QUIZ SECTION
A Red Domical Nodule on the Nose: A Quiz
Fanglin LU1,2#, Minghui SUN3#, Sha ZHAO2, Feifei CHEN2 and Xiaofang ZHU2*
1Department of Dermatology, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Northern Jiangsu People’s Hospital, Yangzhou, Jiangsu Province, 2Department of Dermatology, Northern Jiangsu People’s Hospital, Yangzhou, and 3Department of Dermatology, Shaoxing Hospital of Traditional Chinese Medicine, Shaoxing TCM Hospital Affiliated to Zhejiang Chinese Medical University, Shaoxing, Zhejiang, China.
*E-mail: sharonzhu66@126.com
#These authors contributed equally to this work.
Citation: Acta Derm Venereol 2024; 104: adv41199. DOI https://doi.org/10.2340/actadv.v104.41199.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Published: September 18, 2024
Competing interests and funding: The authors have no conflicts of interest to declare.
A 60-year-old male patient presented to our department with a 2-month history of nasal rash. The patient had experienced recurrent folliculitis-like papules and papulopustules in the same location for the past 2 years. Initially, the rash subsided after using topical iodophor and mupirocin topical, but it had recurred in the past 2 months. Despite repeating the same above-mentioned treatments, the rash did not subside and instead increased in size over time. The patient was in good overall health and did not exhibit any accompanying systemic symptoms. There was no history of insect bites, prolonged sun exposure, or trauma prior to the lesion. Systematic examination did not reveal any superficial lymph node enlargement or other abnormalities. A red domical nodular lesion, roughly 1.5 cm in diameter, was observed on the nose’s dorsum. The lesion had defined edges, a firm texture, and appeared smooth (Fig. 1A).
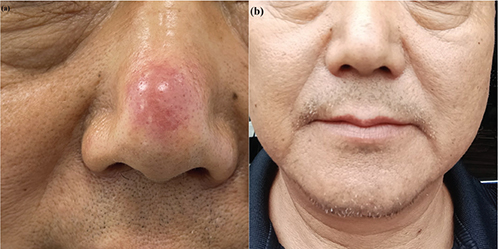
Fig. 1. (A) Clinical picture: red, domical nodule on the nose. (B) Follow-up picture: the rash disappeared. The patient gave consent for his photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Histopathological examination of the biopsy specimen revealed slight atrophy of the epidermis, distorted, hyperplastic hair follicles were observed in the dermis, and dilated, activated pilosebaceous units were surrounded by dense lymphocytes. (Fig. 2A, B). Immunohistochemical staining (Fig. 3A–G).
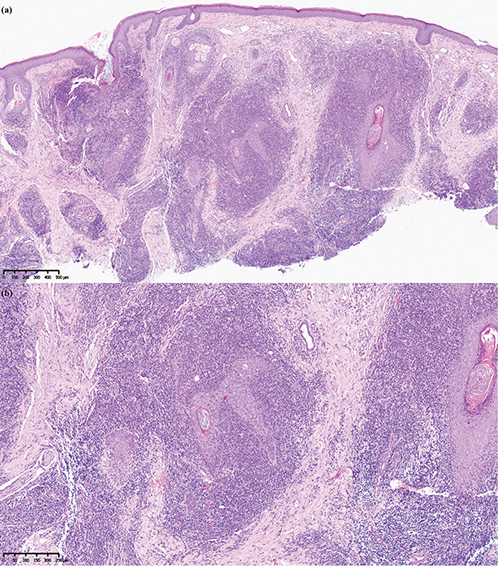
Fig. 2. Haematoxylin-eosin staining. (A) Original magnification x 50. (B) Original magnification x 100. Histopathology: slight atrophy of the epidermis, distorted, hyperplastic hair follicles were observed in the dermis, and dilated, activated pilosebaceous units were surrounded by dense lymphocytes. The majority of infiltrating cells were lymphocytes and some histiocytes, which invaded the hair follicle epithelium and disrupted the structure of the hair follicle. The infiltrate and the epidermis were separated by a Grenz zone, and the infiltrated cells exhibited relatively uniform morphology and size.
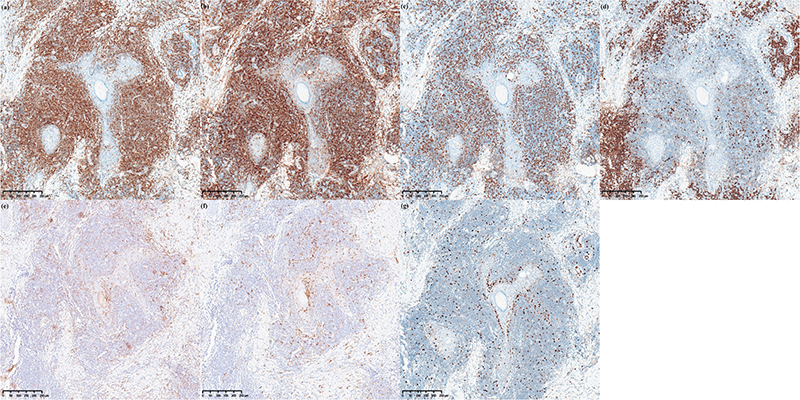
Fig. 3. Immunohistochemical staining. CD3+, CD4+ lymphocytes were diffusely densely distributed, CD20+ cells were distributed in scattered foci in the dermis, CD1a+ and S100+ dendritic cells infiltrated perifollicularly. The infiltrate cells were positive for CD3 (A), CD4 (B), CD8 (C), CD20 (F), CD1a (E), S-100 (F), Ki-67 5%+ (G).
Two months after the rash biopsy, the patient’s skin lesion resolved spontaneously without any additional specific treatment. (Fig. 1B).
What is your diagnosis?
Differential diagnosis 1: Cutaneous marginal zone lymphoma
Differential diagnosis 2: Small and medium-sized pleomorphic cutaneous T-cell lymphoma
Differential diagnosis 3: Follicular mycosis fungoides
Differential diagnosis 4: Other cutaneous pseudolymphomas
See next page for answer.
ANSWERS TO QUIZ
A Red Domical Nodule on the Nose: A Commentary
Diagnosis: Pseudolymphomatous folliculitis
Pseudolymphomatous folliculitis(PLF) is an uncommon skin disorder characterized by lymphoid hyperplasia. It was first clearly identified as a variant of pseudolymphoma 1986 (1). PLF affects individuals of all ages and does not show a clear gender predisposition (2). Typically, the lesions are located on the face, particularly on the nose, cheeks, and forehead. Rashes on the scalp and trunk are infrequently observed. The common clinical manifestation of PLF is the presence of red or purplish domical nodules (3–6). While most cases are asymptomatic, some patients may experience mild itchiness or pain (2). Single lesions are common, while multiple lesions are infrequent (7, 8).
The aetiology of PLF is unknown. The dense distribution of CD1a/S100+ perifollicular dendritic cells has been recognized as an essential characteristic of PLF (3, 9). As is known, dendritic cells, which are specialized antigen-presenting cells in the body, have the ability to efficiently take up and process foreign antigens and subsequently deliver them to T lymphocytes. The significantly increased number of CD1a+ dendritic cells in all follicular segments of PLF suggests that there may be underlying antigenic stimulus residues in the follicle that cause an increase in dendritic cells and thus recruitment of T cells. PLF may be a result of a delayed reaction to antigens situated in the epithelium or follicular lumen, which is supported by the spontaneous regression of PLF after biopsy, as the so-called implied antigens are removed (1, 3). However, the reasons for the antigenic challenge have not been explored and the exact pathogenesis of this disease remains obscure. In our case, the patient had a 2-year history of recurrent localized folliculitis, and this exogenous stimulus may have been involved in the development of PLF (10).
The clinical characteristics of pseudolymphomatous folliculitis are atypical, and the diagnosis relies on histopathological and immunohistochemical analysis.
The pathological feature of pseudolymphomatous folliculitis is nodular dense or diffuse infiltration of lymphocytes extending from the dermis to the subdermis. The infiltrate is separated from the epidermis by the Grenz zone (2, 5). Distorted and hyperplastic hair follicles are seen in the dermis, surrounded by dilated and activated pilosebaceous units encircled by dense lymphocytes, leading to deformation of the follicle wall. Follicular invasion can be classified into 2 types: the first where hair follicles are destroyed and eventually disappear, and the other where the hair follicles are not damaged but become activated, resulting in irregular enlargement of the follicle wall (2, 4, 7, 11, 12). In our case, we observed distorted and hyperplastic hair follicles surrounded by lymphocyte infiltration with some cells invading the hair follicle epithelium, causing damage to the follicle wall. Immunohistochemistry revealed a mixture of CD3, CD4 positive T lymphocytes and CD20 positive B lymphocytes (2, 3, 5). Many dendritic cells with positive expression of CD1a protein and S100 protein were also seen in and around the pilosebaceous units. This is a distinctive histological feature of PLF, but not a diagnostic sign of cutaneous lymphomas (11).
The rarity and atypical clinical manifestations of PLF warrant consideration of multiple differential diagnoses including Rosacea, follicular lymphomatoid papulosis, and lymphoma (13). Rosacea shares similarities with PLF in the presence of surrounding pilosebaceous units accompanied by lymphocytic infiltration. However, distinguishing features include the absence of significant CD1a/S100+ dendritic cell proliferation in Rosacea, by which it can be distinguished from PLF (4,13). Follicular lymphomatoid papulosis, on the other hand, is characterized by immunohistochemical features such as proliferation of CD3+, CD4+, and CD30+ lymphocytes, with CD8 typically being negative. This feature makes possible differentiation from PLF (13, 14).
PLF is a challenging condition to diagnose due to the presence of atypical lymphocytes that can mimic primary cutaneous malignant lymphomas. These include cutaneous marginal zone lymphoma, small- and medium-sized pleomorphic cutaneous T-cell lymphoma, follicular mycosis fungoides, and other cutaneous pseudolymphomas. However, PLF can be distinguished from other types of cutaneous lymphoma by its distinct histopathological and immunohistochemical features.
In most malignant lymphomas, except for follicular mycosis fungoides, the pilosebaceous units are usually typically destroyed or absent. Marginal zone lymphoma can be separated from PLF by the diffuse proliferation of marginal zone cells, often forming sheets or zones of monotonous plasma cells, along with the existence of reactive lymphoid follicles (9, 10, 13). Additionally, features such as facial predisposition and solitary lesions aid in distinguishing PLF from pleomorphic small-/medium-sized T-cell lymphoma (3). The lack of a heterogeneous epidermal infiltrate of atypical cells is also in PLF a distinguishing factor between PLF and follicular mycosis fungoides (6, 12, 13).
The primary treatment for PLF is skin biopsy. In many cases of isolated lesions, the rash has been observed to resolve spontaneously after biopsy (6). Corticosteroid injections are recommended if the rash is incompletely excised (12). Additionally, antimalarial drugs, methotrexate, cyclosporine, triamcinolone, and tacrolimus may be considered as therapeutic options for residual lesions after biopsy or multiple lesions (3, 5, 7, 12, 13).
Although there have been no reports of PLF progressing to a malignant lesion, there is a possibility of spontaneous recurrence. Therefore, some authors recommend a follow-up period of 6 months after biopsy and to monitor the recovery of the rash (15).
In this particular case, the residual rash had resolved on its own without any other further treatment, 2 months after biopsy. We conducted a 6-month follow-up, and observed no recurrence of the lesion.
REFERENCES
- McNutt N. Cutaneous lymphohistiocytic infiltrates simulating malignant lymphoma. In: Murphy G, Mihm M, editors. Lymphoproliferative disorders of the skin. Boston, MA: Butterworths, 1986: p. 256–285.
- Kazakov DV, Belousova IE, Kacerovska D, Sima R, Vanecek T, Vazmitel M, et al. Hyperplasia of hair follicles and other adnexal structures in cutaneous lymphoproliferative disorders: a study of 53 cases, including so-called pseudolymphomatous folliculitis and overt lymphomas. Am J Surg Pathol 2008; 32: 1468–1478. https://doi.org/10.1097/PAS.0b013e31817bdcfb
- Arai E, Okubo H, Tsuchida T, Kitamura K, Katayama I. Pseudolymphomatous folliculitis: a clinicopathologic study of 15 cases of cutaneous pseudolymphoma with follicular invasion. Am J Surg Pathol 1999; 23: 1313–1319. https://doi.org/10.1097/00000478-199911000-00001
- Kwon EJ, Kristjansson AK, Meyerson HJ, Fedele GM, Tung RC, Sellheyer K, et al. A case of recurrent pseudolymphomatous folliculitis: a mimic of cutaneous lymphoma. J Am Acad Dermatol 2009; 60: 994–1000. https://doi.org/10.1016/j.jaad.2008.10.010
- Wu RW, Li MH, Chang CH. Pseudolymphomatous folliculitis presenting as an eruptive nodule over the mandibular area. J Cutan Pathol 2022; 49: 327–330. https://doi.org/10.1111/cup.13951
- Kakizaki A, Fujimura T, Numata I, Hashimoto A, Aiba S. Pseudolymphomatous folliculitis on the nose. Case Rep Dermatol 2012; 4: 27–30. https://doi.org/10.1159/000336207
- Gutte RM. Pseudolymphomatous folliculitis: a distinctive cutaneous lymphoid hyperplasia. Indian J Dermatol 2013; 58: 278–280. https://doi.org/10.4103/0019-5154.113937
- Shah SD, Nikam BP, Ankad BS, Anusha HL. Pseudolymphomatous folliculitis after hair transplantation: dermoscopic view of a rare entity. Int J Dermatol 2023; 62: e81–e83. https://doi.org/10.1111/ijd.16252
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol 2005; 36: 505–511. https://doi.org/10.1016/j.humpath.2005.02.012
- Shojiguchi N, Arai E, Anan T, Ansai SI, Tsuchida T, Yasuda M. Distribution of CD1a-positive cells is not different between pseudolymphomatous folliculitis and primary cutaneous marginal zone lymphoma. J Dermatol 2021; 48: 464–469. https://doi.org/10.1111/1346-8138.15731
- Horikiri M, Abe N, Ueda K. Multiple nodules on the left cheek represented pseudolymphomatous folliculitis. Clin Case Rep 2016; 4: 568–571. https://doi.org/10.1002/ccr3.571
- Lee HW, Ahn SJ, Lee MW, Choi JH, Moon KC, Koh JK. A case of pseudolymphomatous folliculitis. J Eur Acad Dermatol Venereol 2006; 20: 230–232. https://doi.org/10.1111/j.1468-3083.2006.01392.x
- Mendoza Ramírez JB, Ayala D, Heald A, Moreno GYC. Differential diagnoses of pseudolymphomatous folliculitis: considerations as regards one case. BMJ Case Rep 2021; 14: e238291. https://doi.org/10.1136/bcr-2020-238291
- Roque Quintana B, Peñate Y, Montenegro Dámaso T. Pseudolymphomatous folliculitis. Dermatol Online J 2020; 26: 13030/qt85t3f8nw. https://doi.org/10.5070/D3265048782
- Sanchis-Sánchez C, Santos-Alarcón S, Benavente-Villegas FC, Mateu-Puchades A, Soriano-Sarrió MP. Red nodule on the face with “spontaneous” regression. An Bras Dermatol 2017; 92: 135–137. https://doi.org/10.1590/abd1806-4841.20175540