (14.5%), Infections (10.5%), Other (17.5%) | Cohen’s Kappa | Kappa test–retest reliability coefficient of 0.83 | + |
| Khoudri 2013(41) | Morocco | 244 | Psoriasis | ICC | ICC of the test–retest reliability was 0.97 for the overall DLQI and exceeded 0.70 in all scales. | + |
| Liu 2012(42) | China | 131 | Urticaria | ANOVA | ANOVA with Friedman’s test chi2 = 320.61 (p <0.001) indicated good repeatability using the DLQI of Chinese version. | ? |
| Ali 2017(43) | United Kingdom | 104 | Any skin disease | ICC, Wilcoxon’s signed rank test | ICC = 0,98; 95% confidence interval (CI) 0.97–0.99 | + |
| Jesmin 2021(44) | Bangladesh | 80 | Psoriasis | ICC | ICC= 0.97 | + |
| Meneguin 2021(45) | Brazil | 188 | psoriasis, cellulitis/erysipelas, chronic ulcers and eczematous dermatosis, other dermatoses | ICC | For cases that did not show any clinical change in their disease status (n = 44), first interview media n = 9 (4.5–11), second interview after 7 to 14 days media n = 10 (5.5–11.5). ICC 0.95 (CI 0.88–0.98) | + |
| Schwartzman 2021(46) | United States | 994 | Atopic dermatitis | ICC | 0.81 (95% CI 0.76–0.85) | + |
| Internal consistency reliability studies | ||||||
| Finlay 1994(1) | United Kingdom | 100 | Any skin disease | Consistency between all questions when paired was found to be statistically significant (p = 0.002) ranging from Rank correlations of 0-23-0-70 | ? | |
| Badia 1999(34) | Spain | 246 | Eczema and psoriasis | α = 0·83 | + | |
| Jobanputra 2000(35) | South Africa | 660 | 84 different diagnoses were made during the study. Dermatitis, including atopic and contact dermatitis (26%), psoriasis (18%), and acne (10%) were the most common disorders | α = 0.83. The inter-item rank correlation coefficients ranged from 0.04 to 0.54 | + | |
| Zachariae 2000(47) | Denmark | 400 | Psoriasis, Atopic eczema, Other eczema, Urticaria, Bullous disease, Erythroderma, Hyperhidrosis, Collagenosis, Pruritus, Acne, Viral warts, Miscellaneous | α = 0.88 | + | |
| Shikiar 2003(48) | United States | 1095 | Psoriasis | Study A baseline α =0.871, week12 α =0.921; Study B baseline α =0.869, week12 α =0.919 | + | |
| Aghaei 2004(49) | Iran | 70 | Vitiligo | α = 0.77 | Cronbach’s alpha by domain, and by gender, marital status, severity, and extension of disease, and | + |
| Ilgen 2005(50) | Türkiye | 108 | Acne | α = 0.87 | + | |
| Mazzotti 2005(51) | Italy | 900 | Psoriasis | a = 0.83; item-total correlation = 0.40–0.70 | + | |
| Ozturkcan 2006(52) | Türkiye | 79 | Eczema-contact dermatitis, Psoriasis, Urticaria, chronic urticaria, Tinea, Alopecia areata, Acne | Cronbach’s α = 0.87. The item versus total (overall). Spearman’s correlation coefficients ranged from 0.48–0.81 with a median of 0.66, and the subscales versus total (overall) ranged from 0.71–0.83 with a median of 0.77. The α value was 0.84 for the age groups under 20 years and 0.89 for the 21+ years age group | males 0.83 and females 0.88; outpatients 0.86 and inpatients 0.87; eczema/acne 0.90 and other dermatological disorders 0.84). | + |
| Shikiar 2006(53) | United States | 147 | Psoriasis | α was 0.89 at baseline, 0.92 at Week 12 | + | |
| Takahashi 2006(37) | Japan | 197 | Acne | α = 0.83. Exclusion of any one of the 10 items did not increase α by > than 0.01. | + | |
| Baranzoni 2007(38) | Italy | 22 | Any skin disease | α = 0.787 for 1st assessment, 0.828 for 2nd assessment | + | |
| Mazharinia 2007(54) | Iran | 109 | Burns | Cronbach’s α for physical Q1,3,5,7,10, psychological Q2,4,6,8, and sexual domains Q9 and for total DLQI were 0.78, 0.77, 0.72, and 0.75, respectively. | + | |
| Henok 2008(55) | Ethiopia | 74 | Podoconiosis | Overall α value was 0.90, standardized item alpha 0.89. Average inter-item correlation was 0.44, item total correlation ranged from 0.15 to 0.81. Only item 6 (about sport) had a value of <0.2. The average item total correlation was 0.64. | + | |
| Aghaei 2009(56) | Iran | 125 | Psoriasis | α = 0.79 | + | |
| An 2010 (57) | China | 128 | Leprosy | Cronbach’s α = 0.765, standardized item α =0.759, | Average inter-item correlation was 0.240 (> 0.2), Item total correlation ranged from 0.212 to 0.596. Average item total correlation was 0.427. | + |
| Madarasingha 2011(40) | Sri Lanka | 200 | Eczema (24.5%), Psoriasis (23.0%), Acne (10.0%), Vitiligo (14.5%), Infections (10.5%), Other (17.5%) | Cronbach’s α 0.561 to 0.741 (except for the personal relationship domain). Healthy volunteers (n = 40): Symptoms and feelings (0.598), Daily activities (0.654), Leisure (0.569). Personal relationships (0.498). Patients (n = 200): Symptoms and feelings (0.561), Daily activities (0.741, Leisure (0.687). Personal relationships (0.442). | – | |
| Liu 2012(42) | China | 131 | Urticaria | α was 0.82, and it became 0.84 when item 1 was deleted. The α value reached 0.85 after standardization | + | |
| Maksimovic 2012(58) | Serbia | 66 | Atopic dermatitis | α = 0.84 | + | |
| Twiss 2012(59) | United Kingdom | 292 | Psoriasis and Atopic dermatitis | The Person Separation Index (PSI) indicated that the DLQI had adequate internal reliability. | + | |
| An 2013(60) | China | 395 | Neurodermatitis or psoriasis vulgaris | Cronbach’s α =0.889. Average inter-item correlation = 0.415, item-total correlation ranged from 0.483 to 0.711, average item-total correlation was 0.628. | + | |
| He 2013(61) | China | 851 | Psoriasis | α = 0.91. Exclusion of any one of the 10 items did not increase a by more than 0.01. Corrected item-total correlations ranged from 0.51 to 0.79 | + | |
| Khoudri 2013(41) | Morocco | 244 | Psoriasis | Overall 0.70 (α = 0.84) and ranged in all scales from 0.33 to 0.75. Item internal congruency 0.82– 0.90. ICC 0.85–0.97 | + | |
| Lilly 2013(62) | United States | 90 | Vitiligo | Cronbach α = 0.935. Item-total correlations ranged between 0.56 and 0.84 except for VitiQoL question 13 (‘’Has your skin condition affected your sun protection efforts during recreation?’’) with a correlation of 0.36. | + | |
| Liu 2013(63) | China | 106 | Pruritic papular eruption | α = 0.673 for the six dimensions (Symptoms and feelings, Daily activities, Leisure, Work and School, Personal relationships and Treatment) were 0.633, 0.777, 0.771, 0.785, 0.772 and 0.684 respectively. | – | |
| Lockhart 2013(64) | United Kingdom | 85 | Vulval intraepithelial neoplasia | α = 0.93 | + | |
| Thomas 2014(65) | India | 38 | Lymphatic Filariasis | α = 0.73 | + | |
| Wachholz 2014(66) | Brazil | 41 | Leg ulcers | α = 0.729 | + | |
| Qi 2015(67) | China | 698 | Alopecia | α = 0.887, standardized item alpha was 0.881, The average inter-item correlation was 0.425 (> .2), suggesting good reliability. The item total correlation ranged from 0.180 to 0.797. The average item total correlation was 0.617 | + | |
| Chernyshov 2016(68) | Ukraine | 126 | Psoriasis and atopic dermatitis | α =0.81 for AD and 0.86 for psoriasis | + | |
| Solgajová 2016(69) | Slovakia | 104 | Acne or atopic dermatitis | α = 0.82 | Note: Aghaei et al., 2004; (Liu) Zhibin et al., 2013 don’t give this value, it must be from this study! | + |
| Kirby 2017(70) | United States | 154 | Hidradenitis Suppuratvia | α = 0.90 | R, version 3.3.2 | + |
| Cozzani 2018(71) | Italy | 50 | Psoriasis and psoriatic arthritis | α = 0.90 (0.88–0.92 for items). Highest value for the item-test correlation (r = 0.89) was for item 9 (interpersonal relationships), while the lowest corresponded to item 6 (leisure; r = 0.31). α increased to 0.90 only with the deletion of item 5 (sociability) | + | |
| Hunt 2018(72) | Vietnam | 102 | Leprosy | α = 0.78 | + | |
| Shimizu 2018 (73) | Brazil | 116 | Alopecia | α = 0.87 | + | |
| Xiao 2018(74) | China | 465 | Arsenic-related skin lesions and symptoms | Cronbach’s α was 0.79, and the split-half reliability was 0.77 | + | |
| Beamer 2019(75) | United States | 40 | Radio-dermatitis | α = 0.69 with work and study item was removed from analysis because the variance was zero. Inter-item correlation from 0.10 to 0.66 | Removal of treatment subscale (item) would improve alpha by .15. | – |
| Patel 2019(33) | United States | 340 | Atopic dermatitis | Cronbach’s α = 0.89. Spearman rho Interitem correlations 0.30 to 0.62 | + | |
| Satti 2019(76) | Pakistan | 173 | Uremic pruritus | α = 0.71 | + | |
| Storck 2018(77) | Germany | 79 | Pruritus | α Paper based 0.80, iPAD electronic1 0.81, iPAD electronic2 0.81 | + | |
| Temel 2019(78) | Türkiye | 150 | Acne vulgaris (AV) or vitiligo, or alopecia areata (AA) | α acne vulgaris 0.812, vitiligo 0.329, alopecia areata 0.915 | + | |
| Demirci 2020(79) | Türkiye | 100 | Psoriasis | α =0.82 (SPSS 20.0) | + | |
| Jorge 2020(80) | Brazil | 1286 | 14 dermatoses. (Basal cell carcinoma Bullous disorders, Female alopecia Genital warts, Hidradenitis suppurativa, Leprosy, Melasma, Onychocriptosis, Photoaging, Psoriasis, Rosacea, Uremic pruritus, Urticaria, Vitiligo) | Total Cronbach’s α (CI 95%) 0.90 (0.89–0.91); 0.72–0.91 for individual diseases. If any item was excluded, Cronbach’s α for the total sample ranged from 0.87 to 0.89 . | Highlighed cultural difficulty of q9 (sexual life) within the population. IRT analysis indicates that q9 is most affected with severe HRQOL impact. | + |
| Paudel 2020(81) | Nepal | 149 | Urticaria | α = 0.88, standardised 0.89, and did not change with the deletion of any of the items. The interitem correlation matrix revealed that the Pearson’s correlation coefficients (r) ranged from 0.097 to 0.730. All items had a satisfactory correlation with each other. Items 1–4 α = 0.79, items 5–10 α =0.86 | + | |
| Meneguin 2021(45) | Brazil | 188 | Psoriasis, cellulitis/erysipelas, chronic ulcers and eczematous dermatosis, other dermatoses | α = 0.85 (CI 0.82–0.88) | + | |
| Pollo 2021(82) | Brazil | 281 | Psoriasis | α = 0.87 | + | |
| Kolokotsa 2022(83) | Greece | 150 | Acne | α = 0.80 | + | |
| Data was extracted from referenced publications. | ||||||
| For test–retest reliability COSMIN: “+” ICC or weighted Kappa ≥ 0.70; “?” ICC or weighted Kappa not reported; “–” ICC or weighted Kappa < 0.70. The criteria are based on Prinsen et al.(17). | ||||||
| For internal consistency reliability COSMIN: “+” At least low evidence for sufficient structural validitya AND Cronbach’s alpha(s) ≥ 0.70 for each unidimensional scale or subscaleb; “?” Criteria for “At least low evidence for sufficient structural validitya” not met; “–” At least low evidence for sufficient structural validitya AND Cronbach’s alpha(s) < 0.70 for each unidimensional scale or subscaleb. | ||||||
| aThis evidence may come from different studies. | ||||||
| bThe criteria ‘Cronbach alpha < 0.95’ was deleted, as this is relevant in the development phase of a PROM and not when evaluating an existing PROM. The criteria are based on Prinsen et al. (17) | ||||||
Responsiveness to change
Although many clinical trials have demonstrated DLQI score change in patients’ QoL before and after treatment, only 12 studies were included (Table III), where the study was specifically conducted and statistical analysis using anchors performed to assess the responsiveness to change of the DLQI. Effect sizes were reported between 0.3 and 0.82 where effects are considered small 0.2, medium 0.5, large 0.8 and very large 1.3 (28). Pearson’s/Spearman’s correlations with other measures ranged from –0.35 to 0.75 with correlation of ±0.2 small, ±0.5 medium and ±0.8 large (28). Significant responsiveness by ANOVA and Wilcoxon 2-sample (paired) analysis was also demonstrated. Although in assessing responsiveness to change, the effect size of change is not informative according to COSMIN, however, as this is a systematic review we have reported all validation data. The method for calculating effect size is missing for some studies where it was not reported.
| References | Country | DLQI completed | Disease | Methods | Results | Method other | COSMIN |
| Badia 1999(34) | Spain | 246 | Eczema and psoriasis | Effect size (ES) | Effect sizes (ES) for changes in overall DLQI score between visits 1 and 3 were 0·82 for eczema patients and 0·58 for psoriasis patients | ? | |
| Shikiar 2003(48) | United States | 1095 | Psoriasis | Correlation of DLQI with other measure, ANOVA | Pearson’s correlations Among Change Scores of DLQI and change scores of Study 1 PASI (0.47), OLS (0.43) and PGA (0.46); Study 2 PASI (0.54), OLS (0.46) and PGA (0.53) all p <0.001. | ANOVA of DLQI Among Three Groups of PASI Improvement Scores:≥ 75%; Between 50% and 75%; and < 50%: Study A mean change score (N) <50% 4.79 (230), ≥ 50% and <75% 13.53 (96), ≥ 75% 18.63 (110), F statistic=54.61 p <0.0001. Study B mean change score (N) <50% 2.49 (268), ≥ 50% and <75% 6.83 (146), ≥ 75% 10.03 (122), F statistic=75.05, p <0.0001 | + |
| Shikiar 2006(53) | United States | 147 | Psoriasis | Correlation of DLQI with other measure | DLQI Correlations Baseline EQ-5D Index (0.51), EQ-5D VAS (–0.35); Week12 EQ-5D Index (–0.71), EQ-5D VAS (–0.58); Change EQ-5D Index (–0.53), Change EQ-5D VAS (–0.46), all p <0.001. | + | |
| Takahashi 2014(84) | Japan | 119 | Psoriasis | Correlation of DLQI with other measure | Spearman’s correlation PASI and DLQI scores. r = 0.134, P = 0.63. | + | |
| Basra 2015(85) | United Kingdom | 192 | Any skin disease | Paired t-test, Effect size (ES) | Mean DLQI total score BL 9.8 SD 7.8, follow-up 7.4 SD 7.1, mean change 2.4 t-test p = 0.001, Cohen’s effect size = 0.3, SRM 0.4 | + | |
| Richter 2017(86) | Germany | 41 | Acne | Effect size (ES) | Overall ES=0.64. Divided into the responder groups (based on Investigator Static Global Assessment; ISGA), highest ES were detected in the ‘Highly improved’ group (ISGA > =2, ES=0.66. | + | |
| Patel 2019(33) | United States | 340 | Atopic dermatitis | Effect size (ES) | Overall, DLQI scores changed significantly between baseline and the next visit. Cohen’s d = –0.74 for > =1 point POEM improvement, d=–0.72 > =3.4 point POEM improvement (MCID); d=0.28 for > =1 point POEM worsening, d=0.65 for > =0.3.4 points POEM worsening (MCID) | + | |
| Silverberg 2020(87) | United States | 118 | Atopic dermatitis | Correlation of DLQI with other measure, Wilcoxon 2-sample | Changes from baseline in PROMIS Cognitive Function T-scores were weakly inversely correlated (Spearman’s) with changes from baseline DLQI (r = –0.22, p = 0.0003) | The impact of cognitive dysfunction (PROMIS Cognitive Function T-score <=45%) on HRQOL was examined in bivariable models (Mann-Whitney U-test) stratified by Patient’s Global Assessment (PGA). There were generally stepwise increases in DLQI and ItchyQoL scores between mild, moderate, and severe AD | + |
| Silverberg 2020(88) | United States | 410 | Atopic dermatitis | Correlation of DLQI with other measure | NRS worse 0.26, NRS average 0.33, VRS worse 0.27, VRS average 0.28, all p <0.001 | Follow-up visit duration of 0.3 ± 0.4 years (maximum 1.9 years) n = 374. Change in numeric rating scales (NRS) and verbal rating scales (VRS) vs change in DLQI | – |
| Meneguin 2021(45) | Brazil | 188 | Psoriasis, cellulitis/erysipelas, chronic ulcers and eczematous dermatosis, other dermatoses | Correlation of DLQI with other measure, Wilcoxon 2-sample | Spearman’s: correlation (ρ) : Skindex-16 Total r=0.75; Sk-16 symptoms r=0.57; Sk-16 emotions r=0.66; Sk-16 functionality r=0.70 | For patients showing clinical improvements using the Wilcoxon test, First interview media n = 10 (6.5–15.5); second interview after 7 to 14 days media n = 7.50 (4.5–13); p <0.01 | + |
| Schwartzman 2021(46) | United States | 994 | Atopic dermatitis | Correlation of DLQI with other measure, Wilcoxon matched | Change in DLQI score with change PGH T scores. Change in DLQI score with change PO-SCORAD r=0.39, change PHQ-9 r=0.41, change PROMIS sleep Disturbance r=0.40, change PROMIS sleep Related impairment r=0.22, change Objective SCORAD r=0.53, change SCORAD r=0.58, all p <0.001 | + | |
| Papoui 2022(89) | Cyprus | 38 | Pruritus | Effect size (ES) | Control Group Mean DLQI ± SD, Week 1 7.9 ± 6.2 Week 2 9.6 ± 6.2 Week 3 9.7 ± 5.3; Intervention Group Mean DLQI ± SD, Week1 8.7 ± 7.4 Week2 7.9 ± 4.7 Week3 7.5 ± 4.7 Cohen’s d Week1, –0.12 Week2 0.31 Week3 0.44 | + | |
| Data was extracted from referenced publications. | |||||||
| COSMIN: “+” The result is in accordance with the hypothesis OR AUC ≥ 0.70; “?” No hypothesis defined (by the review team); “–” The result is not in accordance with the hypothesis OR AUC < 0.70. The criteria are based on Prinsen et al.(17) | |||||||
Studies assessing known group analysis of the Dermatology Life Quality Index
Table SI(C) shows studies where known group validity (i.e. a type of construct validity) analysis was performed on the DLQI. We included studies where known group analysis was performed, even if the authors had not stated an a priori hypothesis. Only four studies reported effect sizes. A majority of the statistical tests performed in the known group analyses (Student’s t, Pearson’s correlation, Spearman’s correlation, Mann-Whitney U-test, Kruskal-Wallis) to discriminate between the studies groups showed statistical significance. Known-groups validity evidence is essential to provide confidence in the construct and use of a measure, and the DLQI demonstrates this over a wide variety of groups (e.g. disease severity, anxiety, depression, stigma, scarring, well-being, sexual function, disease location, disease duration, race).
Studies assessing the correlation of the Dermatology Life Quality Index with other PRO/QoL instruments
In many studies, the DLQI was used in parallel with other instruments, some generic, some dermatology-specific and disease-specific measures. In this systematic review we captured correlations of the DLQI with PRO/QOL instruments reflecting its construct validity (or more specifically its convergent validity as shown in Table IV). The working definition of PRO/QoL is listed in the Appendix S1. Correlations with non-PRO or non-QoL measures e.g. severity scales were not included. Of 133 studies that published correlations, almost all were Spearman’s or Pearson’s with one Kendall’s tau correlation, one Wilcoxon test and 14 studies did not specify.
| References | Country | DLQI completed | Disease | Measure | Methods | Results | COSMIN hypothesis | |||
| Herd 1997(90) | United Kingdom | 56 | Atopic dermatitis | Patient Generated Index (PGI) | not stated | Correlation between DLQI and PGI was –0.52 (P <0.001). For DLQI Q1 to 10 r= –0.36*, –0.51**, –0.39*, –0.42**, –0.40*, –0.27, –0.20, –0.19, –0.13, –0.32; * p <0.01, ** p <0.001 | 2* | |||
| Badia 1999(34) | Spain | 246 | Eczema and psoriasis | Nottingham Health Profile (NHP) | Spearman’s | Correlations between DLQI scores and NHP dimensions were low to moderate, ranging from 0·32 with the NHP mobility dimension to 0·12 with the energy dimension. | 2 | |||
| Kent 1999(91) | United Kingdom | 614 | Vitiligo | 12-item General Health Questionnaire (GHQ-12), (Perceived) Stigma Questionnaire (adaptation of Ginsberg and Link 1989, some items dropped, replaced “psoriasis” with “vitiligo”); Self Esteem (Rosenberg 1965)(92) | not stated | GHQ-12 r=0.40, p <0.001; Perceived stigma r=0.62 p <0.001; Self Esteem r=-0.45 p <0.001 | 2* | |||
| Mallon 1999(93) | United Kingdom | 111 | Acne | SF36 | Pearson’s | SF-36 dimensions Self-esteem -0.37, Role-emotional -0.46, Social function -0.69, Mental health -0.53, Energy/vitality -0.38, all p <0.001 | 2* | |||
| Lundberg 2000(94) | Sweden | 366 | Psoriasis OR atopic dermatitis | SF36 | Spearman’s | The Spearman’s correlation coefficients between the data of SF-36 and the DLQI showed significant correlations ranging between ± 0.15 and ± 0.41. | 2 | |||
| Williamson 2001(95) | United Kingdom | 70 | Alopecia | Center for Epidemiologic Studies Depression Scale (CES-D) | Spearman’s | r= 0.62 (P <0.0001) | 2* | |||
| Sampogna 2004(96) | Italy | 786 | Psoriasis | Skindex, Impact of Psoriasis Questionnaire (IPSO), Psoriasis Disability Index (PDI), Psoriasis Life Stress Inventory, General Health Questionnaire (GHQ-12) | Pearson’s: Correlation matrix of clinical severity, QOL & psychological distress instruments | Skindex Social functioning r=0.723, Emotions r=0.633, Symptoms r=0.452; Impact of Psoriasis Questionnaire (IPSO) r=0.758; Psoriasis Disability Index (PDI) r=0.805; Psoriasis Life Stress Inventory 0.627; General Health Questionnaire (GHQ-12) r=0.576. No p values given, | Skindex mostly 1 IPSO 1 PDI 1 PLSI 1 GHQ 2 |
|||
| Wittkowski 2004(97) | United Kingdom | 125 | Atopic dermatitis | Stigmatisation and Eczema Questionnaire (SEQ), the Hospital Anxiety and Depression Scale (HADS), the Fear of Negative Evaluation Scale (FNE) and the Rosenberg Self-Esteem Scale (RSE). | Pearson’s | SEQ r=0.56 p <0.01, HADS anxiety r=0.32 p <0.05, HADS depression r=0.49 p <0.01, FNE r=0.27 p <0.01, RSE r=0.38 p <0.01 | SEQ 2* HADS-D 2* FNE 2* RSE 2* |
|||
| Yazici 2004(98) | Türkiye | 61 | Acne | Anxiety and Depression Scale (HADS) | Pearson’s | HAD-A ( r = 0.485, P = 0.0001) and HAD-D ( r = 0.455, P = 0.0001) | HADS 2* | |||
| Ilgen 2005(50) | Türkiye | 108 | Acne | Acne Quality of Life Scale (AQOLS) | Spearman’s | AQOLS and DLQI (r=0.466, p≤0.05). | 2* | |||
| Ferraz 2006(36) | Brazil | 115 | Multiple for reliability. See suppl data for full list. Lupus Erthematous for validity. | SF36 | Pearson’s | The correlation coefficient between DLQI and each SF-36 component score were highly statistically significant (r= -0,30 to -0.56, p <0.001) | 2* | |||
| Vilata 2008(99) | Spain | 247 | Anogenital Condylomata Acuminata | CECA (Specific Questionnaire for Condylomata Acuminata) | Spearman’s | Overall r=-0.670, Emotional dimension r=-0.546, Sexual activity dimension r=-0.676 | 1* | |||
| Aghaei 2009(56) | Iran | 125 | Psoriasis | Psoriasis Disability Index (PDI) | not specified | r = 0.94 | 1* | |||
| Menter 2010(100) | United States | 96 | Psoriasis | Zung Self-rating Depression Scale | Pearson’s | Baseline: r= 0.5 p <0.0001; Score changes from baseline to wk12 r= 0.5 p <0.0001 | 2* | |||
| de Ue 2011(101) | Brazil | 62 | Urticaria | SF-36 | Spearman’s | r = 0.254 to -0.465 between the domains of the DLQI and those of the SF-36. | 2 | |||
| Goreshi 2011(102) | United States | 120 | Dermatomyositis | Skindex-29 | Pearson’s | Each Skindex-29 subscore significantly correlated with DLQI scores (Skindex-29 Symptom r=0.632, Skindex-29 Emotion r=0.674, Skindex-29 Function r=0.856; all p values<0.0001) | 1* | |||
| Kluger 2011(103) | France | 18 | Birt-Hogg-Dube syndrome (facial fibrofolliculomas) | Cardiff Acne Disability Index (CADI) | Spearman’s | r=0.83 | 1 | |||
| Lau 2011(104) | Australia | 119 | Contact dermatitis | ShortFormHealthSurvey (SF-36) | Spearman’s | SF-36 PCS 0.253 (p <0.01); MCS −0.298 (p <0.002) | ||||
| Tadros 2011(105) | Greece | 80 | Psoriasis | Family Dermatology Life Quality Index (FDLQI) | Spearman’s | DLQI was significantly and positively correlated with FDLQI (Spearman r = 0.51, P <0.001) | 1^ | |||
| Fernandez-Penas 2012(31) | Spain | 144 | Psoriasis | Skindex-29 | Spearman’s | r ≥ 0.57 for DLQI total score and Skindex-29 subscales (0.73 symptoms, 0.73 emotions and 0.57 functioning, all p <0.01). Correlations of DLQI items and Skindex-29 subscales 0.37 to 0.73 (all p <0.01, n = 144) | 1* | |||
| Ghajarzadeh 2012(106) | Iran | 300 | Psoriasis, vitiligo, alopecia areata | Beck Depression Inventory (BDI) | Pearson’s | All r=0.44 p <0.001. Significant correlation between DLQI and BDI in all groups: vitiligo (r=0.5, P <0.001), psoriasis (r=0.3, P=0.001), AA (r=0.34, p <0.001) | 2* | |||
| Ghajarzadeh 2012(107) | Iran | 100 | Alopecia | Beck Depression Inventory (BDI) | Pearson’s | r=0.34 p value < 0.001 | 2* | |||
| Kimball 2012(108) | United States | 1212 | Psoriasis | Work Productivity and Activity Impairment Questionnaire for Psoriasis (WPAI-Psoriasis) | Pearson’s | Correlation coefficients = 0.57, 0.58, 0.66, and 0.28 for TAI, TWPI, presenteeism, and absenteeism, respectively | 1 | |||
| Maksimovic 2012(58) | Serbia | 66 | Atopic dermatitis | SF36 | Spearman’s | Correlation coefficients between SF-36 and DLQI scales ranged between -0.26 and -0.38, most p <0.01. The highest correlations were seen between symptoms and feelings and daily activities (q = 0.75; P <0.01), symptoms and feelings and work ⁄school (q = 0.56; p <0.01), and leisure and work ⁄school (q =0.53; P <0.01) for subscales of the DLQI. | 1* | |||
| Norlin 2012(109) | Sweden | 2191 | Psoriasis | EQ-5D | Spearman’s | r = 0.55, p <0.001 (n = 2091; adjusted R2 =0.28; Root Mean Square Error = 0.1989; Probability > F =0<0.0001) | 1* | |||
| Yu 2012(110) | Korea South | 138 | Eczema/Hand eczema | Beck’s Depression Inventory (BDI-II) scoring system | Spearman’s | BDI-II scores also had a positive correlation with DLQI score (p<0.05) | ||||
| Bin Saif 2013(111) | Saudi Arabia | 141 | Vitiligo | Family Dermatology Life Quality Index (FDLQI) | not stated | r = 0.56, p <0.001 | 1* | |||
| Ghaderi 2013(112) | Iran | 70 | Acne | SF-36 | Pearson’s | r=-0.46 p <0.001; Physical functioning (PF) r= −0.20 p = 0.10; role physical (RP) r=-0.37 p = 0.002; role emotional (RE) r=-0.49 p <0.001; vitality (VT) r=-0.36 p = 0.002; mental health (MH) r=-0.19 p = 0.11; social functioning (SF) r=-0.21 p = 0.09; bodily pain (BP) r=-0.31 p = 0.009; general health (GH) r=-0.38 p = 0.001 | 2* | |||
| Lilly 2013(62) | United States | 90 | Vitiligo | Vitiligo-specific quality-of-life instrument (VitiQoL) | Pearson’s | total VitiQOL (0.832), Interpersonal (0.752), Emotion (0.842), Grooming (0.499), all p <0.05 | 1* | |||
| Lindberg 2013(113) | Sweden | 93 | Eczema/Hand eczema | EQ5D | Spearman’s | EQ5D-VAS (−0.62), and the EQ5D-index (−0.67) , both p <0.05 | 1* | |||
| Lockhart 2013(64) | United Kingdom | 85 | Vulval intraepithelial neoplasia | Vulval intraepithelial neoplasia questionnaire (VIN) | not stated | VIN questionnaire score was statistically significantly correlated with the DLQI (r = 0.69). VIN questions which related to symptoms and activities of daily life correlated strongly with the DLQI questionnaire, with correlations ranging from 0.45 to 0.62 | 2* | |||
| Rizwan 2013(114) | United Kingdom | 178 | Photodermatoses | Hospital Anxiety and Depression Scale (HADS), social anxiety using the Fear of Negative Evaluation measure (FNE), coping strategies (brief COPE) | Pearson’s | DLQI scores were significantly associated with anxiety (r = 0.28, p <0.01), depression (r = 0.41, p <0.01), adaptive (r = 0.31, p <0.01) and maladaptive (r = 0.3, p <0.01) coping strategies. | HADS-D 2*, COPE adaptive 2*, maladaptive 2* | |||
| Strand 2013(115) | United States | 352 | Psoriasis | SF-36 | Pearson’s | Correlations between SF-36 scores and DLQI were moderate (r> 0.30 and ≤0.60) | 2* | |||
| Stumpf 2013(116) | Germany | 284 | Pruritus | Frankfurt Body Concept Scales (Frankfurter Körperkonzeptskalen; FKKS) | Pearson’s | Total r=-0.295 p <0.02. DLQI showed negative correlations with all subscales (r=-0.184 to 0.379, all p <0.01) and SKKO (r=-0.131, p <0.05) except SDIS and SPKF | ||||
| Tjokrowidjaja 2013(117) | Australia | 70 | Bullous disease | Treatment of Autoimmune Bullous Disease Quality of Life (TABQOL) | not specified | r = 0.64 | 2* | |||
| Vinding 2013(118) | Denmark | 177 | Non-Melanoma Skin Cancer | Skin Cancer Quality of Life (SCQoL) | Spearman’s | SCQoL Total r=0.45, p <0.0001; SCQoL Function r=0.36, p <0.0001; SCQoL Emotions r=0.44, <0.0001; SCQoL Control r=0.36, <0.001 | 1* | |||
| Yano 2013(119) | Japan | 112 | Atopic dermatitis | Work productivity and activity impairment-specific health problem (WPAI-SHP) | not specified | WPAI total work productivity impairment [TWPI] n = 97 r=0.600 p <0.001; total activity impairment [TAI]) n = 112 r=0.637 p <0.001 | 2* | |||
| Bardazzi 2014(120) | Italy | 240 | Psoriasis | Psoriasis awareness among patients in Italy questionnaire | Spearman’s | Awareness was positively correlated with QoL as measured by DLQI: pathogenesis r=0.02 p = 0.768, diagnosis r=0.11 p = 0.099, clinical course r=0.18 p = 0.005, quality of life r=0.245 p = 0.245, whole scale r=0.13 p = 0.043 | ||||
| Doʇruk Kaçar 2014(121) | Türkiye | 38 | Vitiligo | Feeling of Stigmatization Questionnaire 33-item | Kendall’s tau correlation | r=0.548, p = 0.001 | 2* | |||
| Ghaderi 2014(122) | Iran | 70 | Eczema/Hand eczema | SF-36 | Pearson’s | Correlation with SF-36 domains between -0.226 and -0.442 | ||||
| Ghaderi 2014(123) | Iran | 70 | Vitiligo | SF-36 | Pearson’s | r=-0.472, p <0.001; PF, physical functioning r=-0.199 p = 0.099; RP, role physical r=-0.327 p = 0.006; RE, role emotional r=-0.324 p = 0.006; VT, vitality r=-0.349 p = 0.003; MH, mental health r=-0.365 p = 0.002; SF, social functioning r=-0.296 p = 0.013; BP, bodily pain r=-0.360 p = 0.002; GH, general health r=-0.347 p = 0.003 | SF-36 2* | |||
| Hawro 2014(124) | Poland | 60 | Psoriasis | Basic hope inventory (BHI-12) | Pearson’s | r =-0.281; p = 0.030 | 3* | |||
| Herédi 2014(125) | Hungary | 200 | Psoriasis | EQ-5D score | Spearman’s | -0.48, p <0.05 | 2* | |||
| Susel 2014(126) | Poland | 200 | Uremic pruritus | 36-item Short Form Health Survey (SF-36) | Spearman’s | Significant negative correlation between SF-36 score and DLQI score in HD patients with UP (R = -0.29, p = 0.01) | ||||
| Takahashi 2014(84) | Japan | 119 | Psoriasis | General Health Questionnaire (GHQ)-30 | Spearman’s | GHQ-30 and DLQI (r = 0.487, P <0.01) | 2* | |||
| Boza 2015(127) | Brazil | 74 | Vitiligo | Vitiligo-specific health-related quality of life instrument (VitiQol) | Pearson’s | r = 0.776, p <0.001 | 1* | |||
| Bruer 2015(128) | Germany | 84 | Psoriasis | Short Form Health Survey-8 (SF-8), Patient Health Questionnaire (PHQ-9), Shirom Melamed Burnout Measure (SMBM) | Pearson’s | SF-8 r = -0.603, p <0.001; PHQ-9 depression score r = 0.437, p <0.001; SMBM total r = 0.550, p <0.001; SMBM physical fatigue r = 0.521, p <0.001; SMBM cognitive weariness r = 0.359, p <0.001; SMBM emotional exhaustion r = 0.497, p <0.001 | SF-8 2* PHQ-9 2* SMBM 2* |
|||
| Chiang 2015(129) | United Kingdom | 105 | Alopecia | Anxiety and Depression Scale (HADS) | Pearson’s | HADS Total scores (r = 0.674, p <0.001; HADS-A (r = 0.519, p <0.001), HADS-D (r = 0.711, p <0.001) | 2* | |||
| Durai 2015(130) | India | 140 | Acne | Cardiff Acne Disability Index (CADI) | Spearman’s | r = 0.74 p <0.0001 | 1* | |||
| Heelan 2015(131) | Canada | 94 | Bullous disease | Work Productivity and Activity Impairment Questionnaire- Specific Health Problem (WPAIQ-SHP) | Spearman’s | rs = −0.221, p = 0.032 (n = 94); bivariate correlations of subset of employed persons (n = 48) rs = −0.298, p = 0.040; total activity impairment subscale (TAI) rs = −0.329, p = 0.023 | TAI 2* | |||
| Moradi 2015(132) | Iran | 71 | Psoriasis | EQ-5D | Spearman’s | EQ-5D and EQ VAS showed moderate negative correlations with DLQI (rs = -0.44 p <0.001) | 2* | |||
| Schmitt 2015(133) | Germany | 201 | Psoriasis | Work Limitations Questionnaire (WLQ) | no specified | DLQI scores were significantly correlated with presenteeism (r = 0.47; p <0.0001) and to a lesser degree also with absenteeism (r = 0.29; p <0.001) | DLQI presenteeism 2* | |||
| Sung 2015(134) | Korea South | 66 | Pemphigus | General Health Questionnaire (GHQ-12) | Spearman’s | GHQ positivity was associated with a higher DLQI score (p<0.0001) | ||||
| Tennvall 2015(135) | Denmark | 290 | Acitinic keratosis | Actinic Keratosis Quality of Life Questionnaire (AKQoL); EQ-5D-5; EQ-VAS | Spearman’s | AKQoL n = 283 r=0.52 (p <0.001); EQ-5D-5-L n = 273 r=−0.36 (p <0.001); EQ-VAS r=−0.21 (n = 282 <0.001) | AKQoL 1* EQ-5D-5L 2* |
|||
| Catucci Boza 2016(136) | Brazil | 117 | Vitiligo | Vitiligo-specific quality-of-life instrument (VitiQoL) | Spearman | r = 0.81; p <0.001, r = 0.36 to 0.84 (all p <0.001) for domains | 1* | |||
| Chernyshov 2016(68) | Ukraine | 126 | Psoriasis and atopic dermatitis | Skindex-16 | Spearman’s | atopic dermatitis r=0.66, p <0.001; psoriasis r=0.71, p <0.001 | 1* | |||
| Gawlik 2016(137) | Poland | 130 | Psoriasis | Anxiety and Depression Scale (HADS) | Spearman’s | DLQI and HADS-A scores (r = 0.467; p <0.001) and between the DLQI and HADS-D scores (r = 0.569; p <0.001). | 2* | |||
| Ko 2016(138) | Taiwan | 480 | Psoriasis | EQ5D and VAS | not stated | EQ-5D (r=0.416**, p <0.01) and VAS (r=0.369**, p <0.01) were significantly correlated with every dimension (p <0.01) of the DLQI. Sub-analysis for mild, moderate and severe groups | 2* | |||
| Kong 2016(139) | Korea South | 50 | Atopic dermatitis | Pittsburgh sleep quality index (PSQI) | Pearson’s | r = 0.388, p = 0.04 | 2* | |||
| Kouris 2016(140) | Greece | 80 | Psoriasis | Hospital Anxiety and Depression Scale (HADS) | Pearson ‘s | Within the group of psoriasis patients was a higher quality of life impairment significantly correlated with higher anxiety (r=0.27; p = 0.02), higher loneliness and social isolation (r=0.54, p <0.001), and lower self-esteem (r=-0.48, p <0.001). | ||||
| Maranzatto 2016(141) | Brazil | 154 | Melasma | Melasma Quality of Life Scale (MELASQoL) | Spearman’s | r=0.70 (p <0.01) | 1* | |||
| Salman 2016(142) | Türkiye | 148 | Vitiligo and acne patients with facial involvement | Liebowitz Social Anxiety Scale (LSAS), Hospital Anxiety and Depression Scale (HADS) | Pearson’s | Vitiligo: LSAS r=0.511 p <0.05; HADS r=0.574, p <0.05. Acne: LSAS r=0.478 p <0.05; HADS r=0.401, p <0.05 | LSAS 2* HADS 2* |
|||
| Sarhan 2016(143) | Egypt | 75 | Vitiligo | Arabic Version of the Female Sexual Functioning Index (AVFSFI) | Pearson’s | DLQI score was significantly correlated with AVFGSIS alone and with AVFSFI alone and with both AVFGSIS and AVFSFI (p <0 .01) | ||||
| Alarcon 2017(144) | Spain | 100 | Acitinic keratosis | Actinic Keratosis Quality of Life (AKQoL) | Spearman’s | Total score r=0.87; Function r=0.75; Emotions r=0.78; Control r=0.75; Global item r=0.76 | 1* | |||
| Augustin 2017(145) | Multiple | 340 | Psoriasis | Patient Benefit Index (PBI) | Spearman’s rank correlation | r=-0.29 p <0.001 (week 4) to r=-0.49 p <0.001 (week52, LOCF, last observation carried forward)) | 2* | |||
| Březinová 2017(146) | Czech Republic | 128 | Atopic dermatitis | Brief Illness Perception Questionnaire (B-IPQ), Family Dermatolology Life Quality Index (FDLQI), | not stated | B-IPQ r=0.42, p <0.001; FDLQI r=0.52, p <0.001 | B-IPQ 2* FDLQI 1* |
|||
| Catucci Boza 2016(136) | Brazil | 93 | Vitiligo | Vitiligo-specific quality-of-life instrument (VitiQoL) | Spearman’s | r= 0.81; p <0.001 | 1* | |||
| Janse 2017(147) | Netherlands | 300 | Hidradenitis Suppurativa and Psoriasis | Female Sexual Function Index (FSFI) | Pearson’s | r = -0.20, P =0.003 | ||||
| Masaki 2017(148) | Japan | 133 | Psoriasis | EQ-5D | Pearson’s | R=-0.472 | 2 | |||
| Michelsen 2017(149) | Norway | 141 | Psoriatic arthritis | Rheumatoid Arthritis Impact of Disease (RAID) | Spearman’s | ρ = 0.32, p <0.001 | 2* | |||
| Müller 2017(150) | Germany | 172 | nonmelanoma skin cancer (NMSC) | EORTC Questionnaire - Cancer (QLQ-C30) | Spearman’s | The DLQI total score was significantly associated with all functioning and symptom scales of the QLQ-C30, ranging from r (s) = 0.16 to 0.49. | ||||
| Xu 2017(151) | Korea South | 364 | See supplementary data for full list | Skindex-29, SF-36 | Spearman’s | Skindex-29: psoriasis r=0.794, vitiligo r=-0.677; SF-36: psoriasis r=--0.703, vitiligo r=-0.532, all p <0.01 | Skindex-29 1* SF-36 2* |
|||
| Yfantopoulos 2017(152) | Greece | 396 | Psoriasis | EQ-5D3L, EQ-5D-5L | Spearman’s | Correlations between EQ-5D dimensions and the DLQI sum score were all significant at least at α = 5%, with higher DLQI scores being associated with more problems on the EQ-5D scale. On average, the EQ-5D-5L items were stronger correlated with the DLQI sum score (mean q5L = 0.210 vs. q3L = 0.192, p = 0.039 based on a paired t test). | ||||
| Cozzani 2018(71) | Italy | 50 | Psoriasis and Psoriatic arthritis | PSOdisk | Pearson’s | not given | ||||
| Hassanin 2018(153) | Egypt | 100 | chronic skin disease (on the genitalia or exposed areas) | Female Sexual Function Index (FSFI) | Spearman’s and Pearson’s (unspecified) | Excluding the pain domain (R: −0.16 and P: 0.12), the DLQI score was significantly negatively correlated with all sex domain scores and the total FSFI score. The R values were: −0.35, −0.48, −0.29, −0.44, −0.56, and−0.48 for desire, arousal, lubrication, orgasm, satisfaction, and total scores, respectively; and the P values were: 0.003 for lubrication and < 0.001 for all other scores. | 2* | |||
| Kluger 2018(154) | Finland | 26 | Psoriasis | 15D HRQoL questionnaire (15D), the Dermatology Life Quality Index (DLQI), and the Beck Depression Inventory-21 (BDI-21) | Spearman’s | The 15D score negatively correlated with the DLQI score (r = -0.492; p = 0.011) and the BDI-21 score (r = -0.592; p = 0.001) | 15D 2* BDI-21 2* |
|||
| Morice-Picard 2018(155) | France | 40 | Albinism | SF-36 and Burden of Albinism questionnaire (BoA) | Pearson’s | SF-36 (n = 40)-PCS r=−0.56 p <0.002; SF-36 MCS r= −0.9 p <0.0013; Burden of Albinism questionnaire (BoA) Global score r= 0.68 p <0.0001 | SF-36 2* BoA 1* |
|||
| Tekin 2018(156) | Türkiye | 131 | Psoriasis | Anxiety and Depression Scale HAD-A, HAD-D, Type D Personality Scale (DS-14) and subscales: Negative Affectivity (NA) and Social Inhibition (SI) | Pearson’s | Correlations HAD-A 0.612, HAD-D 0.471, DS-14 0.494, subscales: NA 0.412, SI 0.501, PASI 0.360. All p <0.01 | HADS 2* DS-14 2* SI 2* |
|||
| Vakharia 2018(157) | United States | 210 | Atopic dermatitis | ItchyQoL | Spearman’s | DLQI, ItchyQoL and 5-D itch scale all significantly correlated with each other, ranging from 0.36-0.73 (P <0.0001). | 1* | |||
| Wang 2018(158) | Australia | 61 | Bullous disease | Specific Health Problem (WPAIQ-SHP) | Spearman’s | WPAIQ-SHP Presenteeism rs = 0.90, P = 0.00001; Total work productivity impairment rs = 0.88, P = 0.000035; Total activity impairment rs = 0.47, P = 0.00048 | 2* | |||
| Wu 2018(159) | China | 397 | Rosacea | Anxiety and Depression Scale (HADS) | Pearson’s | Total DLQI score of patients of patients with rosacea was positively related with anxiety (r = 0.526, p <.001) and depression scores (r = .399, p <.001) in HADS. | 2* | |||
| Albuquerque 2019(160) | Brazil | 104 | Leprosy | SF-36 | Spearman. Total and correlation between all DLQI and SF36 items | r = -0.58, p <0,01 | 2* | |||
| Arents 2019(161) | Multiple | 1189 | Atopic dermatitis | Atopic Eczema Score of Emotional Consequences (AESEC), HADS Anxiety and Depression Scale-D7 | Spearman’s ρ | AESEC (0.546, p <0.001, 95%CI =0.505, 0.585), HADS-D7 (ρ=0.461 p <0.001), 95%CI=0.414, 0.505) | AESEC 1* HADS-D7 2* |
|||
| Kalboussi 2019(162) | Tunisia | 150 | Contact dermatitis | Work Productivity and Activity Impairment: Allergy Specific (WPAI:AS) Questionnaire | Pearson’s | The DLQI score was significantly associated with atopy (p = 0.03), relapses strictly greater than 10 (p = 0.02), presenteeism (p <10−3), overall work productivity loss (p = 0.01), and daily activity impairment (p = 0.03) | ||||
| Le 2019(163) | Vietnam | 136 | Eczema/Hand eczema | EQ5D | Spearman’s | r=-0.73 | 2* | |||
| Narang 2019(164) | India | 179 | Superficial cutaneous dermatophytosis | General Health Questionnaire (GHQ-12) | Spearman’s | r = 0.30; P <0.05 | 2* | |||
| Patro 2019(165) | India | 294 | Superficial dermatophytic infection | 5Dpruritus scale | Pearson’s | r=0.802 p <0.0001 | 1* | |||
| Satti 2019(76) | Pakistan | 173 | Uremic pruritus | Public Health Questionnaire-9 (PHQ-9) | Spearman’s | r=0.69, p = 0.01 | 2* | |||
| Stefanidou 2019(166) | Greece | 103 | Pruritus | SF-6D | Spearman’s | rho = − 0.617, p <0.001 | 2* | |||
| Temel 2019(78) | Türkiye | 150 | Acne vulgaris (AV) or vitiligo, or alopecia areata (AA) | Internalized Stigma Scale (ISS) | not specified | Acne: ISS and DLQI (r = 0.596, P <0.001); Vitiligo:ISS and DLQI (r = 0.540, P <0.001) ; Alopecia areata:ISS and DLQI (r = 0.508, P <0.001) | 2* | |||
| Zeidler 2019(167) | Multiple | 535 | See supplementary data for full list | ItchyQoL | Pearson’s | r = 0.72. P = 0.001 | 1* | |||
| Demirci 2020(79) | Türkiye | 100 | Psoriasis | Anxiety and Depression Scale (HADS) | Pearson’s | DLQI scores were significantly and positively correlated with HADS anxiety scores (r=0.205, P <0.05), depression scores (r= 0.269, P <0.01) | ||||
| Gerdes 2020(168) | Germany | 538 | Psoriasis | Beck Depression Inventory (BDI-II) | Wilcoxon test | The correlation of DLQI and BDI-II scores was highly significant (p <0.0001) | ||||
| Namdar 2020(169) | Türkiye | 71 | Psoriasis | Toronto Alexithymia Scale, Beck’s Depression Scale, Beck’s Anxiety Scale | Spearman’s | DLQI score of psoriasis patients and anxiety (r=0.342 P <0.001), depression (r=0.327 P=0.006), alexithymia (r=0.341 P=0.004), and PASI scores (r=0.389 P=0.001) | All 2* | |||
| Oosterhaven 2020(170) | Netherlands | 294 | Eczema/Hand eczema | Quality of Life in Hand Eczema Questionnaire (QOLHEQ) | Pearson’s | r=0.77, no p value given | 1 | |||
| Passlov 2020(171) | Sweden | 21 | Eczema/Hand eczema | Activities of daily living (ADL) | Spearman’s | rho = 0.72, p = 0.00022 | 2* | |||
| Silpa-archa 2020(172) | Thailand | 104 | Vitiligo | Patient Health Questionnaire-9 (PHQ-9) | Pearson’s | r= 0.524, p <0.001 | 2* | |||
| Stepien 2020(173) | Poland | 240 | Pruritus | 12-Item Pruritus Severity Scale (12-PSS) | Spearman’s | p = 0.54 | 1* | |||
| Tawil 2020(174) | Lebanon | 152 | Urticaria | Arabic Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) | Pearson’s | r = 0.86 p <0.001. Correlations between each corresponding domain of the CU-Q2oL and the DLQI were found to be moderate to strong (≥ 0.5, p<0.001). | 1* | |||
| Acar 2021(175) | Türkiye | 200 | Fibromyalgia syndrome (FMS) in rosacea | Fibromyalgia Impact Questionnaire (FIQ) | Spearman’s correlation | r=0.39; p = 0.017 | ||||
| Bakar 2021(176) | Malaysia | 174 | Psoriasis | Malay Hospital Anxiety and Depression Scale (HADS) | Pearson’s | There is positive correlation between HADS-D and DLQI (r = 0.421, p-value <0.001) and between HADS-A and DLQI (r = 0.465, p-value <0.001). | 2* | |||
| Barbieri 2021(177) | United States | 764 | Atopic dermatitis | DLQI-R and SF-12 | Spearman’s | DLQI-R scoring modification had stronger correlation with the SF-12 Physical Health Score p = 0.02) and SF-12 Mental Health Score (−0.44 vs −0.41, Steiger’s Z p <0.001) (−0.09 vs −0.07, Steiger’s Z p = 0.02) | 2* | |||
| Chaudhary 2021(178) | India | 35 | Leprosy | Stigma Assessment and Reduction of Impact (SARI) scale v.1.1 | Spearman’s | r = - 0.272, p = 0.113 | ||||
| Emre 2021(179) | Türkiye | 105 | Urticaria | Beck Depression Inventory (BDI) | Spearman’s | r =0.073 | ||||
| Erol 2021(180) | Türkiye | 105 | Urticaria | Fatigue Severity Scale (FSS) | unspecified | r = 0.302, P = 0.002 | 2* | |||
| Esposito 2021(181) | Italy | 105 | Psoriasis and Psoriatic arthritis | Sheehan Disability Scale (SDS) | not stated | SDS and the DLQI were strongly correlated (r = 0.71, p <0.001) | SDS 2* | |||
| Ferrucci 2021(182) | Italy | 300 | Atopic dermatitis | Anxiety and Depression Scale (HADS) | Pearson’s | HADS depression r = 0.49, p <0.01; HADS anxiety r = 0.47, p <0.01 | 2* | |||
| Gundogdu 2021(183) | Türkiye | 51 | Psoriasis | Psoriasis Disability Index (PDI) | Spearman’s | r = 0.641 P = 0.000 | 1* | |||
| Kirby 2021(184) | United States | 441 | Hidradenitis Suppuratvia | Patient global assessment (PtGA) for hidradenitis suppurativa | Spearman’s | r = 0.78, 95% CI 0.74-0.82 | 1* | |||
| Kurhan 2021(185) | Türkiye | 129 | Contact dermatitis | Social Appearance Anxiety Scale (SAAS), HADS | Pearson’s | SAAS r=0.060, no sig., HADS-A r=0.263 p <0.01, HADS-D r=0.006 not sig. | SAAS 2* | |||
| Morioke 2021(186) | Japan | 48 | Recurrent angiodema | Angioedema Quality of Life Questionnaire (AE-QoL) | Spearman’s | Total AE-QoL r=0.631, p <0.001; Changes in DLQI (delta DLQI) score and those in AE-QoL scores were positively correlated r =0.48, p <0.001 | 1* | |||
| Pollo 2021(82) | Brazil | 281 | Psoriasis | Anxiety and Depression Scale (HADS) | Spearman’s | HADS-A r=0.40 p <0.05; HADS-D- r=0.40 p <0.05 | 2* | |||
| Segal 2021(187) | Israel | 58 | Pemphigus | Revised Illness Perception Questionnaire (IPQ-R) | Pearson’s | Several IP variables (timeline cyclical 0.30 p <0.05, treatment control 0,26 p <0.05, emotional representations 0.31 p <0.05, psychological attributions 0.37 p <0.01) showed correlation with DLQI, and no such correlation was found for Multidimensional Scale of Perceived Social Support (MSPSS). | 2* | |||
| Silverberg 2021(188) | United States | 458 | Atopic dermatitis | Patient Health Questionnaire-9 (PHQ9) & abridged version (PHQ-2) | Spearman’s | PHQ-9 was strongly correlated with DLQI (r=0.50) and PHQ-2 (r=0.48) and change in DLQI with change in PHQ9 (r=0.42) and PHQ-2 (r=0.33), p <0.001 for all | PHQ-9 2* PHQ-2 2* |
|||
| Singh 2021(189) | India | 1392 | Acne | Cardiff Acne Disability Index (CADI) | Spearman’s | r=0.71 | 1 | |||
| Solmaz 2021(190) | Türkiye | 306 | Psoriasis | Revised Illness Perception Questionnaire (IPQ-R) | Spearman’s | DLQI scores and IPQ-R subscales of Illness identity (r = 0.420) and Consequences (r = 0.408, p<0.001), Personal attributions (r = 0.277), Chance factor (r = 0.222), and External attributions (r = 0.212, p<0.001). | IPQ-R Illness Identity and Conseq. 2* | |||
| Talamonti 2021(191) | Italy | 174 | Atopic dermatitis | Beck Depression Inventory (BDI), Toronto Alexithymia Scale (TAS-20) | Pearson’s | BDI r=0.306 (p = 0.001); TAS-20 r=0.1874 (p = 0.040) | BDI 2* | |||
| Zhao 2021(192) | China | 182 | Vitiligo | Vitiligo specific quality of life instrument (VitiQoL) | method not specified | r= 0.70 (P <0.01) | 1* | |||
| Aminizadeh 2022(193) | Iran | 200 | Any skin disease | Skindex-29 | Spearman’s | r=0.719, subscales Skindex-29, r from 0.24 to 0.71, P <0.01) | Overall Skindex-29 1* |
|||
| Benny 2022(194) | India | 69 | Vitiligo | General Health Questionnaire-28 (GHQ-28) | Spearman’s | r = 0.54 (P <0.001) | 2* | |||
| Ito 2022(195) | Japan | 400 | Alopecia | Anxiety and Depression Scale (HADS), SF36v2 | not stated | HADS-A r=0.42, HADS-D r=0.47, both p <0.01. SF36: PF, physical functioning -0.34, RP, role physical -0.47, BP, bodily pain -0.27, GH, general health -0.30, VT, vitality -0.28, SF, social functioning -0.43, RE, role emotional -0.48, MH, mental health -0.41, all p <0.01 | HADS 2* SF-36 all except pain and vitality 2* |
|||
| Koszoru 2023(196) | Hungary | 218 | Atopic dermatitis | Skindex-16, EQ-5D-5L, EQ VAS (0-100) | Spearman’s | Total score r=0.839; Symptoms subscale r=0.730; Emotions subscale r=0.697; Functioning subscale r=0.827; EQ-5D-5L (r= −0.848 to 1) Total r=−0.731; EQ VAS r=−0.598; all p <0.05 | 1* | |||
| Nahidi 2022(197) | Iran | 80 | Psoriasis | Family Dermatology Life Quality Index (FDLQI) | Spearman’s | Meaningful relationship was noted between the quality of life of patients and their spouses (r = 0.48, P = 0.001) | 1* | |||
| Saeki 2022(198) | Japan | 73 | Psoriasis | Work Productivity and Activity Impairment- Psoriasis (WPAI- PSO) | Partial Spearman correlation coefficient ([ρ]; | In the adjusted model, the WPL score correlated with the DLQI ρ = 0.608, p <0.0001. The presenteeism score correlated with the DLQI ρ = 0.568 p <0.0001. activity impairment score correlated with the DLQI ρ = 0.530, p <0.0001 | 2* | |||
| Tan 2022(30) | Multiple | 723 | Acne | The Facial Acne Scar Quality of Life (FASQoL); Dysmorphic Concern Questionnaire (DCQ) | Pearson’s | significant correlation between DLQI and FASQoL scores (r = 0.683; P <0.001). DCQ score moderately correlated with DLQI (r = 0.47; P <0.001) | FASQoL 2* DCQ 1* |
|||
| Tee 2022(199) | Malaysia | 30 | Pemphigus | Autoimmune Bullous Disease Quality of Life (ABQOL), | Spearman’s | DLQI correlated positively with ABQOL (r = 0.84, p <0.001) | 1* | |||
| Tuchinda 2022(200) | Thailand | 130 | Chronic urticaria or eczema | 5-D itch scale | Spearman’s | all r = 0.76, p <0.0001, (CI 0.62-0.82); chronic urticaria r= 0.76 p <0.0001 (CI 0.63-0.85); eczema r=0.72 p <0.0001 (CI 0.58-0.81) | 1* | |||
| Xavier 2022(201) | Brazil | 397 | Skin picking disorder | Generalized Anxiety Disorder Assessment Scale (GAD-7) | Pearson’s | r = 0.73 | 2 | |||
| Yang 2022(202) | Taiwan | 143 | Vitiligo | SF36 | Spearman’s | PF, physical function r=−0.079 p = 0.351 ; RP, role limitation related to physical problems r=−0.173 p = 0.039; BP, bodily pain r=−0.134 p = 0.112; GH, general health r=−0.280 p = 0.001; SF, social functioning −0.284 p = 0.001; VT, vitality r=−0.331 p <0.001; RE, role limitation related to emotional problems r=−0.289 p <0.001; MH, mental health r=−0.466 p <0.001 | ||||
| Ye 2022(203) | Korea South | 500 | Urticaria | EQ-5D | Pearson’s | r = -0.545 p <0.001 | 2* | |||
| Zhao 2022(204) | China | 325 | Urticaria | Chronic urticaria quality of life questionnaire (CU-Q2oL) | Spearman’s | r = 0.769, p <0.001 | 1* | |||
| Koszoru 2023(205) | Hungary | 218 | Atopic dermatitis | EQ5D, Skindex-16 | Spearman’s | EQ-5D-3L rs = 0.267 to 0.570 by EQ5D item; EQ-5D-5L rs = 0.354 to 0.670 by EQ5D item; EQ-5D-3L index rs = −0.669; EQ-5D-5L rs =− 0.731; Skindex-16 rs= − 0.622 | EQ-5D-3L 2* EQ-5D-5L 2* EQ-5D Index 2* Skindex-16 1* |
|||
| Data in this table was extracted from the referenced publications. | ||||||||||
| COSMIN generic hypotheses taken from Table 4 Generic hypotheses to evaluate construct validity and responsiveness (17). Number indicates hypothesis level was supported by correlation, and a * significance at p <0.05. | ||||||||||
| 1. Correlations with (changes in) instruments measuring similar constructs should be ≥ 0.50, | ||||||||||
| 2. Correlations with (changes in) instruments measuring related, but dissimilar constructs should be lower, i.e., 0.30–0.50, | ||||||||||
| 3. Correlations with (changes in) instruments measuring unrelated constructs should be < 0.30, | ||||||||||
| 4. Correlations with (changes in) instruments measuring similar constructs should differ by a minimum of 0.10 from correlations, with (changes in) instruments measuring related but dissimilar constructs, | ||||||||||
| 5. Correlations with (changes in) instruments measuring related but dissimilar constructs should differ by a minimum of 0.10 from correlations with (changes in) instruments measuring unrelated constructs, | ||||||||||
| 6. Meaningful changes between relevant (sub)groups (e.g., patients with expected high versus low levels of the construct of interest). * indicates statistically significant correlation at p <0.05. | ||||||||||
Studies assessing the Differential Item Functioning (DIF) of the Dermatology Life Quality Index
Limited or no DIF was observed over gender or age, but many studies found DIF in some items (Table SID), as is generally found in most health-related QoL measures (29). Significantly, the study of Tan 2022 (30) reported that no significant differences were observed in DLQI scores in 723 acne patients across six countries in Europe, north America and Brazil.
Translations and cross-cultural adaptations
Thirteen publications that addressed validation of translations and cross-cultural adpatations included adaptation to 11 languages from the original English version, one illustrated version, and one considering dimensionality across language versions were included in this study (Table SI(€)).
Appraisal of representation of minorities ethnic participants.
The results of analysis of patients included in studies by Naicker’s Critically Appraisal Tool are shown in Table V.
| Question | Yes | No | Unclear | N/A | Total |
| Were minoritised ethnic participants recruited | 31 (15%) | 3 (1.4%) | 172 (83.1%) | 1 (0.5%) | 207 (100%) |
| Were minoritised ethnic participants representative? | 15 (7.2%) | 1 (0.5%) | 189 (93.1%) | 2 (1%) | 207 (100%) |
| Were results data stratified by race/ethnicity and if so, was this justified/appropriate/explained by the author? | 8 (3.9%) | 199 (96.1%) | 0 (0%) | 0 (1%) | 207 (100%) |
| Were any differences in study outcomes for minoritised ethnic populations appropriately addressed and interpreted? | 6 (2.9%) | 201 (97.1%) | 0 (0%) | 0 (0%) | 207 (100%) |
| Did researchers avoid assigning race as a variable, a risk factor or a proxy for genetic ancestry? | 1 (0.5%) | 1 (0.5%) | 4 (1.9%) | 201 (97.1%) | 207 (100%) |
| Naicker, R (2022) (23) Critically Appraising for Antiracism Tool. Available at: https://www.criticallyappraisingantiracism.org/. | |||||
Risk of bias
Data for the COSMIN criteria for good measurement properties are given in the Appendix S1 and individual COSMIN ratings are given in the last column of most tables.
Floor and ceiling effects
Two studies reported floor effects (31, 32), one study reported neither (33) and none reported ceiling effects.
Dermatology Life Quality Scores
There were 152 datasets for patients with a dermatological diagnosis where mean DLQI was reported. Additionally, six datasets were reported from healthy control groups (1, 34, 35, 143, 159). More details are given in Table SI(F).
Interpretability or clinical meaningfulness of the scores
The clinical meaningfulness of the DLQI is interpreted using validated score bands with band 0 DLQI scores 0–1 no effect on patient’s life, band 1 DLQI scores 2–5 small effect on patient’s life, band 2 DLQI scores 6–10 moderate effect on patient’s life, band 3 DLQI scores 11–20 very large effect on patient’s life, band 4 DLQI scores 21–30 extremely large effect on patient’s life (206). Narang et al. (164) interpreted their data using banding of the DLQI scores accordingly, and found that superficial cutaneous dermatophytosis had a small effect on the QoL of 41.3% of patients (band 2–5), while it caused an extremely large effect in the lives of 23.9% patients (band 21–30). Shakiar et al. 2006 (53) derived estimates of the MID of the DLQI using both the PASI and the Physician Global Assessment (PGA), as well as two distributional approaches to derive estimates of the MID of the DLQI. Their estimates ranged from 2.33–6.95, but they considered the PASI 50 is too conservative for estimating the minimum change that was beneficial to patients. Further information determining estimates of MID of the DLQI is reported by Basra et al. (85).
DISCUSSION
This systematic review compiles data from 207 peer reviewed studies describing research on 58,828 patients across 49 different countries on the validation of the DLQI over the 27 years of its global use. In contrast, the previous (non-systematic) review (7) of DLQI validation reported on only 115 studies, some of which were not published in English. Others were not full peer-reviewed papers.
The DLQI demonstrated strong test–retest reliability, assessed over 13 studies, reassuring researchers that completion is non-random and consistent. In 43 studies good internal consistency was confirmed, informing researchers of high correlation among the DLQI item scores. Furthermore, the DLQI score change in longitudinal design has been demonstrated in vast numbers of studies, including in randomised controlled trials (2). In addition, this review identified 12 studies performed using anchors to assess change responsiveness, with a range of effect sizes from small to large. Significant responsiveness by ANOVA and Wilcoxon 2-sample (paired) analysis was also demonstrated. Researchers can be confident of the DLQI responding appropriately to change. Concerning responsiveness of the DLQI, patients’ feelings about and perception of their skin disease may change less rapidly than the physical state of their disease. The psychosocial impact of skin disease may persist despite improvement in the disease.
Known-groups validity (207) was tested in 42 studies: such evidence is essential to provide confidence in the construct and use of a measure. The DLQI demonstrates this over a wide variety of groups, including disease severity, anxiety, depression, stigma, scarring, well-being, sexual function, disease location, disease duration and race. Correlation of the DLQI with other 119 different PRO/QoL measures in 207 studies were found in our current review, and demonstrated a range of correlations with other measures, adding to DLQI construct validation. This wide range reflected differences between the DLQI construct and that of comparator measures.
There are currently more than 138 DLQI language translations (208), all based on the original English language measure. Multiple publications demonstrate widespread DLQI use across many of these languages. Translations are all validated by independent forward and backward translations, checked by Cardiff University. Many have been fully culturally validated. This review found twelve publications that investigated DLQI translation validation and cross-cultural adaptations.
Factor analysis or item response theory was used to examine the dimensionality of the DLQI in 28 studies. These reached different conclusions, identifying from one to four factors. Generally studies with too few data did not find unidimensionality (found multiple factors), while the majority of those with sufficient data (N> 250) for these analyses, located only a single factor, supporting the unidimensionality of the DLQI. The exception was an Italian translation (51) and some Chinese translations (67, 74), where there may be some cross-cultural translation issues. Even with a 20:1 subject to item ratio (giving n = 200 for the DLQI), error rates may be well above alpha = 0.05 level (209). In contrast, the concept of DLQI score descriptor bands (206) transformed the DLQI into a useful clinical outcome assessment tool, informing clinical decisions. The DLQI score descriptor bands have informed a quality of life parameter incorporated in a wider definition of current psoriasis severity (210). Score banding, combined with knowledge of the DLQI Minimal Clinically Important Difference (85), allows appropriate and simple score interpretation.
Validation methodology continues to change, with many new methods, stricter criteria for acceptance of validation models, and new reporting of model fit criteria. COSMIN (211) is a standardized framework used to evaluate the quality of patient-reported outcome measures (PROMs). COSMIN includes guidelines and criteria for evaluating reliability, validity, and responsiveness of PROMs, as well as the quality of studies using them (17, 212). The FDA Guidance for Industry Patient-Reported Outcome Measures (213) requires comprehensive validation studies, covering all aspects of validation (validity, accuracy and reliability), including classical test theory, item response theory and clinical meaning. These high standards of reporting (17, 213) are relatively recent, and adoption of such rigorous methodology has been slow.
There is no absolute definition of what range of assessments should contribute to “validation”. Assessments, not normally considered, may be important, for example, whether PROM responses are influenced by frequency or number of times used in a subject (214). Face validity, the sense of a measure making sense to subjects, is clearly important but seldom reported. Perhaps very wide acceptance and use of a measure is itself an aspect of validation: “validation through widespread use”, standing the test of time. This concept applies Darwinian evolutionary theory to validation: a measure not fit for purpose may be soon forgotten, whereas a very popular measure likely has some positive characteristics leading to its widespread adoption, including some not focused on by PROM scientists (215).
Most studies reviewed had limited datasets, often with low participant numbers, validation lacked completeness and good metrics for analyses particularly in model goodness of fit and were therefore rated poorly using COSMIN guidelines (17). Studies often lacked reporting measurement error. Many studies used translated versions of the DLQI, complicating consideration of construct validity. There was a lack of a priori hypotheses associated with statistical tests, and little interpretation on the basis of hypotheses. Some other dermatology measures have poor developmental methodological quality, quality assessment of results, content validity and poor methodological quality of measurement properties (216). Few have complete coverage at domain level. Most, including the DLQI, were classed as Category B and can be recommended for use pending further validation (216). However, the body of validation of the DLQI as revealed by this systematic review gives stronger support for the underlying psychometric properties of the DLQI.
Although test–retest and internal consistency reliability, responsiveness, correlation with other measures and known group analysis provided a wealth of positively supportive data for the validity of the DLQI, further validation data is desired for factor structure (unidimensionality), responsiveness to change over time (longitudinal studies with effect sizes) and DIF, conducted with larger datasets and better metrics. Additionally, item response theory models using large data sets should investigate item fit using Rasch (infit and outfit) and graded response models, Chi-square statistics, and local dependencies, and compare responses across different language adaptations using t-scored data. This could also provide a calibration for the DLQI based on the original English-language construct, and cross-walks translation of sum scores to t-scores.
It is difficult to assess validity differences across diseases, as studies either focused on specific diseases or included any relevant diseases that patients presented with. Usually when multiple disease were included, sub-analysis was not performed, probably because the dataset sizes were too small for all but the most common diseases. Interpretation of results is also problematic, as a-priori hypothesis of whether one disease would be expected to have higher or lower DLQI scoring depends as much on individual severity as on the disease, making the framework multifactorial. Differential item functioning (DIF) using item response theory (IRT) would be a useful approach, but no studies undertook this.
Only 15% of studies explicitly recruited minority ethnic participants, with recruitment strategy unclear in 83.1%. Usually, it was not clear whether the recruitment ethnic mix was representative of the populations studied. Only 3.9% of study reports justified or appropriately explained results stratified by race/ethnicity. Differences in study outcomes for minority ethnic populations were appropriately addressed and interpreted in only 2.9% of studies. It is important to publish data on subjects’ ethnicity as many dermatological conditions are affected by race and skin color (217–219). Possibly most validation methods are not affected by a minority representation within a dataset, but only purposefully designed studies and methods such as DIF can reveal the relevance of such an effect. Rather than there being bias in validation studies, there is a lack of datasets with sufficient minority representation and appropriate methods to differentiate outcomes. An exemplary treatment of race and ethnicity was by Nagpal et al. (220) who discussed comparisons between racial/ethnic groups in detail. The study by Tan et al. (30) on the impact of facial atrophic acne scars on QoL, assessed “ethnicity” indirectly (but possibly inappropriately) (221) and observed no significant differences in DLQI scores between countries.
Strengths of this review include the large number of relevant articles identified, and the provision of direct access to previously scattered information. Limitations include: only English language articles being reviewed, though often reporting on validation using different DLQI translations; and because of the very broad set of concepts relevant to validation, we cannot be certain that all articles describing aspects of DLQI validation were identified. An alternate more complicated scoring system for the DLQI has been proposed, taking into account the influence of items scored “not relevant” (222), but this scoring method was not included as a search term in this systematic review.”
Future validation studies should use modern, accepted psychometric methods and appropriate metrics of fit for models used. They should also use reliable anchors for known groups (i.e. anchors that show good correlation with the DLQI) and improve responsiveness analyses. These methods and metrics are clearly outlined in the COSMIN guidelines (17,211). IRT studies with sufficiently large datasets are also required.
In conclusion this systematic review has brought together a wide range of data to illustrate current knowledge concerning validation of the DLQI. This review confirms many strengths of the DLQI and identifies areas for which further validation studies would be useful.
ACKNOWLEDGEMENTS
We wish to thank Dr. R K Singh for her contribution to the planning of this systematic review.
REFERENCES
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x
- Vyas J, Johns JR, Ali FM, Singh RK, Ingram JR, Salek S, et al. A systematic review of 454 randomized controlled trials using the Dermatology Life Quality Index: experience in 69 diseases and 43 countries. Br J Dermatol 2024; 190: 315–339. https://doi.org/10.1093/bjd/ljad079
- Finlay A, Singh R. DLQI guidelines, registries and reimbursement guidelines. Cardiff University: https://www.cardiff.ac.uk/__data/assets/pdf_file/0008/1744793/DLQI-guidelines-worldwide-Jan-2020.pdf, 2020.
- Kamudoni P, Johns N, Salek S. Living with Chronic Disease: Measuring Important Patient-Reported Outcomes. Singapore: Adis, Springer Nature; 2018. https://doi.org/10.1007/978-981-10-8414-0
- Rencz F, Szabo A, Brodszky V. Questionnaire Modifications and Alternative Scoring Methods of the Dermatology Life Quality Index: A Systematic Review. Value Health 2021; 24: 1158–1171. https://doi.org/10.1016/j.jval.2021.02.006
- Ali FM, Johns N, Salek S, Finlay AY. Correlating the Dermatology Life Quality Index with psychiatric measures: A systematic review. Clin Dermatol 2018; 36: 691–697. https://doi.org/10.1016/j.clindermatol.2018.08.014
- Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035. https://doi.org/10.1111/j.1365-2133.2008.08832.x
- Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004; 9: 169–180. https://doi.org/10.1111/j.1087-0024.2004.09113.x
- Streiner DL, Norman GR, Cairney J. Health Measurement Scales. 5th edition ed. Oxford: Oxford University Press;; 2015. https://doi.org/10.1093/med/9780199685219.001.0001
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. IHRev Esp Cardiol (Engl Ed) 2021; 74: 790–799. https://doi.org/10.1016/j.recesp.2021.06.016
- Schiavo JH. PROSPERO: An International Register of Systematic Review Protocols. Med Ref Serv Q 2019; 38: 171–180. https://doi.org/10.1080/02763869.2019.1588072
- Peters MD. Managing and Coding References for Systematic Reviews and Scoping Reviews in End-Note. Med Ref Serv Q 2017; 36: 19–31. https://doi.org/10.1080/02763869.2017.1259891
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Version 6.3 ed: Available from www.training.cochrane.org/handbook., 2022.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. https://doi.org/10.1016/j.jbi.2008.08.010
- Van Bulck L, Wampers M, Moons P. Research Electronic Data Capture (REDCap): tackling data collection, management, storage, and privacy challenges. Eur J Cardiovasc Nurs 2022; 21: 85–91. https://doi.org/10.1093/eurjcn/zvab104
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. https://doi.org/10.1016/j.jbi.2019.103208
- Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 2018; 27: 1147–1157. https://doi.org/10.1007/s11136-018-1798-3
- Cooper RS. Race in biological and biomedical research. Cold Spring Harb Perspect Med 2013; 3. https://doi.org/10.1101/cshperspect.a008573
- Evans BC. Content validation of instruments: are the perspectives of Anglo reviewers different from those of Hispanic/Latino and American Indian reviewers? J Nurs Educ 2005; 44: 216–224. https://doi.org/10.3928/01484834-20050501-04
- Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008; 112: 228–242. https://doi.org/10.1002/cncr.23157
- Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, et al. Race and Genetic Ancestry in Medicine – A Time for Reckoning with Racism. N Engl J Med 2021; 384: 474–480. https://doi.org/10.1056/NEJMms2029562
- Narla S, Heath CR, Alexis A, Silverberg JI. Racial disparities in dermatology. Arch Dermatol Res 2022; 10.1007/s00403-022-02507-z: 1–9. https://doi.org/10.1007/s00403-022-02507-z
- Naicker R. Critically appraising for antiracism. Educ Inf 2022; 38: 291–308. https://doi.org/10.3233/EFI-220052
- Mokkink LB, Prinsen CA, Patrick DL, Alonso J, Bouter LM, de Vet HCW, et al. COSMIN methodology for systematic reviews of Patient-Reported Outcome Measures (PROMs) user manual,. online] 2018 [cited 2023. Available from https://www.cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018-1.pdf https://doi.org/10.1007/978-3-319-69909-7_2972-2
- Portney LG. Chapter 32. Measurement Revisited: Reliability and Validity Statistics. Foundations of clinical research: Applications to Evidence-Based Practice. 4th ed. Philadelphia: F.A. Davies; 2020: p. p.489.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012; 22: 276–282. https://doi.org/10.11613/BM.2012.031
- Dancey CP, Reidy J. Chapter 15: Non-parametric statistics. Statistics without Maths for Psychology. 5th ed. London: Prentice Hall; 2011: p. p.523–524.
- Sullivan GM, Feinn R. Using Effect Size-or Why the P Value Is Not Enough. J Grad Med Educ 2012; 4: 279–282. https://doi.org/10.4300/JGME-D-12-00156.1
- Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, et al. Differential item functioning (DIF) analyses of health-related quality of life instruments using logistic regression. Health Qual Life Outcomes 2010; 8: 81. https://doi.org/10.1186/1477-7525-8-81
- Tan J, Beissert S, Cook-Bolden F, Chavda R, Harper J, Hebert A, et al. Impact of Facial Atrophic Acne Scars on Quality of Life: A Multi-country Population-Based Survey. Am J Clin Dermatol 2022; 23: 115–123. https://doi.org/10.1007/s40257-021-00628-1
- Fernandez-Penas P, Jones-Caballero M, Espallardo O, Garcia-Diez A. Comparison of Skindex-29, Dermatology Life Quality Index, Psoriasis Disability Index and Medical Outcome Study Short Form 36 in patients with mild to severe psoriasis. Br J Dermatol 2012; 166: 884–887. https://doi.org/10.1111/j.1365-2133.2012.10806.x
- Szabó A, Brodszky V, Rencz F. A comparative study on the measurement properties of Dermatology Life Quality Index (DLQI), DLQI-Relevant and Skindex-16. Br J Dermatol 2022; 186: 485–495. https://doi.org/10.1111/bjd.20765
- Patel KR, Singam V, Vakharia PP, Chopra R, Sacotte R, Patel N, et al. Measurement properties of three assessments of burden used in atopic dermatitis in adults. Br J Dermatol 2019; 180: 1083–1089. https://doi.org/10.1111/bjd.17243
- Badia X, Mascaro JM, Lozano R, Cavide Res G. Measuring health-related quality of life in patients with mild to moderate eczema and psoriasis: clinical validity, reliability and sensitivity to change of the DLQI. Br J Dermatol 1999; 141: 698–702. https://doi.org/10.1046/j.1365-2133.1999.03112.x
- Jobanputra R, Bachmann M. The effect of skin diseases on quality of life in patients from different social and ethnic groups in Cape Town, South Africa. Int J Dermatol 2000; 39: 826–831. https://doi.org/10.1046/j.1365-4362.2000.00073.x
- Ferraz LB, Almeida FA, Vasconcellos MR, Faccina AS, Ciconelli RM, Ferraz MB. The impact of lupus erythematosus cutaneous on the Quality of life: The Brazilian-Portuguese version of DLQI. Qual Life Res 2006; 15: 565–570. https://doi.org/10.1007/s11136-005-2638-9
- Takahashi N, Suzukamo Y, Nakamura M, Miyachi Y, Green J, Ohya Y, et al. Japanese version of the Dermatology Life Quality Index: validity and reliability in patients with acne. Health Qual Life Outcomes 2006; 4: 46. https://doi.org/10.1186/1477-7525-4-46
- Baranzoni N, Scalone L, Mantovani LG, De Portu S, Monzini MS, Giannetti A. Validation of the Italian version of the Dermatology Life Quality Index. G Ital Dermatol Venereol 2007; 142: 209–214.
- Mackenzie H, Thavaneswaran A, Chandran V, Gladman DD. Patient-reported Outcome in Psoriatic Arthritis: A Comparison of Web-based Versus Paper-completed Questionnaires. J Rheumatol 2011; 38: 2619–2624. https://doi.org/10.3899/jrheum.110165
- Madarasingha NP, de Silva P, Satgurunathan K, Madarasingha NP, de Silva P, Satgurunathan K. Validation study of Sinhala version of the dermatology life quality index (DLQI). Ceylon Med J 2011; 56: 18–22. https://doi.org/10.4038/cmj.v56i1.2890
- Khoudri I, Lamchahab FZ, Ismaili N, Senouci K, Hassam B, Abouqal R. Measuring quality of life in patients with psoriasis using the Arabic version for Morocco of the Dermatology Life Quality Index. Int J Dermatol 2013; 52: 795–802. https://doi.org/10.1111/j.1365-4632.2011.05450.x
- Liu JB, Yao MZ, Si AL, Xiong LK, Zhou H. Life quality of Chinese patients with chronic urticaria as assessed by the dermatology life quality index. J Eur Acad Dermatol Venereol 2012; 26: 1252–1257. https://doi.org/10.1111/j.1468-3083.2011.04277.x
- Ali FM, Johns N, Finlay AY, Salek MS, Piguet V. Comparison of the paper-based and electronic versions of the Dermatology Life Quality Index: evidence of equivalence. Br J Dermatol 2017; 177: 1306–1315. https://doi.org/10.1111/bjd.15314
- Jesmin A, Sultana A, Bhuiyan MSI, Yasir Arafat SM. Validation of bangla dermatology life quality index among patients with psoriasis. J Pak Assoc Derm 2021; 31: 165–172.
- Meneguin S, Matos TDD, Pollo CF, Garuzi M, Miot HA, de Oliveira C. Psychometric characteristics of DLQI-BRA and Skindex-16 to measure the impact of dermatological diseases on quality of life in Brazilian patients. PLOS ONE 2021; 16. https://doi.org/10.1371/journal.pone.0254882
- Schwartzman G, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. Validity and reliability of Patient-Reported Outcomes Measurement Information System Global Health scale in adults with atopic dermatitis. J Am Acad Dermatol 2021; 85: 636–644. https://doi.org/10.1016/j.jaad.2021.01.033
- Zachariae R, Zachariae C, Ibsen H, Mortensen JT, Wulf HC. Dermatology Life Quality Index: Data from Danish inpatients and outpatients. Acta Derm Venereol 2000; 80: 272–276. https://doi.org/10.1080/000155500750012153
- Shikiar R, Bresnahan BW, Stone SP, Thompson C, Koo J, Revicki DA. Validity and reliability of patient reported outcomes used in psoriasis: Results from two randomized clinical trials. Health Qual Life Outcomes 2003; 1: 53. https://doi.org/10.1186/1477-7525-1-53
- Aghaei S, Sodaifi M, Jafari P, Mazharinia N, Finlay AY. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol 2004; 4: 8. https://doi.org/10.1186/1471-5945-4-8
- Ilgen E, Derya A. There is no correlation between acne severity and AQOLS/DLQI scores. J Dermatol 2005; 32: 705–710. https://doi.org/10.1111/j.1346-8138.2005.tb00829.x
- Mazzotti E, Barbaranelli C, Picardi A, Abeni D, Pasquini P. Psychometric properties of the Dermatology Life Quality Index (DLQI) in 900 Italian patients with psoriasis. Acta Derm Venereol 2005; 85: 409–413. https://doi.org/10.1080/00015550510032832
- Ozturkcan S, Ermertcan AT, Eser E, Sahin MT. Cross validation of the Turkish version of dermatology life quality index. Int J Dermatol 2006; 45: 1300–1307. https://doi.org/10.1111/j.1365-4632.2006.02881.x
- Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health Qual Life Outcomes 2006; 4: 71. https://doi.org/10.1186/1477-7525-4-71
- Mazharinia N, Aghaei S, Shayan Z, Mazharinia N, Aghaei S, Shayan Z. Dermatology Life Quality Index (DLQI) scores in burn victims after revival. J Burn Care Res 2007; 28: 312–317. https://doi.org/10.1097/BCR.0B013E318031A151
- Henok L, Davey G. Validation of the Dermatology Life Quality Index among patients with podoconiosis in southern Ethiopia. Br J Dermatol 2008; 159: 903–906. https://doi.org/10.1111/j.1365-2133.2008.08773.x
- Aghaei S, Moradi A, Ardekani GS, Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol 2009; 75: 220–220. https://doi.org/10.4103/0378-6323.48689
- An JG, Ma JH, Xiao SX, Xiao SB, Yang F. Quality of life in patients with lepromatous leprosy in China. J Eur Acad Dermatol Venereol 2010; 24: 827–832. https://doi.org/10.1111/j.1468-3083.2009.03534.x
- Maksimovic N, Jankovic S, Marinkovic J, Sekulovic LK, Zivkovic Z, Spiric VT. Health-related quality of life in patients with atopic dermatitis. J Dermatol 2012; 39: 42–47. https://doi.org/10.1111/j.1346-8138.2011.01295.x
- Twiss J, Meads DM, Preston EP, Crawford SR, McKenna SP. Can we rely on the Dermatology Life Quality Index as a measure of the impact of psoriasis or atopic dermatitis? J Invest Dermatol 2012; 132: 76–84. https://doi.org/10.1038/jid.2011.238
- An JG, Liu YT, Xiao SX, Wang JM, Geng SM, Dong YY. Quality Of Life of Patients with Neurodermatitis. Int J Med Sci 2013; 10: 593–598. https://doi.org/10.7150/ijms.5624
- He Z, Lu C, Basra MKA, Ou A, Yan Y, Li L. Psychometric properties of the Chinese version of Dermatology Life Quality Index (DLQI) in 851 Chinese patients with psoriasis. J Eur Acad Dermatol Venereol 2013; 27: 109–115. https://doi.org/10.1111/j.1468-3083.2011.04371.x
- Lilly E, Lu PD, Borovicka JH, Victorson D, Kwasny MJ, West DP, et al. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL). J Am Acad Dermatol 2013; 69: e11–e18. https://doi.org/10.1016/j.jaad.2012.01.038
- Liu ZB, Xie Z, Zhang L, Jin YT, Guo HJ, Jiang ZQ, et al. Reliability and validity of dermatology life quality index: assessment of quality of life in human immunodeficiency virus/acquired immunodeficiency syndrome patients with pruritic papular eruption. J Tradit Chin Med 2013; 33: 580–583. https://doi.org/10.1016/S0254-6272(14)60024-8
- Lockhart J, Gray NM, Cruickshank ME. The development and evaluation of a questionnaire to assess the impact of vulval intraepithelial neoplasia: a questionnaire study. BJOG 2013; 120: 1133–1142. https://doi.org/10.1111/1471-0528.12229
- Thomas C, Narahari SR, Bose KS, Vivekananda K, Nwe S, West DP, et al. Comparison of Three Quality of Life Instruments in Lymphatic Filariasis: DLQI, WHODAS 2.0, and LFSQQ. PLoS Negl Trop Dis 2014; 8. https://doi.org/10.1371/journal.pntd.0002716
- Wachholz PA, Masuda PY, Nascimento DC, Taira CMH, Cleto NG. Quality of life profile and correlated factors in chronic leg ulcer patients in the mid-west of São Paulo state, Brazil. An Bras Dermatol 2014; 89: 73–81. https://doi.org/10.1590/abd1806-4841.20142156
- Qi S, Xu F, Sheng Y, Yang Q. Assessing quality of life in Alopecia areata patients in China. Psychol Health Med 2015; 20: 97–102. https://doi.org/10.1080/13548506.2014.894641
- Chernyshov PV. Health-related quality of life in adult atopic dermatitis and psoriatic patients matched by disease severity. G Ital Dermatol Venereol 2016; 151: 37–43.
- Solgajová A, Sollár T, Vörösová G, Zrubcová D. The incidence of anxiety, depression, and quality of life in patients with dermatological diseases. Cent Eur J Nurs Midwifery 2016; 7: 476–483. https://doi.org/10.15452/CEJNM.2016.07.0018
- Kirby JS, Butt M, Esmann S, Jemec GBE. Association of Resilience With Depression and Health-Related Quality of Life for Patients With Hidradenitis Suppurativa. JAMA Dermatol 2017; 153: 1263–1269. https://doi.org/10.1001/jamadermatol.2017.3596
- Cozzani E, Linder D, Burlando M, Gallo F, Sampogna F, Bruzzone M, et al. PSOdisk is a reliable, intuitive instrument for the evaluation of psychological distress, which strongly correlates with DLQI: a preliminary study. Eur J Dermatol 2018; 28: 332–337. https://doi.org/10.1684/ejd.2018.3301
- Hunt WTN, Hùng NT, Tru’Ò’Ng NN, Nikolaou V, Khoa NDD, Hôngly T. A case-control study comparing the Dermatology Life Quality Index (DLQI) ratings of patients undergoing leprosy treatment, people cured of leprosy, and controls in Vietnam. Lepr Rev 2018; 89: 46–55. https://doi.org/10.47276/lr.89.1.46
- Shimizu GKM, Wedy GF, Schaefer LV, Ramos PM, Miot HA. Translation into Portuguese language (Brazil), transcultural adaptation and validation of the quality of life questionnaire in female pattern hair loss (WAA-QoL-BP). An Bras Dermatol 2018; 93: 701–706. https://doi.org/10.1590/abd1806-4841.20187452
- Xiao Y, Huang X, Jing D, Huang Y, Zhang X, Shu Z, et al. Assessment of the Dermatology Life Quality Index (DLQI) in a homogeneous population under lifetime arsenic exposure. Qual Life Res 2018; 27: 3209–3215. https://doi.org/10.1007/s11136-018-1969-2
- Beamer LC, Grant M. Using the Dermatology Life Quality Index to Assess How Breast Radiodermatitis Affects Patients’ Quality of Life. Breast Cancer (Auckl) 2019; 13: 1–7. https://doi.org/10.1177/1178223419835547
- Satti MZ, Arshad D, Javed H, Shahroz A, Tahir Z, Ahmed MMH, et al. Uremic Pruritus: Prevalence and Impact on Quality of Life and Depressive Symptoms in Hemodialysis Patients. Cureus 2019; 11. https://doi.org/10.7759/cureus.5178
- Storck M, Zeidler C, Rehr M, Riepe C, Dugas M, Ständer S, et al. Validation of pruritus measures gathered with the electronic patient-reported outcome system mopat. Acta Derm Venereol 2018; 98: 38–43. https://doi.org/10.2340/00015555-2799
- Temel AB, Bozkurt S, Senol Y, Alpsoy E. Internalized stigma in patients with acne vulgaris, vitiligo, and alopecia areata. Turk J Dermatol 2019; 13: 109–116. https://doi.org/10.4103/TJD.TJD_14_19
- Demirci OO, Ate B, Sagaltici E, Ocak ZG, Altunay IK. Association of the attachment styles with depression, anxiety, and quality of life in patients with psoriasis. Dermatol Sin 2020; 38: 81–87. https://doi.org/10.4103/ds.ds_35_19
- Jorge MFS, Sousa TD, Pollo CF, Paiva BSR, Ianhez M, Boza JC, et al. Dimensionality and psychometric analysis of DLQI in a Brazilian population. Health Qual Life Outcomes 2020; 18: 268. https://doi.org/10.1186/s12955-020-01523-9
- Paudel S, Parajuli N, Sharma RP, Dahal S, Paudel S. Chronic Urticaria and Its Impact on the Quality of Life of Nepalese Patients. Dermatol Res Pract 2020; 10.1155/2020/6694191: 1–5. https://doi.org/10.1155/2020/6694191
- Pollo CF, Miot HA, Matos TDdS, Souza JM, Jorge MFS, Miot LDB, et al. Prevalence and factors associated with depression and anxiety in patients with psoriasis. J Clin Nurs 2021; 30: 572–580. https://doi.org/10.1111/jocn.15577
- Kolokotsa EN, Kapaki V, Kotsopoulos N. Assessment of quality of life in patients with acne vulgaris in Greece. Archives of Hellenic Medicine 2022; 39: 827–831.
- Takahashi H, Iinuma S, Tsuji H, Honma M, Iizuka H. Biologics are more potent than other treatment modalities for improvement of quality of life in psoriasis patients. J Dermatol 2014; 41: 686–689. https://doi.org/10.1111/1346-8138.12544
- Basra MKA, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the Minimal Clinically Important Difference and Responsiveness of the Dermatogy Life Quality Index (DLQI): Further Data. Dermatol 2015; 230: 27–33. https://doi.org/10.1159/000365390
- Richter C, Trojahn C, Hillmann K, Dobos G, Kanti V, Vogt A, et al. Sensitivity to change of the Dermatology Life Quality Index in adult females with facial acne vulgaris: a validation study. J Eur Acad Dermatol Venereol 2017; 31: 169–174. https://doi.org/10.1111/jdv.13757
- Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. Association of atopic dermatitis severity with cognitive function in adults. J Am Acad Dermatol 2020; 83: 1349–1359. https://doi.org/10.1016/j.jaad.2020.05.041
- Silverberg JI, Lai JS, Patel KR, Singam V, Vakharia PP, Chopra R, et al. Measurement properties of the Patient-Reported Outcomes Information System (PROMIS®) Itch Questionnaire: itch severity assessments in adults with atopic dermatitis. Br J Dermatol 2020; 183: 891–898. https://doi.org/10.1111/bjd.18978
- Papoui E, Papastavrou E, Merkouris A, Charalambous A. A pilot randomized controlled study of the effects of an educational training program on skin reactions induced by chemotherapy, Epidermal Growth Factor Inhibitor (EGFRI) treatments, and immunotherapy. Eur J Oncol Nurs 2022; 60: 102194. https://doi.org/10.1016/j.ejon.2022.102194
- Herd RM, Tidman MJ, Ruta DA, Hunter JAA. Measurement of quality of life in atopic dermatitis: Correlation and validation of two different methods. Br J Dermatol 1997; 136: 502–507. https://doi.org/10.1046/j.1365-2133.1997.d01-1225.x
- Kent G. Correlates of perceived stigma in vitiligo. Psychol Health 1999; 14: 241–251. https://doi.org/10.1080/08870449908407325
- Rosenberg M. Society and the adolescent self-image. Princeton: Princeton University Press; 1965. https://doi.org/10.1515/9781400876136
- Mallon E, Newton JN, Klassen A, Stewart-Brown SL, Ryan TJ, Finlay AY. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol 1999; 140: 672–676. https://doi.org/10.1046/j.1365-2133.1999.02768.x
- Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol 2000; 80: 430–434. https://doi.org/10.1080/000155500300012873
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss in quality of life. J Eur Acad Dermatol Venereol 2001; 15: 137–139. https://doi.org/10.1046/j.1468-3083.2001.00229.x
- Sampogna F, Sera F, Abeni D. Measures of clinical severity, quality of life, and psychological distress in patients with psoriasis: A cluster analysis. J Invest Dermatol 2004; 122: 602–607. https://doi.org/10.1046/j.0022-202X.2003.09101.x
- Wittkowski A, Richards HL, Griffiths CEM, Main CJ. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res 2004; 57: 195–200. https://doi.org/10.1016/S0022-3999(03)00572-5
- Yazici K, Baz K, Yazici AE, Kokturk A, Tot S, Demirseren D, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol 2004; 18: 435–439. https://doi.org/10.1111/j.1468-3083.2004.00946.x
- Vilata JJ, Varela JA, Olmos L, Colombo JA, Llorens MA, de los Terreros MS, et al. Validation and clinical use of the CECA, a disease-specific quality of life questionnaire for patients with anogenital condylomata acuminata. Acta Derm Venereol 2008; 88: 257–262.
- Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: A randomized clinical trial. J Am Acad Dermatol 2010; 62: 812–818. https://doi.org/10.1016/j.jaad.2009.07.022
- de Ue APF, de Souza PK, Rotta O, Furlani WD, de Lima ARM, Sabbag D. Quality of life assessment in patients with chronic urticaria. An Bras Dermatol 2011; 86: 897–904. https://doi.org/10.1590/S0365-05962011000500006
- Goreshi R, Chock M, Foering K, Feng R, Okawa J, Rose M, et al. Quality of life in dermatomyositis. J Am Acad Dermatol 2011; 65: 1107–1116. https://doi.org/10.1016/j.jaad.2010.10.016
- Kluger N, Letois F, Picot MC, Guillot B, Bessis D. How much disability is caused by fibrofolliculomas during Birt-Hogg-Dubé Syndrome? J Eur Acad Dermatol Venereol 2011; 25: 940–944. https://doi.org/10.1111/j.1468-3083.2010.03887.x
- Lau MYZ, Matheson MC, Burgess JA, Dharmage SC, Nixon R. Disease severity and quality of life in a follow-up study of patients with occupational contact dermatitis. Contact Dermatitis 2011; 65: 138–145. https://doi.org/10.1111/j.1600-0536.2011.01896.x
- Tadros A, Vergou T, Stratigos AJ, Tzavara C, Hletsos M, Katsambas A, et al. Psoriasis: is it the tip of the iceberg for the quality of life of patients and their families? J Eur Acad Dermatol Venereol 2011; 25: 1282–1287. https://doi.org/10.1111/j.1468-3083.2010.03965.x
- Ghajarzadeh M, Ghiasi M, Kheirkhah S. Associations between skin diseases and quality of life: A comparison of psoriasis, vitiligo, and alopecia areata. Acta Med Iran 2012; 50: 511–515.
- Ghajarzadeh M, Ghiasi M, Kheirkhah S. Depression and quality of life in iranian patients with alopecia areata. Iran J Dermatol 2012; 14: 140–143.
- Kimball AB, Yu AP, Signorovitch J, Xie J, Tsaneva M, Gupta SR, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol 2012; 66: e67–76. https://doi.org/10.1016/j.jaad.2010.10.020
- Norlin JM, Steen Carlsson K, Persson U, Schmitt-Egenolf M. Analysis of three outcome measures in moderate to severe psoriasis: a registry-based study of 2450 patients. Br J Dermatol 2012; 166: 797–802. https://doi.org/10.1111/j.1365-2133.2011.10778.x
- Yu M, Han TY, Lee JH, Son SJ. The Quality of Life and Depressive Mood among Korean Patients with Hand Eczema. Ann Dermatol 2012; 24: 430–437. https://doi.org/10.5021/ad.2012.24.4.430
- Bin Saif GA, Al-Balbeesi AO, Binshabaib R, Alsaad D, Kwatra SG, Alzolibani AA, et al. Quality of life in family members of vitiligo patients: A questionnaire study in Saudi Arabia. Am J Clin Dermatol 2013; 14: 489–495. https://doi.org/10.1007/s40257-013-0037-5
- Ghaderi R, Saadatjoo A, Ghaderi F. Evaluating of Life Quality in Patients with Acne Vulgaris Using Generic and Specific Questionnaires. Dermatol Res Pract 2013; 10.1155/2013/108624: 1–6. https://doi.org/10.1155/2013/108624
- Lindberg M, Bingefors K, Meding B, Berg M. Hand eczema and health-related quality of life; a comparison of EQ-5D and the Dermatology Life Quality Index (DLQI) in relation to the hand eczema extent score (HEES). Contact Dermatitis 2013; 69: 138–143. https://doi.org/10.1111/cod.12067
- Rizwan M, Reddick CL, Bundy C, Unsworth R, Richards HL, Rhodes LE. Photodermatoses: Environmentally induced conditions with high psychological impact. Photochem Photobiol Sci 2013; 12: 182–189. https://doi.org/10.1039/c2pp25177a
- Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements in patient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health Qual Life Outcomes 2013; 11: 82. https://doi.org/10.1186/1477-7525-11-82
- Stumpf A, Stander S, Phan NQ, Tanneberger A, Heuft G, Schneider G. Body Concept of Patients with Chronic Pruritus in Relation to Scratch Lesions and Psychic Symptoms. Dermatol 2013; 227: 263–269. https://doi.org/10.1159/000354911
- Tjokrowidjaja A, Daniel BS, Frew JW, Sebaratnam DF, Hanna AM, Chee S, et al. The development and validation of the treatment of autoimmune bullous disease quality of life questionnaire, a tool to measure the quality of life impacts of treatments used in patients with autoimmune blistering disease. Br J Dermatol 2013; 169: 1000–1006. https://doi.org/10.1111/bjd.12623
- Vinding GR, Christensen KB, Esmann S, Olesen AB, Jemec GBE. Quality of life in non-melanoma skin cancer – The skin cancer quality of life (SCQoL) questionnaire. Dermatol Surg 2013; 39: 1784–1793. https://doi.org/10.1111/dsu.12353
- Yano C, Saeki H, Ishiji T, Ishiuji Y, Sato J, Tofuku Y, et al. Impact of disease severity on work productivity and activity impairment in Japanese patients with atopic dermatitis. J Dermatol 2013; 40: 736–739. https://doi.org/10.1111/1346-8138.12220
- Bardazzi F, Amerio P, Amoruso G, Campanati A, Conti A, De Simone C, et al. Investigating psoriasis awareness among patients in Italy: Validation of a questionnaire. Eur Rev Med Pharmacol Sci 2014; 18: 3435–3452.
- Doʇruk Kaçar S, Özuʇuz P, Baʇcioʇlu E, Coşkun KŞ, Polat S, Karaca Ş. Is a poor dermatology life quality index score a sign of stigmatization in patients with vitiligo? Turkiye Klinikleri J Dermatol 2014; 24: 45–50.
- Ghaderi R, Saadatjoo A. Quality of Life in Patients with Hand Eczema as Health Promotion: A Case Control Study. Acta Dermatovenerol Croat 2014; 22: 32–39.
- Ghaderi R, Saadatjoo A. Evaluating of life quality in Iranian patients with vitiligo using generic and special questionnaires. Shiraz E Med J 2014; 15. https://doi.org/10.17795/semj22359
- Hawro T, Maurer M, Hawro M, Kaszuba A, Cierpialkowska L, Krolikowska M, et al. In psoriasis, levels of hope and quality of life are linked. Arch Dermatol Res 2014; 306: 661–666. https://doi.org/10.1007/s00403-014-1455-9
- Herédi E, Rencz F, Balogh O, Gulácsi L, Herszényi K, Holló P, et al. Exploring the relationship between EQ-5D, DLQI and PASI, and mapping EQ-5D utilities: a cross-sectional study in psoriasis from Hungary. Eur J Health Econ 2014; 15: 111–119. https://doi.org/10.1007/s10198-014-0600-x
- Susel J, Batycka-Baran A, Reich A, Szepietowski JC. Uraemic Pruritus Markedly Affects the Quality of Life and Depressive Symptoms in Haemodialysis Patients with End-stage Renal Disease. Acta Derm Venereol 2014; 94: 276–281. https://doi.org/10.2340/00015555-1749
- Boza JC, Kundu RV, Fabbrin A, Horn R, Giongo N, Cestari TF. Translation, cross-cultural adaptation and validation of the vitiligo-specific health-related quality of life instrument (VitiQoL) into Brazilian Portuguese. An Bras Dermatol 2015; 90: 358–362. https://doi.org/10.1590/abd1806-4841.20153684
- Breuer K, Goldner FM, Jager B, Werfel T, Schmid-Ott G. Chronic stress experience and burnout syndrome have appreciable influence on health-related quality of life in patients with psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1898–1904. https://doi.org/10.1111/jdv.12999
- Chiang YZ, Bundy C, Griffiths CEM, Paus R, Harries MJ. The role of beliefs: lessons from a pilot study on illness perception, psychological distress and quality of life in patients with primary cicatricial alopecia. Br J Dermatol 2015; 172: 130–137. https://doi.org/10.1111/bjd.13259
- Durai PCT, Nair DG. Acne Vulgaris and Quality of Life among Young Adults in South India. Indian J Dermatol 2015; 60: 33–40. https://doi.org/10.4103/0019-5154.147784
- Heelan K, Hitzig SL, Knowles S, Drucker AM, Mittmann N, Walsh S, et al. Loss of Work Productivity and Quality of Life in Patients With Autoimmune Bullous Dermatoses. J Cutan Med Surg 2015; 19: 546–554. https://doi.org/10.1177/1203475415582317
- Moradi M, Rencz F, Brodszky V, Moradi A, Balogh O, Gulácsi L. Health Status and Quality of Life in Patients with Psoriasis: An Iranian Cross-Sectional Survey. Arch Iran Med 2015; 18: 153–159.
- Schmitt J, Kuster D. Correlation between Dermatology Life Quality Index (DLQI) scores and Work Limitations Questionnaire (WLQ) allows the calculation of percent work productivity loss in patients with psoriasis. Arch Dermatol Res 2015; 307: 451–453. https://doi.org/10.1007/s00403-015-1567-x
- Sung JY, Roh MR, Kim SC. Quality of Life Assessment in Korean Patients with Pemphigus. Ann Dermatol 2015; 27: 492–498. https://doi.org/10.5021/ad.2015.27.5.492
- Tennvall GR, Norlin JM, Malmberg I, Erlendsson AM, Haedersdal M. Health related quality of life in patients with actinic keratosis – an observational study of patients treated in dermatology specialist care in Denmark. Health Qual Life Outcomes 2015; 13: 111. https://doi.org/10.1186/s12955-015-0295-4
- Catucci Boza J, Giongo N, Machado P, Horn R, Fabbrin A, Cestari T. Quality of Life Impairment in Children and Adults with Vitiligo: A Cross-Sectional Study Based on Dermatology-Specific and Disease-Specific Quality of Life Instruments. Dermatol 2016; 232: 619–625. https://doi.org/10.1159/000448656
- Gawlik MM, Topczewska B, Kurpas D. Quality of life of psoriatic patients – modulatory variables. Fam Med Prim Care Rev 2016; 10.5114/fmpcr/62559: 235–240. https://doi.org/10.5114/fmpcr/62559
- Ko WC, Tsai TF, Tang CH. Health state utility, willingness to pay, and quality of life among Taiwanese patients with psoriasis. Dermatol Sin 2016; 34: 185–191. https://doi.org/10.1016/j.dsi.2016.05.004
- Kong TS, Han TY, Lee JH, Son SJ. Correlation between Severity of Atopic Dermatitis and Sleep Quality in Children and Adults. Ann Dermatol 2016; 28: 321–326. https://doi.org/10.5021/ad.2016.28.3.321
- Kouris A, Christodoulou C, Efstathiou V, Tsatovidou R, Torlidi-Kordera E, Zouridaki E, et al. Comparative study of quality of life and psychosocial characteristics in patients with psoriasis and leg ulcers. Wound Repair Regen 2016; 24: 443–446. https://doi.org/10.1111/wrr.12416
- Maranzatto CFP, Miot HA, Miot LDB, Meneguin S. Psychometrican analysis and dimensional structure of the Brazilian version of melasma quality of life scale (MELASQoL-BP)*. An Bras Dermatol 2016; 91: 422–428. https://doi.org/10.1590/abd1806-4841.20165014
- Salman A, Kurt E, Topcuoglu V, Demircay Z. Social Anxiety and Quality of Life in Vitiligo and Acne Patients with Facial Involvement: A Cross-Sectional Controlled Study. Am J Clin Dermatol 2016; 17: 305–311. https://doi.org/10.1007/s40257-016-0172-x
- Sarhan D, Mohammed GFA, Gomaa AHA, Eyada MMK. Female Genital Dialogues: Female Genital Self-Image, Sexual Dysfunction, and Quality of Life in Patients With Vitiligo With and Without Genital Affection. J Sex Marital Ther 2016; 42: 267–276. https://doi.org/10.1080/0092623X.2015.1010678
- Alarcon I, Vinding GR, Christensen KB, Esmann S, Malvehy J, Puig S, et al. Spanish version of the Actinic Keratosis Quality of Life questionnaire. J Eur Acad Dermatol Venereol 2017; 31: 986–991. https://doi.org/10.1111/jdv.14127
- Augustin M, Blome C, Paul C, Puig L, Luger T, Lambert J, et al. Quality of life and patient benefit following transition from methotrexate to ustekinumab in psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 294–303. https://doi.org/10.1111/jdv.13823
- Březinová E, Nečas M, Vašků V. Quality of life & psychological disturbances in adults with atopic dermatitis in the Czech Republic. J Biol Regul Homeost Agents 2017; 31: 227–233.
- Janse IC, Deckers IE, van der Maten AD, Evers AWM, Boer J, van der Zee HH, et al. Sexual health and quality of life are impaired in hidradenitis suppurativa: a multicentre cross-sectional study. Br J Dermatol 2017; 176: 1042–1047. https://doi.org/10.1111/bjd.14975
- Masaki S, Tatsukawa R, Uryu M, Takahara M, Furue M, Ohata C, et al. Treatment satisfaction, willingness to pay and quality of life in Japanese patients with psoriasis. J Dermatol 2017; 44: 143–146. https://doi.org/10.1111/1346-8138.13541
- Michelsen B, Diamantopoulos AP, Hoiberg HK, Soldal DM, Kavanaugh A, Haugeberg G. Need for Improvement in Current Treatment of Psoriatic Arthritis: Study of an Outpatient Clinic Population. J Rheumatol 2017; 44: 431–436. https://doi.org/10.3899/jrheum.160973
- Müller K, Karrer S, Szeimies RM, Steinbauer J, Kohl E, Steinbauer D, et al. Quality of life assessment in patients with nonmelanoma skin cancer – psychometric validation of the EORTC QLQ-C30 questionnaire. J Dtsch Dermatol Ges 2017; 15: 1090–1100. https://doi.org/10.1111/ddg.13357
- Xu ST, Oh EH, Kim JE, Ko JY, Ro YS. Comparative study of quality of life between psoriasis, vitiligo and autoimmune bullous disease. Hong Kong J Dermatol Venereol 2017; 25: 57–64.
- Yfantopoulos J, Chantzaras A, Kontodimas S. Assessment of the psychometric properties of the EQ-5D-3L and EQ-5D-5L instruments in psoriasis. Arch Dermatol Res 2017; 309: 357–370. https://doi.org/10.1007/s00403-017-1743-2
- Hassanin AM, Ismail NN, el Guindi A, Sowailam HA. The emotional burden of chronic skin disease dominates physical factors among women, adversely affecting quality of life and sexual function. J Psychosom Res 2018; 115: 53–57. https://doi.org/10.1016/j.jpsychores.2018.10.011
- Kluger N, Sintonen H, Ranta M, Serlachius M. Health-Related Quality of Life of Patients with Hidradenitis Suppurativa Measured with the 15D Instrument and Comparison with the General Population and Patients with Psoriasis. Skin Appendage Disord 2018; 4: 131–135. https://doi.org/10.1159/000481117
- Morice-Picard F, Taieb C, Marti A, Gliksohn A, Bennani M, Bodemer C, et al. Burden of albinism: development and validation of a burden assessment tool. Orphanet J Rare Dis 2018; 13. https://doi.org/10.1186/s13023-018-0894-3
- Tekin A, Atis G, Yasar S, Goktay F, Aytekin S. The relationship of type D personality and quality of life in patients with psoriasis: a cross-sectional study in Turkish population. Acta Med Mediterr 2018; 34: 1009–1013.
- Vakharia PP, Chopra R, Sacotte R, Patel N, Immaneni S, White T, et al. Severity strata for five patient-reported outcomes in adults with atopic dermatitis. Br J Dermatol 2018; 178: 925–930. https://doi.org/10.1111/bjd.16078
- Wang EQ, Radjenovic M, Castrillon MA, Feng GHY, Murrell DF. The effect of autoimmune blistering diseases on work productivity. J Eur Acad Dermatol Venereol 2018; 32: 1959–1966. https://doi.org/10.1111/jdv.15062
- Wu Y, Fu C, Zhang W, Li C, Zhang J. The dermatology life quality index (DLQI) and the hospital anxiety and depression (HADS) in Chinese rosacea patients. Psychol Health Med 2018; 23: 369–374. https://doi.org/10.1080/13548506.2017.1361540
- Albuquerque RG, Buratto GG, Hirotsu C, Maeda SM, Floriano MC, Andersen ML, et al. Comparison of quality of life evaluated by SF-36 and DLQI in multibacillary and paucibacillary leprosy patients from Sao Paulo, Brazil. Int J Dermatol 2019; 58: 1415–1422. https://doi.org/10.1111/ijd.14489
- Arents BWM, Mensing U, Seitz IA, Wettemann N, Fink-Wagner AH, De Carlo G, et al. Atopic eczema score of emotional consequences – a questionnaire to assess emotional consequences of atopic eczema. Allergo J 2019; 28: 58–69. https://doi.org/10.1007/s15007-019-1952-4
- Kalboussi H, Kacem I, Aroui H, El Maalel O, Maoua M, Brahem A, et al. Impact of Allergic Contact Dermatitis on the Quality of Life and Work Productivity. Dermatol Res Pract 2019; 2019. https://doi.org/10.1155/2019/3797536
- Le PH, Vo TQ, Nguyen NH. Quality of life measurement alteration among Vietnamese: Impact and treatment benefit related to eczema. J Pak Med Assoc 2019; 69: S49–S56.
- Narang T, Bhattacharjee R, Singh S, Jha K, Kavita, Mahajan R, et al. Quality of life and psychological morbidity in patients with superficial cutaneous dermatophytosis. Mycoses 2019; 62: 680–685. https://doi.org/10.1111/myc.12930
- Patro N, Panda M, Jena AK. The Menace of Superficial Dermatophytosis on the Quality of Life of Patients Attending Referral Hospital in Eastern India: A Cross-sectional Observational Study. Indian Dermatol Online J 2019; 10: 262–266. https://doi.org/10.4103/idoj.IDOJ_342_18
- Stefanidou M, Evangelou G, Kontodimopoulos N, Koumaki D, Krueger-Krasagakis SE, Yosipovitch G, et al. Willingness to pay and quality of life in patients with pruritic skin disorders. Arch Dermatol Res 2019; 311: 221–230. https://doi.org/10.1007/s00403-019-01900-5
- Zeidler C, Steinke S, Riepe C, Bruland P, Soto-Rey I, Storck M, et al. Cross-European validation of the ItchyQoL in pruritic dermatoses. J Eur Acad Dermatol Venereol 2019; 33: 391–397. https://doi.org/10.1111/jdv.15225
- Gerdes S, Wilsmann-Theis D, Celis D, Kromer C, Mossner R. Two questions may be enough - screening for depression in patients with psoriasis: a multicenter study. J Dtsch Dermatol Ges 2020; 18: 1115–1125. https://doi.org/10.1111/ddg.14203
- Namdar ND, Arıkan İ. The relationship between alexithymia, anxiety, depression, and severity of the disease in psoriasis patients. J Surg Med 2020; 4: 226–229.
- Oosterhaven JAF, Ofenloch RF, Schuttelaar MLA. Validation of the Dutch Quality of Life in Hand Eczema Questionnaire (QOLHEQ). Br J Dermatol 2020; 183: 86–95. https://doi.org/10.1111/bjd.18558
- Passlov HM, Ponten A, Bjork J, Rosen B, Bruze M, Svedman C, et al. Hand strength and dexterity in individuals with hand eczema. J Eur Acad Dermatol Venereol 2020; 34: 2856–2862. https://doi.org/10.1111/jdv.16401
- Silpaarcha N, Pruksaeakanan C, Angkoolpakdeekul N, Chaiyabutr C, Kulthanan K, Rattaapha W, et al. Relationship Between Depression and Quality of Life Among Vitiligo Patients: A Self-assessment Questionnaire-based Study. Clin Cosmet Investig Dermatol 2020; 13: 511–520. https://doi.org/10.2147/CCID.S265349
- Stepien K, Reich A. The 12-Item Pruritus Severity Scale – Determining the Severity Bands. Front Med 2020; 7. https://doi.org/10.3389/fmed.2020.614005
- Tawil S, Irani C, Kfoury R, Salameh P, Baiardini I, Weller K, et al. The Arabic Urticaria Activity Score and Chronic Urticaria Quality of Life Questionnaire: validation and correlations. Int J Dermatol 2020; 59: 893–901. https://doi.org/10.1111/ijd.15006
- Acar EM, Erdogan HK, Sas S, Acer E. Evaluation of fibromyalgia syndrome in patients with rosacea. Arch Rheumatol 2021; 36: 252–257. https://doi.org/10.46497/ArchRheumatol.2021.8280
- Bakar RS, Jaapar SZS, Azmi AF, Aun YC. Depression and anxiety among patients with psoriasis: A correlation with quality of life and associated factors. J Taibah Univ Med Sci 2021; 16: 491–496. https://doi.org/10.1016/j.jtumed.2021.02.008
- Barbieri JS, Fuxench ZCC, Shin DB, Takeshita J. Frequency and influence of “not relevant” responses on the Dermatology Life Quality Index among adults with atopic dermatitis. Qual Life Res 2021; 30: 1705–1713. https://doi.org/10.1007/s11136-021-02770-z
- Chaudhary RG, Changhulani AR, Malhotra SD, Parikh NR, Shah MJ, Chaudhary AR, et al. Dermatology Life Quality Index and Social Stigma among Patients of Hansen’s Disease. Indian J Lepr 2021; 93: 349–360.
- Emre E, Tazegul G. Evaluation of Anxiety, Depression and Quality of Life in Patients with Chronic Urticaria. Astim Allerji Immunoloji 2021; 19: 6–11. https://doi.org/10.21911/aai.543
- Erol K, Ertas SK, Ertas R. Fatigue Is Common and Predicted by Female Gender and Sleep Disturbance in Patients with Chronic Spontaneous Urticaria. J Allergy Clin Immunol Pract 2021; 9: 469–476. https://doi.org/10.1016/j.jaip.2020.08.020
- Esposito M, Giunta A, Nanni RC, Criscuolo S, Manfreda V, Del Duca E, et al. Depressive symptoms and insecure attachment predict disability and quality of life in psoriasis independently from disease severity. Arch Dermatol Res 2021; 313: 431–437. https://doi.org/10.1007/s00403-020-02116-8
- Ferrucci SM, Tavecchio S, Angileri L, Surace T, Berti E, Buoli M. Factors Associated with Affective Symptoms and Quality of Life in Patients with Atopic Dermatitis. Acta Derm Venereol 2021; 101: adv00590. https://doi.org/10.2340/00015555-3922
- Gundogdu M, Kundakci N. Evaluation of the correlation between scales determining disease severity in patients with moderate-severe chronic plaque-type psoriasis. J Cosmet Dermatol 2021; 20: 2328–2331. https://doi.org/10.1111/jocd.13827
- Kirby JS, Hereford B, Thorlacius L, Villumsen B, Ingram JR, Garg A, et al. Validation of global item for assessing impact on quality of life of patients with hidradenitis suppurativa. Br J Dermatol 2021; 184: 681–687. https://doi.org/10.1111/bjd.19344
- Kurhan F. Social appearance anxiety in patients with contact dermatitis. East J Med 2021; 26: 589–594. https://doi.org/10.5505/ejm.2021.52280
- Morioke S, Takahagi S, Kawano R, Fukunaga A, Harada S, Ohsawa I, et al. A validation study of the Japanese version of the Angioedema Activity Score (AAS) and the Angioedema Quality of Life Questionnaire (AE-QoL). Allergol Int 2021; 70: 471–479. https://doi.org/10.1016/j.alit.2021.04.006
- Segal O, Goldzweig G, Tako E, Barzilai A, Lyakhovitsky A, Baum S. Illness Perception, Perceived Social Support and Quality of Life in Patients with Pemphigus Vulgaris: What Should Dermatologists Know? Acta Derm Venereol 2021; 101. https://doi.org/10.2340/00015555-3785
- Silverberg JI, Lee B, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, et al. Measurement Properties of Patient Health Questionnaire 9 and Patient Health Questionnaire 2 in Adult Patients With Atopic Dermatitis. Dermatitis 2021; 32: 225–231. https://doi.org/10.1097/DER.0000000000000653
- Singh IP, Poonia K, Bajaj K. Quality of life in young adults with acne: Results of a cross-sectional study. J Cosmet Dermatol 2021; 20: 4017–4023. https://doi.org/10.1111/jocd.14540
- Solmaz N, Ilhan N, Bulut HM. The effect of illness perception on life quality in psoriasis patients. Psychol Health Med 2021; 26: 955–967. https://doi.org/10.1080/13548506.2020.1847300
- Talamonti M, Galluzzo M, Silvaggio D, Lombardo P, Tartaglia C, Bianchi L. Quality of Life and Psychological Impact in Patients with Atopic Dermatitis. J Clin Med 2021; 10. https://doi.org/10.3390/jcm10061298
- Zhao H, Zhao N, Zhou BN, Chen G, Wang YR, Zhang HM, et al. Reliability and validity of the Chinese version of the vitiligo specific quality of life instrument (VitiQoL). Dermatol Sin 2021; 39: 13–19. https://doi.org/10.4103/ds.ds_48_20
- Aminizadeh S, Askarizadeh G, Bagheri M. The Persian Version of Skindex-29 Health-related Quality of Life Index: Translation and Psychometric Validation. J Res Health 2022; 12: 279–290. https://doi.org/10.32598/JRH.12.4.2021.1
- Benny D, Makkuni A, Thyvalappil A, Mathew P, Sridharan R, Druhin A. Quality of Life and Psychiatric Comorbidity in Vitiligo: A Hospital-based Cross-sectional Study from A Tertiary Care Center in South India. JDDS 2022; 25: 114–118. https://doi.org/10.4103/jdds.jdds_113_20
- Ito T, Kamei K, Yuasa A, Matsumoto F, Hoshi Y, Okada M, et al. Health-related quality of life in patients with alopecia areata: Results of a Japanese survey with norm-based comparisons. J Dermatol 2022; 49: 584–593. https://doi.org/10.1111/1346-8138.16364
- Koszoru K, Hajdu K, Brodszky V, Szabo A, Borza J, Bodai K, et al. General and Skin-Specific Health-Related Quality of Life in Patients With Atopic Dermatitis Before and During the COVID-19 Pandemic. Dermatitis 2022; 33: S92–S103. https://doi.org/10.1097/DER.0000000000000908
- Nahidi Y, Kiafar B, Sadeghinejad Z, Jarahi L, Mallakifard T. Quality of life of psoriasis patients and their partners in Mashhad, Iran. Iran J Dermatol 2022; 25: 17–23.
- Saeki H, Kanai Y, Murotani K, Ito K, Miyagi T, Takahashi H, et al. Work productivity in real-life employed patients with plaque psoriasis: Results from the ProLOGUE study. J Dermatol 2022; 49: 970–978. https://doi.org/10.1111/1346-8138.16517
- Tee CT, Lee CS, Gunabalasingam P. Characteristics and quality of life in pemphigus patients. Med J Malaysia 2022; 77: 324–330.
- Tuchinda P, Kulthanan K, Chularojanamontri L, Rujitharanawong C, Subchookul C, Trakanwittayarak S. The validity and reliability of the Thai-version of 5-D itch scale. Asian Pac J Allergy Immunol 2022; 40: 254–262.
- Xavier ACM, Prati C, Brandao MG, Ebert AB, Macedo MJD, Fernandes MJB, et al. Comorbidity of psychiatric and dermatologic disorders with skin picking disorder and validation of the Skin Picking Scale Revised for Brazilian Portuguese. Braz J Psychiatry 2022; 44: 621–627. https://doi.org/10.47626/1516-4446-2021-2400
- Yang TT, Lee CH, Lan CCE. Impact of Vitiligo on Life Quality of Patients: Assessment of Currently Available Tools. Int J Environ Res Public Health 2022; 19. https://doi.org/10.3390/ijerph192214943
- Ye Y-M, Koh Y-I, Choi J-H, Kim M-A, Park J-W, Kim T-B, et al. The burden of symptomatic patients with chronic spontaneous urticaria: a real-world study in Korea. Korean J Intern Med 2022; 37: 1050–1060. https://doi.org/10.3904/kjim.2022.078
- Zhao ZT, Zhang C, Jiang Y, Peng C, Zhu W, Zhao S, et al. Chinese version of the chronic urticaria quality of life questionnaire: cultural adaptation, factor analysis, assessment of reliability and validity. Arch Dermatol Res 2022; 314: 847–855. https://doi.org/10.1007/s00403-021-02300-4
- Koszoru K, Hajdu K, Brodszky V, Bato A, Gergely LH, Kovacs A, et al. Comparing the psychometric properties of the EQ-5D-3L and EQ-5D-5L descriptive systems and utilities in atopic dermatitis. Eur J Health Econ 2023; https://doi.org/10.1007/s10198-022-01460-y
- Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J Invest Dermatol 2005; 125: 659–664. https://doi.org/10.1111/j.0022-202X.2005.23621.x
- Tabachnick BG, Fidell LS. Using multivariate statistics (7th ed.). London: Pearson; 2019: Chapter 10.
- Cardiff University. Dermatology Life Quality Index https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/dermatology-life-quality-index. 2023.
- Costello A, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Prac Assess Res Eval 2019; 10: 1–9.
- Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol 2005; 152: 861–867. https://doi.org/10.1111/j.1365-2133.2005.06502.x
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010; 19: 539–549. https://doi.org/10.1007/s11136-010-9606-8
- Gagnier JJ, Lai J, Mokkink LB, Terwee CB. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Qual Life Res 2021; 30: 2197–2218. https://doi.org/10.1007/s11136-021-02822-4
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. FDA Guidance Documents. Silver Spring, Maryland, 2009.
- Baker A, Mitchell EJ, Partlett C, Thomas KS. Evaluating the effect of weekly patient-reported symptom monitoring on trial outcomes: results of the Eczema Monitoring Online randomized controlled trial. Br J Dermatol 2023; 189: 180–187. https://doi.org/10.1093/bjd/ljad163
- Finlay AY. Broader concepts of quality of life measurement, encompassing validation. J Eur Acad Dermatol Venereol 2017; 31: 1254–1259. https://doi.org/10.1111/jdv.14254
- Pattinson RL, Trialonis-Suthakharan N, Gupta S, Henry AL, Lavallee JF, Otten M, et al. Patient-Reported Outcome Measures in Dermatology: A Systematic Review. Acta Derm Venereol 2021; 101: adv00559. https://doi.org/10.2340/00015555-3884
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol 2003; 48: S143–148. https://doi.org/10.1067/mjd.2003.274
- Torres V, Herane MI, Costa A, Martin JP, Troielli P. Refining the ideas of “ethnic” skin. An Bras Dermatol 2017; 92: 221–225. https://doi.org/10.1590/abd1806-4841.20174846
- Taylor SC. Epidemiology of skin diseases in people of color. Cutis 2003; 71: 271–275.
- Nagpal N, Gordon-Elliott J, Lipner S. Comparison of quality of life and illness perception among patients with acne, eczema, and psoriasis. Dermatol Online J 2019; 25. https://doi.org/10.5070/D3255044060
- Ling TC, Ayer J. Photosensitivity. In: Chowdhury MMU, T. G, Finlay AY, editors. Dermatology Training – The Essentials. Chichester: Wiley; 2022: p. 333–334.
- Rencz F, Gulacsi L, Pentek M, Poor AK, Sardy M, Hollo P, et al. Proposal of a new scoring formula for the Dermatology Life Quality Index in psoriasis. Br J Dermatol 2018; 179: 1102–1108. https://doi.org/10.1111/bjd.16927
- Boulard C, Duvert Lehembre S, Picard-Dahan C, Kern JS, Zambruno G, Feliciani C, et al. Calculation of cut-off values based on the Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) and Pemphigus Disease Area Index (PDAI) pemphigus scoring systems for defining moderate, significant and extensive types of pemphigus. Br J Dermatol 2016; 175: 142–149. https://doi.org/10.1111/bjd.14405
- da Silva N, Augustin M, Hilbring C, Braren-von Stulpnagel CC, Sommer R. Psychological (co)morbidity in patients with psoriasis: the impact of pruritus and anogenital involvement on symptoms of depression and anxiety and on body dysmorphic concerns – a cross-sectional study. BMJ Open 2022; 12. https://doi.org/10.1136/bmjopen-2021-055477
- Gabes M, Apfelbacher C. IDQoL, CDLQI and the 45-item CADIS received a sufficient content validity rating during the HOME VII meeting in Japan: a group discussion study. J Eur Acad Dermatol Venereol 2021; 35: 458–463. https://doi.org/10.1111/jdv.16848
- Gergely LH, Gaspar K, Brodszky V, Kinyo A, Szegedi A, Remenyik E, et al. Validity of EQ-5D-5L, Skindex-16, DLQI and DLQI-R in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2020; 34: 2584–2592. https://doi.org/10.1111/jdv.16642
- Kage P, Poblotzki L, Zeynalova S, Zarnowski J, Simon JC, Treudler R. Depression, Anxiety, and Suicidal Ideation in Patients with Atopic Eczema in a Prospective Study in Leipzig, Germany. Int Arch Allergy Immunol 2022; 183: 409–414. https://doi.org/10.1159/000520159
- Lei JY, Pulimood SA, Idris FI. Impact of acne vulgaris on the quality of life among adult acne patients in Brunei Darussalam. Brunei International Medical Journal 2016; 12: 164–170.
- Long SQ, Fang J, Shu HL, Xia DM, Wang ZQ, Mi WY, et al. Correlation of catecholamine content and clinical influencing factors in depression among psoriasis patients: a case-control study. Biopsychosocial Med 2022; 16. https://doi.org/10.1186/s13030-022-00245-2
- Loo WJ, Diba V, Chawla M, Finlay AY. Dermatology Life Quality Index: Influence of an illustrated version. Br J Dermatol 2003; 148: 279–284. https://doi.org/10.1046/j.1365-2133.2003.05158.x
- Martínez-Ortega JM, Nogueras P, Muñoz-Negro JE, Gutiérrez-Rojas L, González-Domenech P, Gurpegui M. Quality of life, anxiety and depressive symptoms in patients with psoriasis: A case-control study. J Psychosom Res 2019; 124. https://doi.org/10.1016/j.jpsychores.2019.109780
- Mazzotti E, Picardi A, Sampogna F, Sera F, Pasquini P, Abeni D, et al. Sensitivity of the Dermatology Life Quality Index to clinical change in patients with psoriasis. Br J Dermatol 2003; 149: 318–322. https://doi.org/10.1046/j.1365-2133.2003.05378.x
- McKenzie SA, Harview CL, Truong AK, Grogan TR, Shi VY, Bennett RG, et al. Physical symptoms and psychosocial problems associated with hidradenitis suppurativa: Correlation with hurley stage. Dermatol Online J 2020; 26. https://doi.org/10.5070/D3269050156
- Park SY, Kim KH. What Factors Influence on Dermatology-Related Life Quality of Psoriasis Patients in South Korea? Int J Environ Res Public Health 2021; 18. https://doi.org/10.3390/ijerph18073624
- Rencz F, Poor AK, Pentek M, Hollo P, Karpati S, Gulacsi L, et al. A detailed analysis of “not relevant’ responses on the DLQI in psoriasis: potential biases in treatment decisions. J Eur Acad Dermatol Venereol 2018; 32: 783–790. https://doi.org/10.1111/jdv.14676
- Safikhani S, Sundaram M, Bao Y, Mulani P, Revicki DA. Qualitative assessment of the content validity of the Dermatology Life Quality Index in patients with moderate to severe psoriasis. J Dermatolog Treat 2013; 24: 50–59. https://doi.org/10.3109/09546634.2011.631980
- Sahin E, Hawro M, Weller K, Sabat R, Philipp S, Kokolakis G, et al. Prevalence and factors associated with sleep disturbance in adult patients with psoriasis. J Eur Acad Dermatol Venereol 2022; 36: 688–697. https://doi.org/10.1111/jdv.17917
- Sojevic Timotijevic Z, Jankovic S, Trajkovic G, Majcan P, Perisic S, Dostanic N, et al. Identification of psoriatic patients at risk of high quality of life impairment. J Dermatol 2013; 40: 797–804. https://doi.org/10.1111/1346-8138.12201
- Solak B, Aydin B, Yuksekal G, Yaldiz M. Restless legs syndrome in patients with psoriasis: association with inflammation and sleep quality. Int J Dermatol 2022; 10.1111/ijd.16532. https://doi.org/10.1111/ijd.16532
- Wallenhammar LM, Nyfjall M, Lindberg M, Meding B. Health-related quality of life and hand eczema – A comparison of two instruments, including factor analysis. J Invest Dermatol 2004; 122: 1381–1389. https://doi.org/10.1111/j.0022-202X.2004.22604.x
- Yang FJ, Zhang Q, Song DY, Liu X, Wang L, Jiang X. A Cross-Sectional Study on the Relationship Between Rosacea Severity and Quality of Life or Psychological State. Clin Cosmet Investig Dermatol 2022; 15: 2807–2816. https://doi.org/10.2147/CCID.S390921
- Yi OS, Huan KY, Har LC, Ali NM, Chiang TW. Genital Psoriasis: A Prospective, Observational, Single-Centre Study on Prevalence, Clinical Features, Risk Factors, and Its Impact on Quality of Life and Sexual Health. Indian J Dermatol 2022; 67: 205. https://doi.org/10.4103/ijd.ijd_754_21
- Nijsten T, Meads DM, de Korte J, Sampogna F, Gelfand JM, Ongenae K, et al. Cross-cultural inequivalence of dermatology-specific health-related quality of life instruments in psoriasis patients. J Invest Dermatol 2007; 127: 2315–2322. https://doi.org/10.1038/sj.jid.5700875
- Schmitt J, Ford DE. Understanding the relationship between objective disease severity, psoriatic symptoms, illness-related stress, health-related quality of life and depressive symptoms in patients with psoriasis – a structural equations modeling approach. Gen Hosp Psychiatry 2007; 29: 134–140. https://doi.org/10.1016/j.genhosppsych.2006.12.004
- Ofenloch RF, Diepgen TL, Weisshaar E, Elsner P, Apfelbacher CJ. Assessing health-related quality of life in hand eczema patients: how to overcome psychometric faults when using the dermatology life quality index. Acta Derm Venereol 2014; 94: 658–662. https://doi.org/10.2340/00015555-1842
- Yale L, Tian L, Jingang A, Weihui Z, Shengxiang X, Liu Y, et al. Rasch analysis holds no brief for the use of the Dermatology Life Quality Index (DLQI) in Chinese neurodermatitis patients. Health Qual Life Outcomes 2016; 14: 1–9. https://doi.org/10.1186/s12955-016-0419-5
- He Z, Lo Martire R, Lu C, Liu H, Ma L, Huang Y, et al. Rasch analysis of the dermatology life quality index reveals limited application to chinese patients with skin disease. Acta Derm Venereol 2018; 98: 59–64. https://doi.org/10.2340/00015555-2742
- Stull DE, Griffiths CEM, Gilloteau I, Zhao Y, Guana A, Finlay AY, et al. Differential effects of secukinumab vs. ustekinumab for treatment of psoriasis on quality of life, work productivity and activity impairment: a structural equation modelling analysis. Br J Dermatol 2018; 178: 1297–1307. https://doi.org/10.1111/bjd.16366
- Jesmin A, Sultana A, Bhuiyan MSI, Yasir Arafat SM. Validation of Bangla dermatology life quality index among patients with psoriasis. J Pak Assoc Dermatol 2021; 31: 165–172.
- Rencz F, Mitev AZ, Szabo A, Beretzky Z, Poor AK, Hollo P, et al. A Rasch model analysis of two interpretations of ‘not relevant’ responses on the Dermatology Life Quality Index (DLQI). Qual Life Res 2021; 30: 2375–2386. https://doi.org/10.1007/s11136-021-02803-7
- Tosun M, Yasak Guner R, Yurtseven ED, Ozpinar S, Akyol M. The Multidimensional Assessment of Interoceptive Awareness-2 Scale: A Turkish Validity and Reliability Study in Patients Admitted to the Dermatology Outpatient Clinic. Turkiye Klinikleri Dermatoloji 2022; 32: 79–88. https://doi.org/10.5336/dermato.2021-87742
- Terwee CB, Bot SDM, de Boer MR, van der Windt DAWM, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60: 34–42. https://doi.org/10.1016/j.jclinepi.2006.03.012
- Prinsen CAP, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials 2016; 17: 449. https://doi.org/10.1186/s13063-016-1555-2