Sensitive skin is a prevalent syndrome, characterized by discomfort in response to mild stimuli, which impacts on quality of life. Pruritus is one of the major symptoms of sensitive skin. However, the pathomechanism of sensitive skin is insufficiently understood. As an experimental model for pruritus, the cowhage skin prick test might provide insight into the understanding of sensitive skin. This study aimed to specify the characteristics of cowhage-induced pruritus in sensitive skin. Female volunteers, 20 with sensitive skin and 20 controls, were recruited. Self-report questionnaires were distributed and the responses evaluated; moreover, alongside assessments by dermatologists, skin physiology assessments, lactic acid sting test, capsaicin test and cowhage skin challenge were performed. Pruritus in sensitive skin was perceived as more intense and longer-lasting than in normal skin, with different qualities of accompanying sensations. Cowhage skin challenge results showed moderate consistency with clinical assessments. The results suggest that cowhage skin challenge could be a new tool for the assessment of sensitive skin.
Key words: sensitive skin; pruritus; cowhage.
Accepted Nov 1, 2021; Epub ahead of print Nov 1, 2021
Acta Derm Venereol 2021; 101: adv00587.
doi: 10.2340/actadv.v101.413
Corr: Wei Hua, Department of Dermatology, West China Hospital, Sichuan University, 610041 Chengdu, China. E-mail: weihua1007@outlook.com
SIGNIFICANCE
Pruritus is one of the major symptoms of sensitive skin. By comparing 40 volunteers, 20 with and 20 without sensitive skin, this study showed that pruritus induced by cowhage is perceived as more intense and longer-lasting in sensitive skin, with different qualities of accompanying sensations. Cowhage skin challenge results showed moderate consistency with clinical assessment results. The results of this study suggest that cowhage skin challenge could be used as a new tool for the assessment of sensitive skin.
INTRODUCTION
Defined by the special interest group (SIG) on sensitive skin of the International Forum for the Study of Itch (IFSI), sensitive skin is characterized as “a syndrome defined by the occurrence of unpleasant sensations (stinging, burning, pain, pruritus and tingling sensations) in response to stimuli that normally should not provoke such sensations” (1). These unpleasant sensations cannot be explained by lesions attributable to any skin disease. The skin can appear normal or be accompanied by erythema. Sensitive skin can affect all body locations, especially the face (2, 3). The self-declared prevalence of sensitive skin affects approximately 60–70% of women and 50–60% of men (4). The non-uniform diagnostic standard might account for the variation in the incidence among different studies (2, 5–8). The pathophysiology of sensitive skin remains insufficiently understood. Impaired epidermal barrier integrity, neurosensory hyperactivity, vascular hyper-reactivity and psychophysiological factors, such as stress, have been proposed to be involved (9). Furthermore, transcriptomics indicated significant difference in skin samples between sensitive skin and normal skin (10, 11). Metabolic homeostasis dysfunction, and pain-related transcripts, such as TRPV1, ASIC3 and CGRP, were significantly upregulated in sensitive skin (10). Downregulation or upregulation of 20 long non-coding RNAs were addressed in sensitive skin, few of which were known (11).
To measure the symptoms of sensitive skin, questionnaires are a useful assessment tool (7). Currently, self-assessment questionnaires are used as the most common test to examine the prevalence and symptoms of sensitive skin. However, the results of the questionnaire can be affected by subjective factors (5). Apart from subjective assessment, a number of physical tests could help with the clinical diagnosis of sensitive skin; among these, sting tests, including the lactic acid sting test (LAST) and capsaicin test (CT), are used most widely (12). However, the reliability of these tests is unclear.
Pruritus, as one of the symptoms of sensitive skin, may provide a better understanding of the pathomechanism of hypersensitivity of sensitive skin. As previously identified, the activation of peripheral sensory nerve fibres (C and Aδ) might be involved in sensitive skin (13). One of the well-established experimental models of pruritus is skin provocation tests with cowhage (Mucuna pruriens). Pruritus can be generated by cowhage spicules containing the protease mucunain (14) that also activates the C and Aδ fibres (15). The intensity of cowhage-induced itch might therefore correlate with the neurosensitivity of the skin. Thus, cowhage skin challenge could be employed as a new evaluation method for sensitive skin.
This study assessed pruritus in sensitive skin induced by cowhage. Intensity and time course of pruritus, and the associated sensory qualities were compared between sensitive skin and healthy controls. Diagnostic methods for sensitive skin were tested and evaluated in comparison with cowhage skin challenge.
MATERIALS AND METHODS
Study population
Twenty Chinese female volunteers with sensitive skin (mean ± standard deviation (SD) age 30.4 ± 6.9 years) and 20 healthy female volunteers with resistant skin (28.75 ± 8.2 years) were recruited. Specific occupations, such as outdoor workers, were excluded. None of the 2 groups had facial inflammatory dermatosis that might affect the test results. Volunteers were also required not to take any medication that could influence their perception of itch (such as antihistamines or antidepressants) for 1 week prior to and during the study.
Participants were required to complete self-assessment questionnaires and were evaluated by 3 dermatologists. A clinical examination was performed to confirm the absence of any inflammatory skin diseases (e.g. acne, seborrhoeic dermatitis, rosacea) or any visible skin lesions in the test area on the subjects’ faces. The questionnaire was adapted from Baumann skin type system (16), which was assumed to be more applicable to Chinese people (17) (see Appendix S1). Scores from the questionnaire (summed as a total score) were categorized as follows: scores ≥ 18 were considered as sensitive skin and ≤ 17 as healthy resistant skin. Participants with total scores ≥ 18 were then examined by dermatologists; the definition of sensitive skin proposed by the IFSI was explained (1); those who met the diagnostic criteria were considered to have sensitive skin, while the others were classified as non-sensitive and were placed in the healthy control group.
Ethics and registration
The trial was performed from February to March 2021, according to the principles of the Declaration of Helsinki and was approved by the ethics committee of West China Hospital, Sichuan University (2021–200). The volunteers provided written informed consent prior to the start of the study. This study has been registered on the Chinese Clinical Trial Registry (www.chictr.org.cn); identifier ChiCTR2100043580.
Procedure and timeline
Volunteers were asked to wash their faces with clean water 30 min before measurements and tests, and to rest for at least 1 h until they reported no perceptible sensations, such as itching or stinging, before each test (for a timeline of the tests, see Fig. S1). The study was performed in a temperature-controlled room at 21 ± 1°C and relative humidity 45 ± 5% for 30 min, and the measurements were performed under the same conditions.
Tests and measurements
The most commonly used tests for sensitive skin were conducted, including skin physiological measurements, lactic acid sting test and capsaicin test, in comparison with cowhage skin challenge. Test sites are marked respectively in Fig. S2.
Skin physiological measurements. The baseline skin physiological parameters of each participant were measured. Skin erythema intensity was measured using a Mexameter (MX 18; Courage and Khazaka Electronic GmbH, Kӧln, Germany; values given in arbitrary units). Mexameter is a reflectance spectroscopy-based device measuring melanin content and erythema level of the skin, the latter indirectly reflecting the haemoglobin content of the skin and thus its blood perfusion. Transepidermal water loss (TEWL) was recorded with the open chamber system Tewameter (TM 300; Courage and Khazaka Electronic GmbH). TEWL is expressed in g/m2/h, and reflects the density gradient of water evaporation from the skin and its increase through water loss due to damage to the barrier function. Corneometer (CM 825; Courage and Khazaka Electronic GmbH) was used to quantify skin hydration through measurement of the electrical capacitance of the skin (in arbitrary units). All 3 devices were attached to a central unit (MPA 9) and subsequently to a PC (Windows). Published guidelines were followed (18–20). All devices went through regular calibration before the tests.
Lactic acid sting test. A 10% aqueous lactic acid (Sigma Chemical, St Louis, MO, USA) solution was applied to the nasolabial fold, followed by evaluation of the intensity of the subjective symptoms. Lactic acid solution was applied randomly to one side of the nasolabial fold, and an equal volume of saline was applied to the contralateral site. The participants were asked to grade the intensity of stinging, using a 4-point scale (where 0 = no stinging; 1 = slight stinging; 2 = moderate stinging; and 3 = strong stinging) at 0 s, 2.5 min and 5 min after application of lactic acid solution. Cumulative scores ≥ 3 at 2.5 and 5 min were considered as positive for sensitive skin.
Capsaicin test. A solution of 0.001% capsaicin (w/v) was prepared from pure-grade capsaicin powder (8-methyl-N-vanillyl-6-nonenamide ≥ 98%, Solarbio Co, Beijing, China) in 10% ethanol solution. The vehicle was a 10% ethanol/90% water (v/v) solution prepared using distilled water and absolute ethanol. The capsaicin solution (50 µl) was randomly applied through filter paper (0.8 cm diameters) on 1 side of the face 1 cm from the nasolabial fold and the vehicle was applied on the other side. All subjects were asked to report any abnormal sensation and assess its severity on the following 6-point scale: 0 = none; 1 = doubtful, barely perceptible; 2 = slightly perceptible; 3 = moderately perceptible; 4 = strongly perceptible; 5 = painful. Scores of burning sensation ≥ 3 that were persistent for more than 30 s were considered as positive.
Skin challenge by cowhage and itch measurement. Pruritus was induced at sites 1 cm outside the corner of the mouth on the surface of the face. Considering the hyper-reactivity of sensitive skin on the face, previously described protocols were modulated (21). Only approximately 20 cowhage spicules (derived from the Department of Gardening and Horticulture, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Xishuangbanna, Yunnan Province, China), which is less than half of the normal dose used to induce pruritus on the forearm of healthy subjects, were applied with a gloved finger on a 2-cm2 area on the face. Spicules were then gently rubbed on the skin for 20 s with a circular motion. Inactivated spicules were autoclaved for 1 h according to Reddy et al. (22) as semi-face control. Pruritus assessment started immediately after the challenge and was recorded every minute for the following 30 min. A 100-mm visual analogue scale (pruritus-VAS, ranging from 0 “no pruritus at all” to 100 “worst pruritus imaginable”) was used to record pruritus intensity on both sides of the face. The spicules were completely removed after 30 min using adhesive tape. Subjects were asked to rate the type and overall intensities of pruritus qualities following cowhage provocation. The different items chosen to rate pruritus quality were adapted from the Eppendorf Itch Questionnaire (23). Itching sensation scores ≥ 8 (VAS) that persisted for more than 3 min were considered positive.
Statistical analyses
Mean values from both sides of the face were used for statistical analysis. Normality of distribution of variables was tested with the Kolmogorov–Smirnov test. Differences between 2 groups of parametric variables were tested with the 2-sample t-test and analysis of variance for repeated measurements. Multiple comparisons within the time curve of the cowhage-induced itch were made using Fisher’s least significant difference test.
Correlation was assessed with the Pearson correlation test for parametric variables and with the Spearman correlation test for non-parametric variables. Consistency in each test with clinical assessment results was evaluated with Cohen’s kappa coefficient. Quantitative data are presented as mean ± standard deviation (SD) unless otherwise indicated. Statistical significance was set at p < 0.05.
RESULTS
Cowhage-induced pruritus in sensitive skin
During the cowhage skin challenge, 15 participants in the sensitive skin group and 6 in the healthy control group reported itching of varying intensities (Table I). Cowhage spicules induced markedly more intense pruritus in the sensitive skin group compared with the healthy control group (Fig. 1). At the time-point of 2 min after itch provocation, VAS difference between the 2 groups showed the most significance. Higher peak scores of visual analogue scale (p < 0.001, Table I) and longer duration of pruritus (p < 0.01, Table I) were observed in the sensitive skin group. Furthermore, cowhage-provoked pruritus in the sensitive skin group contained more pricking and warm sensations, which showed a distinct pattern of wavelike feelings, with a significantly stronger urge to scratch (Fig. 2). The VAS score of the sensitive skin group showed significant difference on the side of natural spicules compared with inactivated spicules as semi-face control (p < 0.05, Table I), while no difference was detected in the healthy control group (p = 0.10, Table I). Age did not impact the maximum pruritus intensities provoked by cowhage in either the sensitive skin group or the healthy control group.



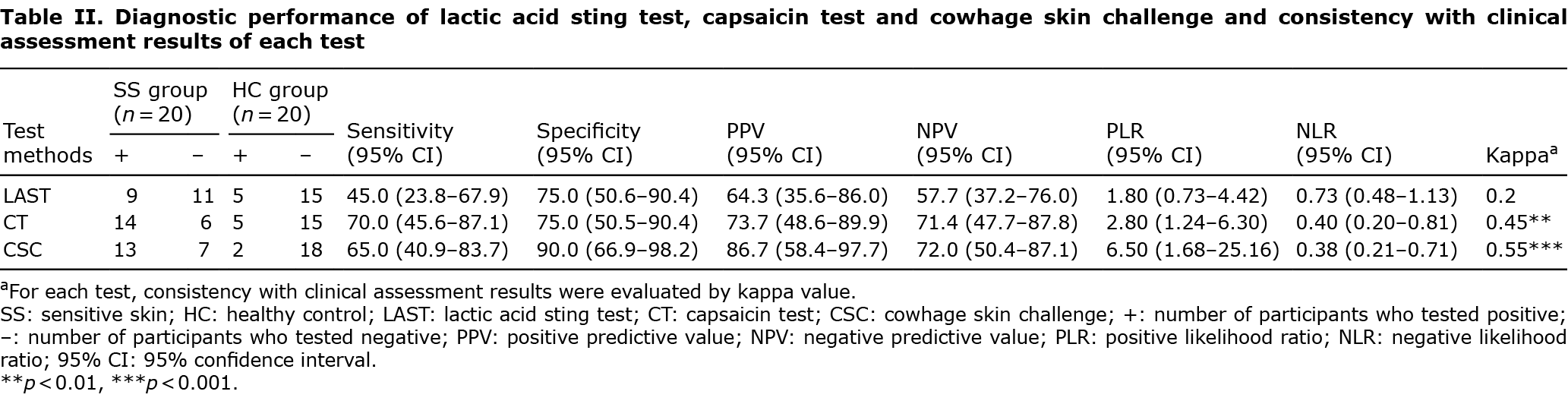
Consistency in clinical assessment results with lactic acid sting test, capsaicin test and cowhage skin challenge
Classification of sensitive skin was made by dermatologists through clinical assessment, after clinical evaluation and investigation by the sensitive skin questionnaire for the participants. Results of cowhage skin challenge showed moderate consistency with clinical assessment (Table II). By comparison, the results of the capsaicin test revealed similar consistency, while the results of the lactic acid sting test indicated poor consistency with clinical assessment results (Table II). The mean scores of questionnaires in the sensitive skin group were significantly higher than those in the healthy control group (22.1 ± 2.2 vs 14.6 ± 2.0, p < 0.001). Intensity of pruritus induced by cowhage in sensitive skin showed no significant correlation with questionnaire scores (r = 0.30, p = 0.20).
Skin physiology parameters
Facial erythema assessed by Mexameter was slightly increased in the sensitive skin group, but showed no statistically significant difference compared with the healthy control group (310.21 ± 85.60 vs 275.75 ± 54.30, p = 0.137). Skin barrier function, as measured with a Tewameter showed no significant difference between the sensitive skin and healthy control groups (16.38 ± 4.60 vs 16.99 ± 4.37 g/h/m2, p = 0.670). Skin hydration, as assessed with the Corneometer, indicated no significant difference between the 2 groups (55.67 ± 10.99 vs 58.06 ± 12.38 arbitrary unit, p = 0.523). Skin barrier integrity, as assessed by TEWL, showed no correlation with intensity of pruritus, either in the SS group (r = 0.22, p = 0.36) or in the healthy control group (r = 0.32, p = 0.17).
DISCUSSION
This study found that cowhage-induced pruritus was more intense and sensorially different in sensitive skin, including more pricking, wavelike, warmer sensation and a greater urge to scratch, compared with in healthy resistant skin.
Sensitive skin is not an immunological disorder, but is related to alterations in the skin nervous system (24). Biopsies of sensitive skin have shown significant reduction in intra-epidermal nerve fibre density and alteration in C fibres, in contrast with those of healthy subjects (25). Since pruritus is mediated by unmyelinated C-fibres in the epidermis (26, 27), and the activation of mechano-sensitive C-fibres by cowhage has been observed (28), it is likely that a neurosensory dysfunction in the skin represents one of the pathological mechanisms of more intense pruritus in sensitive skin when triggered by cowhage. Along with itchy sensation, the specific quality of pruritus was also investigated, as cowhage spicules were previously indicated to provoke sensations in healthy volunteers, such as burning, pricking, tingling, warmth with wavelike patterns, and urge to scratch (29). In the current study, a greater intensity of these specific sensations in the sensitive skin group coincided with the complicated symptoms of sensitive skin in response to mild irritations.
In sensitive skin, sensory proteins, such as TRPV1 and ASIC3, have been reported to be overexpressed on the surface of keratinocytes and intra-epidermal nerve endings (30). Multiple environmental factors can trigger proteins, such as TRPV1, which can be activated by capsaicin or heat (31). However, other mechanisms remain unexplored in the complicated syndrome of sensitive skin. Mucunain, the cysteine protease that is contained in the spicules of cowhage, induces pruritus through protease-activated receptors (PAR)-2, PAR-4 and Mas-related G-protein coupled receptors (Mrgprs) expressed on keratinocytes and dermal neurones (21, 32). Cowhage spicules resulted in more enhanced pruritus in the sensitive skin group, even at a very low dose. This indicates that the activation of PAR and Mrgprs may play a part in the itching sensation of sensitive skin, and warrants further studies.
The results of the current study indicate that there is no correlation between age and maximum itch intensity in sensitive skin. Kwa et al. (33) and André et al. (34) found no difference in age or sexes on itch intensity score. Bahali et al. (35) reported no association between age and pruritus among patients with psoriasis, but a more prominent pruritus in females. Male subjects were not included in the current study, since it was considered that the presence of facial hair bristles, which share a similar appearance to cowhage spicules, might interfere with the test results, and because female participants were much easier to enrol in the study due to the higher prevalence of skin sensitivity in females and a higher tendency for females to pay closer attention to their skin condition. Thus, sex bias could have interfered with the test scores. The effect of sex on intensity of pruritus in sensitive skin requires further study.
The sensitive scale (36) and its cut-off scores (37) have been used for measuring the severity of sensitive skin. A modified questionnaire (17) was used, which is more applicable to Chinese people with sensitive skin. In the current study, less than half of the sensitive skin group and one-quarter of the healthy control group tested positive for LAST. Marriott et al. (38) suggested that the nasolabial sting test was a poor predictor of general skin sensitivity. They tested 4 substances in order to induce different sensory effects: lactic acid (stinging), capsaicin (burning), menthol (cooling) and ethanol (a mixture of burning and stinging), on 58 individuals. A high level of variation in reactivity to the stimuli was observed, and, in general, an elevated response to one substance was not predictive of increased reactivity to another stimulus. Consistent with Jourdain et al. (39), the capsaicin test in the current study is more strongly linked to self-declared sensitive skin than is the lactic acid sting test. Capsaicin is a natural agonist of TRPV1 expressed in skin tissue, including keratinocytes and peripheral sensory nerve fibres (C and Aδ) (40), and the capsaicin test is usually used to identify the neurosensitive subtype of sensitive skin. Among the 3 physical tests in the current study, the results of cowhage skin challenge showed the highest consistency with clinical assessment results. Inactivated spicules were designed as semi-face control to exclude the needle-like effects of cowhage spicules. The significant difference in VAS between the 2 sides of the face in the sensitive skin group indicated the role of mucunain in pruritus induction in this group.
Increased TWEL were observed in a few studies in sensitive skin, but the overall results remain controversial (41). Meanwhile, the application of lactic acid or capsaicin did not enhance differences in erythema responses (41). Skin barrier abnormalities or vascular response did not explain the distinct results of the 3 physical tests in the current study, since no significant differences were detected in physiological parameters between the 2 groups.
A few factors may have contributed to the lower intensity of reported itch compared with previous studies (21, 29, 34). Firstly, itch induction usually takes place in the forearm according to the commonly used protocol (21). Since the face is the most frequent site of sensitive skin (2), and also to better compare with the other standard tests for sensitive skin, the face was chosen as the test site. Different perception threshold of nerves and other factors, such as abundant sebum in the face, may account for the lower intensity of itch. In our preliminary experiments (with approximately 20 cowhage spicules), cowhage induced a higher intensity of itch in the forearm than in the face in the healthy control group (14.28 ± 21.10 vs 2.33 ± 5.17, p = 0.009; n = 18; 13 of 18 subjects reported itch in the forearm, while only 6 of 18 reported itch in the face; data not show); while there was no significant difference between the 2 sites in the sensitive skin group (18.67 ± 23.10 vs18.33 ± 24.10, p = 0.676, n = 15; 10 and 9 out of 15 subjects reported itch in the face and the forearm, respectively). Moreover, according to Maki Ozawa et al. (42), electronic-evoked perception threshold differs in terms of body sites and nerves, respectively. The required current intensity of neuroselective transcutaneous electrical stimulator to evoke perception in the face was stronger than in the forearm. Thus, we assume the neuronal sensitivity of the forearm is higher than that of the face. Secondly, during the experiment, although 20 spicules were applied by gently rubbing in a circular motion, no more than 7 spicules pierced the skin. This was confirmed by re-checking the application sites using a magnifying glass. The remainder of the spicules remained on the surface. Perhaps further improvement in the application method is required, such as making use of the “spicule inserter” according to LaMotte et al. (15), to ensure that more spicules are placed correctly. Lastly, an aim of the current study was to distinguish sensitive skin from the population. Considering that “minor” stimulation can cause significant irritation, which is the main feature of sensitive skin, the dosage of cowhage application was cut down in half, so as to result in “sub-induction” rather than “induction” of itch for normal skin. Meanwhile, the intensity and duration of pruritus provoked by cowhage varied highly between individuals. Seven participants in the sensitive skin group did not perceive remarkable itching (VAS < 8, or duration < 3 min), 4 of whom were also negative for the capsaicin test. These participants may be classified to the non-neurosensitive subtype or itching-resistant subtype of sensitive skin (12).
Since a limited number of volunteers were included in this study, a larger sample is required to verify the use of cowhage skin challenge for sensitive skin assessment in the future. To set up a standard method, further investigation is needed to address the itch-inducing capacity of cowhage plants and its application to different human anatomical sites.
In conclusion, cowhage induced a more intense, longer duration of pruritus and different accompanying sensations in the sensitive skin group in this study compared with healthy controls. To the best of our knowledge, there is no final consensus regarding standard methods for diagnosis of sensitive skin. Cowhage skin challenge could be used as a new test method for assessment of sensitive skin, and is highly recommended to be combined with clinical assessment and other physical tests. The pathophysiological mechanism of pruritus in sensitive skin is unknown, and cowhage could serve as a suitable experimental model in future research.
ACKNOWLEDGEMENTS
This work was supported by the project of Science and Technology Department of Sichuan Province (2021YFS0199). The authors thank the Department of Gardening and Horticulture, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, who generously provided us with cowhage.
The authors have no conflicts of interest to declare.
REFERENCES
- Misery L, Ständer S, Szepietowski JC, Reich A, Wallengren J, Evers AWM, et al. Definition of sensitive skin: an expert position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch. Acta Derm Venereol 2017; 97: 4–6.
- Farage MA. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clin Exp Dermatol 2009; 34: e521–e530.
- Saint-Martory C, Roguedas-Contios AM, Sibaud V, Degouy A, Schmitt AM, Misery L. Sensitive skin is not limited to the face. Br J Dermatol 2008; 158: 130–133.
- Farage MA. The prevalence of sensitive skin. Front Med (Lausanne) 2019; 6: 98.
- Guinot C, Malvy D, Mauger E, Ezzedine K, Latreille J, Ambroisine L, et al. Self-reported skin sensitivity in a general adult population in France: data of the SU.VI.MAX cohort. J Eur Acad Dermatol Venereol 2006; 20: 380–390.
- Misery L, Myon E, Martin N, Verrière F, Nocera T, Taieb C, et al. Sensitive skin in France: an epidemiological approach. Ann Dermatol Venereol 2005; 132: 425–429.
- Willis CM, Shaw S, De Lacharrière O, Baverel M, Reiche L, Jourdain R, et al. Sensitive skin: an epidemiological study. Br J Dermatol 2001; 145: 258–263.
- Sparavigna A, Di Pietro A, Setaro M. ‘Healthy skin’: significance and results of an Italian study on healthy population with particular regard to ‘sensitive’ skin. Int J Cosmetic Sci 2005; 27: 327–331.
- Do LHD, Azizi N, Maibach H. Sensitive skin syndrome: an update. Am J Clin Dermatol 2020; 21: 401–409.
- Kim EJ, Lee DH, Kim YK, Kim M, Kim JY, Lee MJ, et al. Decreased ATP synthesis and lower pH may lead to abnormal muscle contraction and skin sensitivity in human skin. J Dermatol Sci 2014; 76: 214–221.
- Yang L, Lyu L, Wu W, Lei D, Tu Y, Xu D, et al. Genome-wide identification of long non-coding RNA and mRNA profiling using RNA sequencing in subjects with sensitive skin. Oncotarget 2017; 8: 114894–114910.
- Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmetic Sci 2013; 35: 2–8.
- Kueper T, Krohn M, Haustedt LO, Hatt H, Schmaus G, Vielhaber G. Inhibition of TRPV1 for the treatment of sensitive skin. Exp Dermatol 2010; 19: 980–986.
- Shelley WB, Arthur RP. Mucunain, the active pruritogenic proteinase of cowhage. Science 1955; 122: 469–470.
- LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol 2009; 101: 1430–1443.
- Baumann L. The skin type solution: a revolutionary guide to your best skin ever. New York: Bantam Books 2006.
- Luan M, Dai R, Fan L, Hua W, Xie H, Li L. Developing a new sensitive skin questionnaire and compare reliability, validity of new Questionnaire and Baumann Sensitive Skin Questionnaire. Ch J Dermatol 2018; 32: 80–83.
- Fullerton A, Fischer T, Lahti A, Wilhelm KP, Takiwaki H, Serup J. Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dermatitis 1996; 35: 1–10.
- Berardesca E, Loden M, Serup J, Masson P, Rodrigues LM. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res Technol 2018; 24: 351–358.
- Rogiers V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol 2001; 14: 117–128.
- Papoiu ADP, Tey HL, Coghill RC, Wang H, Yosipovitch G. Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLoS One 2011; 6: e17786.
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 2008; 28: 4331–4335.
- Darsow U, Scharein E, Simon D, Walter G, Bromm B, Ring J. New aspects of itch pathophysiology: component analysis of atopic itch using the ‘Eppendorf Itch Questionnaire’. Int Arch Allergy Imm 2001; 124: 326–331.
- Misery L, Weisshaar E, Brenaut E, Evers AWM, Huet F, Ständer S, et al. Pathophysiology and management of sensitive skin: position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J Eur Acad Dermatol Venereol 2020; 34: 222–229.
- Buhé V, Vié K, Guéré C, Natalizio A, Lhéritier C, Le Gall-Ianotto C, et al. Pathophysiological study of sensitive skin. Acta Derm Venereol 2016; 96: 314–318.
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci 2006; 7: 535–547.
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci 1997; 17: 8003–8008.
- Wei JY, Tuckett RP. Response of cat ventrolateral spinal axons to an itch-producing stimulus (cowhage). Somatosens Mot Res 1991; 8: 227–239.
- Hawro T, Lehmann S, Deuring E, Weller K, Altrichter S, Church MK, et al. Comparison of pruritus and sensory qualities induced by capsaicin, histamine and cowhage. J Eur Acad Dermatol Venereol 2019; 33: 1755–1761.
- Kim EJ, Lee DH, Kim YK, Eun HC, Chung JH. Adiponectin deficiency contributes to sensitivity in human skin. J Invest Dermatol 2015; 135: 2331–2334.
- Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel) 2016; 9: 77.
- Reddy VB, Azimi E, Chu L, Lerner EA. Mas-related G-protein coupled receptors and cowhage-induced itch. J Invest Dermatol 2018; 138: 461–464.
- Kwa KAA, Pijpe A, Rashaan ZM, Tuinebreijer WE, Breederveld RS, van Loey NE. Course and predictors of pruritus following burns: a multilevel analysis. Acta Derm Venereol 2018; 98: 636–640.
- André F, Fluhr JW, Hawro T, Church MK, Maurer M, Metz M. Characterization of cowhage-induced pruritus in inflamed and non-inflamed skin. J Eur Acad Dermatol Venereol 2020; 34: 202–206.
- Bahali AG, Onsun N, Su O, Ozkaya DB, Dizman D, Topukcu B, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol 2017; 92: 470–473.
- Misery L, Jean-Decoster C, Mery S, Georgescu V, Sibaud V. A new ten-item questionnaire for assessing sensitive skin: the Sensitive Scale-10. Acta Derm Venereol 2014; 94: 635–639.
- Legeas C, Misery L, Fluhr JW, Roudot A, Ficheux A, Brenaut E. Proposal for cut-off scores for sensitive skin on sensitive scale-10 in a group of adult women. Acta Derm Venereol 2021; 101: adv00373.
- Marriott M, Holmes J, Peters L, Cooper K, Rowson M, Basketter DA. The complex problem of sensitive skin. Contact Dermatitis 2005; 53: 93–99.
- Jourdain R, Bastien P, de Lacharrière O, Rubinstenn G. Detection thresholds of capsaicin: a new test to assess facial skin neurosensitivity. J Cosmet Sci 2005; 56: 153–166.
- Gibson RA, Robertson J, Mistry H, McCallum S, Fernando D, Wyres M, et al. A randomised trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. Plos One 2014; 9: e100610.
- Richters R, Falcone D, Uzunbajakava N, Verkruysse W, van Erp P, van de Kerkhof P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Phys 2015; 28: 75–83.
- Ozawa M, Tsuchiyama K, Gomi R, Kurosaki F, Kawamoto Y, Aiba S. Neuroselective transcutaneous electric stimulation reveals body area-specific differences in itch perception. J Am Acad Dermatol 2006; 55: 996–1002.