REVIEW ARTICLE
Safety of Dupilumab Therapy for Atopic Dermatitis during Pregnancy: A Systematic Review and Meta-analysis
Verónica SÁNCHEZ-GARCÍA1 , Eva DE-MIGUEL-BALSA2
, Eva DE-MIGUEL-BALSA2 , José-Manuel RAMOS-RINCÓN2
, José-Manuel RAMOS-RINCÓN2 and Isabel BELINCHÓN-ROMERO1,2,
and Isabel BELINCHÓN-ROMERO1,2,
1Dermatology Department, Dr Balmis General University Hospital, Alicante Institute for Health and Biomedical Research (ISABIAL), Alicante, and 2Department of Clinical Medicine, Miguel Hernández University, Alicante, Spain
Atopic dermatitis (AD) is the most common skin condition among pregnant women. However, there is limited information on the safety of biologicals during pregnancy. A systematic review and meta-analysis was conducted following the PRISMA guidelines to evaluate the effects of exposure to biologicals during pregnancy and/or preconception in women with AD, and to estimate the pooled prevalence of spontaneous abortions and congenital malformations in their newborns. MEDLINE, Embase, Scopus, and Web of Science to 31 May 2024 were searched to identify randomized controlled trials and non-randomized studies. To test the robustness of our findings, sensitivity analyses were performed. Fifteen observational studies involving 115 pregnant women with a mean age of 33.46 years (standard deviation [SD] 3.02 were included). All studies evaluated dupilumab. The mean duration of exposure to dupilumab during pregnancy was 27.52 weeks (SD 11.16). The weighted prevalence of spontaneous abortions was 18.9% (95% confidence interval 5.3 to 38.2). There were no reports of congenital malformations. The sensitivity analyses showed no significant differences in weighted prevalences. In conclusion, the current scientific evidence suggests that dupilumab is probably safe during pregnancy and preconception in women with AD, with no significant increase in the risk of miscarriage or congenital malformations compared to the general population. However, the results of this review are inconclusive due to the limited number of large, well-designed clinical studies.
SIGNIFICANCE
This study was undertaken to evaluate whether use of biological therapy for atopic dermatitis in pregnant women increases the risk of spontaneous abortion and congenital malformations. The studies included in the meta-analysis do not show that exposure to dupilumab for atopic dermatitis during pregnancy and/or preconception increases the risk of spontaneous abortion and congenital malformations, so may pose an acceptable risk for pregnant women and their foetuses/newborns. These findings may facilitate clinical decision-making for women with a good therapeutic response to dupilumab who become pregnant or are planning to conceive, or for pregnant women who require biological therapy to control atopic dermatitis.
Key words: atopic dermatitis; biological therapy; pregnancy; spontaneous abortion; congenital abnormalities.
Citation: Acta Derm Venereol 2025; 105: adv41307. DOI: https://doi.org/10.2340/actadv.v105.41307.
Copyright: © 2025 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Aug 8, 2024; Accepted after revision: Jan 16, 2025; Published: Feb 12, 2025.
Corr: José-Manuel Ramos-Rincón, MD, PhD, Department of Clinical Medicine, Miguel Hernández University, C/ Crta. Nacional, 332, ES-03550 Alicante, Spain. Email: jose.ramosr@umh.es
Competing interests and funding: The authors have no conflicts of interest to declare.
This research received external funding from the X Call for Aid for the Support and Promotion of Research by the Alicante Health Research Institute (ISABIAL).
INTRODUCTION
A topic dermatitis (AD) is the most common skin condition among pregnant women. It accounts for around 50% of all dermatoses of pregnancy and usually develops in the second or third trimester (1, 2). During pregnancy, immune system deviation towards T helper 2 (Th2) response helps ensure tolerance of the foetus and reduces the risk of spontaneous abortion (3, 4). Unfortunately, as AD is a Th2-driven disease, women with AD are at increased risk of experiencing flares during pregnancy (3). Untreated AD can lead to serious complications for the mother and foetus, including eczema herpeticum, premature rupture of membranes (PROM), and neonatal staphylococcal septicaemia, in addition to reduced quality of life and increased anxiety for the mother (5, 6).
To treat moderate-to-severe AD during pregnancy, the European Task Force on Atopic Dermatitis recommends systematic corticosteroids, cyclosporine, and azathioprine (4, 7–9). Currently, 2 biological drugs are approved for moderate-to-severe AD: dupilumab (approved in 2017), a monoclonal antibody that blocks the IL-4 and IL-13 signalling pathways; and tralokinumab (approved in 2021), an anti-IL-13 antibody. However, current guidelines advise against biological therapy during pregnancy owing to a lack of clinical data on the potential risks (4, 7–10). Most current evidence on the safety of biologicals during pregnancy comes from studies of women with inflammatory bowel disease (IBD) and rheumatic diseases.
In view of the high prevalence of AD in women of childbearing age, it is crucial to determine the safety of biological therapy during pregnancy and breastfeeding to optimize maternal and neonatal outcomes (11). We carried out a systematic review and meta-analysis to evaluate the current evidence on the impact of exposure to biologicals in women with AD during pregnancy and preconception.
MATERIALS AND METHODS
Study design
We conducted a systematic review and prevalence meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (12, 13). Our protocol was prospectively registered in the international prospective register of systematic reviews (PROSPERO; CRD42023457685).
Inclusion criteria
We selected studies that were published or pending publication; written in English, Spanish, or Italian; and that met the following inclusion criteria (PICOS):
- Population: women with a diagnosis of AD who were pregnant or planning to conceive.
- Intervention: exposure to biological drugs approved for the treatment of AD in the 3 months before pregnancy (preconception period) or during pregnancy (any trimester).
- Comparison: not applicable.
- Outcomes: pregnancy, foetal and neonatal outcomes.
- Study type: randomized controlled trials and non-randomized studies (cohort studies, case-control studies, case series, clinical case studies, and patient registries).
Our exclusion criteria were as follows:
- Design: reviews (narrative or systematic) and animal studies.
- Population: atopic diseases or immune-mediated inflammatory skin diseases other than AD.
- Outcomes: articles with incomplete data on foetal impact.
- Intervention: exposure to biological during the postpartum period only, or paternal exposure to biological.
If we found more than 1 article reporting results of the same population, we included the most recent article. We applied no restrictions related to sample size or publication date.
Sources of information and search strategy
We searched the databases PubMed, Embase, Scopus, and Web of Science to 31 May 2024. The search strategy was based on a combination of Medical Subject Headings (MeSH) terms extracted from our research question. Table SI shows the search strategy for each database. Two authors (VSG and IBR) performed the searches independently and archived the references using the reference management software Mendeley (Elsevier). We reviewed conference abstracts and presentations and manually checked the reference lists of all selected articles and relevant systematic reviews to identify additional citations.
Study selection
After removing duplicates, 2 review authors (VSG and IBR) independently screened the title and abstract of each reference and excluded those that were clearly irrelevant. They then retrieved the full text of all potentially relevant records and assessed them against our eligibility criteria. We resolved any disagreements by consensus.
Variables and data extraction
The first author (VSG) extracted data from the included studies to an Excel spreadsheet (Microsoft Corp, Redmond, WA, USA). The senior author (IBR) then revised all extracted data. We contacted study authors to request further information or clarifications where necessary. Table SII lists the variables collected from each study.
Risk of bias assessment
Two review authors (VSG and IBR) assessed the methodological quality of the included studies independently and in duplicate using the Cochrane tool Risk of Bias in Non-randomized Studies - of Interventions (ROBINS-I; Table SIII) (14). We resolved any disagreements by consensus.
Data analysis and synthesis methods
To analyse the extracted data, we used SPSS version 25.0 (IBM Corp, Armonk, NY, USA). We presented the results as absolute and relative frequencies in tables and graphs. For quantitative data, we calculated means with standard deviations (SDs) or medians with interquartile ranges (IQRs). The choice of measure depended on normality of distribution, which we tested using the Kolmogorov–Smirnov test (where p-values below 0.05 represented non-normal distribution). We also provided a narrative synthesis of the individual outcomes in the Results section and Table I.
| 1st author, year | Study design | Pregnancies and women exposed/mean age | Dosage, frequencya | Exposure interval or time of last dose of biological therapy | Classic systemic treatments for AD before or during pregnancy | Pregnancy, foetal, or neonatal outcomes | Live births (%); abortions (%) |
| EMA 2017 (17) | PV database from 1 phase 2b and 3 phase 3 studies | 23 pregnancies in 23 women (1 twin pregnancy)/NR | 300 mg every 2 weeks | NR | NR | Live births (n = 8) Twin pregnancy (n = 1) Elective abortion (n = 2) Spontaneous abortion (n = 6)b Ongoing pregnancy (n = 5) Participants lost to follow-up (n = 3) |
8/16 (50); 8/16 (50) |
| Mian 2020 (18) | Case report | 1 pregnancy in 1 woman/28 years | 300 mg every 2 weeks | From week 24 to week 37 of pregnancy and during breastfeeding. The woman decided to stop breastfeeding shortly after the birth | Before pregnancy: SCS, phototherapy During pregnancy: SCS |
Gestational hypertension Gestational diabetes AD exacerbation during pregnancy SGA LBW Live term birth Postpartum AD flare |
1 (100); 0 (0) |
| Kage 2020 (19) | Case report | 1 pregnancy in 1 woman/35 years | 300 mg every 2 weeks | During preconception until week 2 of pregnancy, from week 20 to week 40 of pregnancy, and during breastfeeding | Before pregnancy: SCS, phototherapy, CsA | AD exacerbation during preg-nancy (due to discontinuation of dupilumab) Live term birth without complications |
1 (100); 0 (0) |
| Kage 2021 (20) | Case report | 1 pregnancy in 1 woman/36 years | 300 mg every 2 weeks | During preconception, whole pregnancy, and breastfeeding | Before pregnancy: SCS, phototherapy, CsA | Live term birth without complications | 1 (100); 0 (0) |
| Lobo 2021 (21) | Case report | 1 pregnancy in 1 woman/36 years | 300 mg every 3 weeks (dose reduction due to ocular AEs) | During preconception until 24 weeks of pregnancy The woman decided not to breastfeed while receiving dupilumab |
Before pregnancy: SCS, UVB phototherapy, MTX, CsA During pregnancy: oral antibiotics and UVB phototherapy |
Gestational diabetes AD exacerbation during pregnancy (due to discontinuation of dupilumab) Live term birth without complications |
1 (100); 0 (0) |
| Gracia-Darder 2021 (22) | Case report | 1 pregnancy in 1 woman/28 years | 300 mg every 2 weeks | During preconception and throughout pregnancy The woman decided not to breastfeed while receiving dupilumab |
Before pregnancy: CsA, SCS, IVIg, oral antibiotics During pregnancy: IVIg every 14 days |
Live birth without complications | 1 (100); 0 (0) |
| Costley 2021 (23) | Case report | 1 pregnancy in 1 woman/NR | 300 mg every 2 weeks | During preconception, throughout pregnancy, and during breastfeeding | Before pregnancy: CsA, phototherapy | Live term birth without complications | 1 (100); 0 (0) |
| Khamisy-Farah 2021 (24) | Global PV database. (VigiBaseTM) | 36 pregnancies, puerperium and perinatal adverse drug reactions/NR | 300 mg every 2 weeks | NR | NR | Unspecified abortion (n = 2) Spontaneous abortion (n = 21) Ectopic pregnancy (n = 1) Heterotopic pregnancy (n = 1) Pre-eclampsia (n = 1) PROM (n = 1) Neonatal jaundice (n = 1) Other adverse drug reactions during pregnancy, puerperium, and perinatal period (n = 8)c |
— |
| Akhtar 2022 (25) | Case report | 1 pregnancy in 1 woman/33 years | 300 mg every 2 weeks | From 12 weeks before pregnancy to week 36 of pregnancy, with temporary discontinuation between week 27 and week 29 Self-discontinuation of dupilumab at 36 weeks and during the postpartum period |
Before pregnancy: SCS, CsA, MTX, phototherapy, and antibiotics against staphylococcal infection | AD exacerbation during pregnancy (due to discontinuation of dupilumab between week 27 and 29) IUGR LBW Emergency Caesarean delivery (breech position) Live term birth |
1 (100); 0 (0) |
| Kojanova 2022 (26) | Multicentric database (BIOREP registry) | 4 pregnancies in 4 women/NR | 300 mg every 2 weeks | Discontinuation (n = 4) | NR | Ruptured ectopic pregnancy (n = 1) Spontaneous abortion (n = 1) |
2 (50); 2 (50) |
| Escolà 2023 (11) | Multicentre case series | 11 pregnancies (1 twin pregnancy) in 11 women/34 years 2 neonates exposed only during breastfeeding in 2 women (excluded) |
300 mg every 2 weeks | Mean time of exposure to dupilumab during pregnancy was 6.8 (SD 2.9) months Conception (n = 9) 1st trimester (n = 2) 1st and 2nd trimester (n = 1) 2nd and 3rd trimester (n = 1) 1st, 2nd, and 3rd trimester (n = 2) Throughout pregnancy (n = 5) During breastfeeding (n = 7) |
Before pregnancy: CsA (n = 10), MTX (n = 1), AZA (n = 1), phototherapy (n = 2), SCS (n = 11), IVIg (n = 2), MMF (n = 1), apremilast (n = 1), ustekinumab (n = 1) | LBW (n = 2)d Elective Caesarean delivery (n = 2)d Premature (n = 2)d Twin pregnancy (n = 1)d Instrumental delivery (n = 1) Term births, without complications during delivery and with newborns of normal weight (n = 10) |
12 (100); 0 (0) |
| Alvarenga 2023 (30) | Case report | 1 pregnancy in 1 woman/37 years | 300 mg every 2 weeks | During preconception, throughout pregnancy, and during breastfeeding | Before pregnancy: SCS, CsA, phototherapy | Live term birth without complications | 1 (100); 0 (0) |
| Avallone 2024 (27) | Multicentre retrospective cohort study | 28 pregnancies in 28 women/32.4 years (range 19–45) | 300 mg every 2 weeks | Median time of exposure to the drug during pregnancy was 6 weeks (range 2–24) All the documented pregnancies were unplanned, and the drug was discontinued in all cases once pregnancy status was reported |
Before pregnancy: CsA (n = 27) During pregnancy: prednisone (n = 2) |
Gestational diabetes (n = 1) AD recurrence (n = 13) Poor control of AD (n = 1) Postpartum haemorrhage (n = 1) Oligohydramnios (n = 2) Miscarriage (n = 5) Prematurity (n = 7) Respiratory distress (n = 1) Pulmonary hypertension (n = 1) Postpartum depression (n = 1) Idiopathic anaphylaxis (n = 1) AD in offspring (n = 2) Solitary cutaneous mastocytoma in offspring (n = 1) |
23 (82.1); 5 (17.9) |
| Hong 2024 (28) | Case series | 4 pregnancies in 4 women/34 years (range: 29–39) | 300 mg every 2 weeks | Preconception (n = 4) 2nd trimester (n = 3) 3rd trimester (n = 1) |
Before pregnancy: CsA (n = 3), SCS (n = 3), MTX (n = 1) | Mild aggravation of AD symptoms (n = 1) Facial erythema (n = 1) Live term births without complications |
4 (100); 0 (0) |
| Di Lernia 2024 (29) | Case report | 1 pregnancy in 1 woman/35 years | 300 mg every 2 weeks | During preconception, pregnancy (discontinued for 2 weeks), and breastfeeding | Before pregnancy: SCS, CsA | Mild aggravation of AD symptoms (due to discontinuation of dupilumab) | 1 (100); 0 (0) |
| aThe biological drug was dupilumab in all studies. bOf the 6 women with spontaneous abortion, 2 had 1 or more factors known to increase the risk of spontaneous abortion (elevated parathyroid hormone, clotting disorders, and a history of infertility). cIncludes exposure to the drug during pregnancy. dOne was a twin pregnancy that required a Caesarean delivery, with 2 premature babies (week 35) with low weight (1.5 kg and 2.0 kg) but with adequate development and weight gain. AD: atopic dermatitis; AE: adverse effect; AZA: azathioprine; CsA: cyclosporine A; IVIg: intravenous immunoglobulin; IUGR: intrauterine growth restriction; LBW: low birthweight; MMF: mycophenolate mofetil; MTX: methotrexate; NR: not reported; PROM: premature rupture of membranes; PV: pharmacovigilance; SCS: systemic corticosteroids; SD: standard deviation; SGA: small for gestational age; UVB: ultraviolet B. |
|||||||
To combine data on the number of live births, spontaneous abortions, and newborns with congenital malformations relative to the total number of pregnancies exposed to biological therapy, we performed a prevalence meta-analysis using the statistical software StatsDirect v. 3.3.5 (Merseyside, UK; https://www.statsdirect.co.uk/) and applying the Stuart-Ord method (inverse double arcsine square root). We pooled the results of studies that included more than 1 pregnant woman, excluding isolated clinical case reports. We then performed a sensitivity analysis including all studies, to check whether this modified the pooled result (15, 16).
We used forest plots to display the individual and pooled prevalences (with 95% confidence intervals [CIs]).
To evaluate statistical heterogeneity between the studies, we used the I2 statistic and Q test. We considered I2 values above 50% representative of heterogeneity. Because we expected to find substantial heterogeneity, we used the random-effects model for all meta-analyses.
To explore the possibility of publication bias/small study effects, we created funnel plots and performed Egger’s regression test, considering a p-value below 0.10 indicative of statistically significant publication bias.
RESULTS
Results of the search
Our search strategies returned 1,541 references. After the screening process, we included 15 eligible publications in our systematic review (Fig. 1) (11, 17–30).
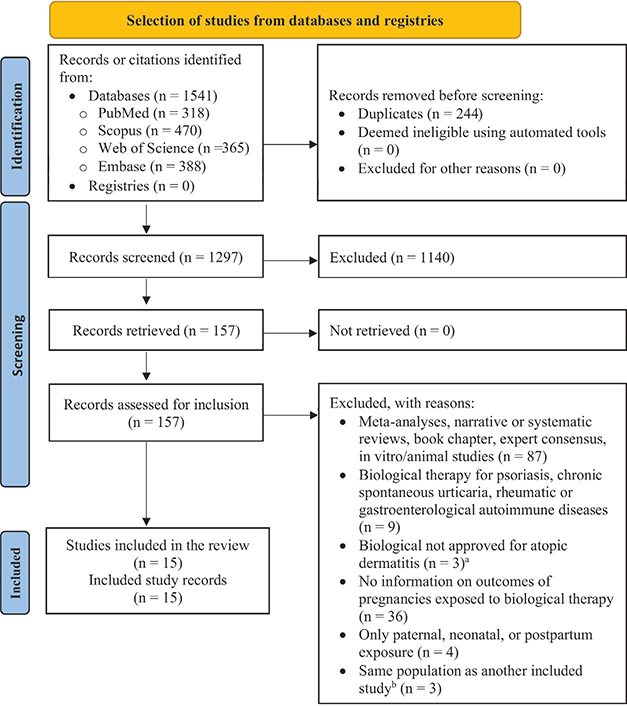
Fig. 1. PRISMA flowchart illustrating the study selection process (12). aOmalizumab (n = 2) (71,72) and rituximab (n = 1) (73). bIn these situations, the most recent article was included in the review.
Characteristics of the included studies
Table SIV presents a descriptive analysis of the characteristics of the included studies. The biological evaluated in all studies was dupilumab. We found no studies on exposure to tralokinumab.
The overall methodological quality of the studies was low (Table SIII).
Table I presents the participant characteristics, intervals of exposure to biological therapy, concomitant systemic treatments used before and/or during pregnancy, and pregnancy outcomes reported in each included study.
Clinical characteristics of participants
Table II presents the characteristics of the study participants.
| Maternal clinical characteristics | n (%)a |
| Number of pregnant women | 115 (100) |
| Mean age in years (SD) (n = 51) | 33.46 (3.02) |
| Age range | 19–45 |
| Age at AD diagnosis, mean (SD) (n = 18) | 1.63 (1.77) |
| Comorbidities | |
| Asthma (n = 18) | 14 (77.8) |
| Rhinitis (n = 18) | 14 (77.8) |
| Conjunctivitis (n = 18) | 11 (61.1) |
| Food allergy (n = 18) | 4 (22.2) |
| Obesity (n = 45) | 6 (13.3) |
| Mental health disorders (n = 18)b | 2 (11.1) |
| Gastrointestinal diseases (n = 18)b | 2 (11.1) |
| Smoking (past or current) (n = 39) | 3 (7.7) |
| Cancer (n = 18)b | 1 (5.6) |
| Endocrine diseases (n = 46)b | 2 (4.4) |
| Allergic contact dermatitis (n = 18) | 0 (0) |
| Hives (n = 18) | 0 (0) |
| Hypertension (n = 18) | 0 (0) |
| Diabetes mellitus (n = 18) | 0 (0) |
| Dyslipidaemia (n = 18) | 0 (0) |
| Liver disease (n = 18) | 0 (0) |
| Cardiovascular diseases (v = 18) | 0 (0) |
| Kidney disease (n = 18) | 0 (0) |
| Neurological diseases (n = 18) | 0 (0) |
| Others (n = 51)b | 14 (27.5) |
| Previous abortions (n = 42) | 2 (4.8) |
| Age at initiation of dupilumab, mean (SD) (n = 8) | 32.4 (3.54) |
| Severity score before initiation of dupilumab, mean (SD) | |
| EASI (n = 17) | 36.3 (18.72) |
| SCORAD (n = 4) | 55 (4.89) |
| IGA (n = 4) | 4 (0) |
| BSA (n = 3) | 61 (34.22) |
| DLQI (n = 2) | 24 (5.66) |
| Systemic treatments prior to pregnancy | |
| Cyclosporine (n = 52) | 48 (92.3) |
| Systemic corticosteroids (n = 24) | 22 (91.7) |
| Phototherapy (n = 24) | 9 (37.5) |
| Methotrexate (n = 24) | 4 (16.7) |
| Intravenous immunoglobulin (n = 24) | 3 (12.5) |
| Azathioprine (n = 24) | 1 (4.2) |
| Mycophenolate mofetil (n = 24) | 1 (4.2) |
| Apremilast (n = 24) | 1 (4.2) |
| Ustekinumab (n = 24) | 1 (4.2) |
| Previous treatments, mean (SD) (n = 24) | 2.77 (0.78) |
| aUnless otherwise specified. bMental health disorders: anxiety (n = 2); gastrointestinal diseases: ulcerative colitis (n = 2); endocrine diseases: hyperparathyroidism (n = 1), thyroid disease (n = 1); cancers: anaplastic lymphoma (n = 1); others: gynaecological diseases (n = 11), autoimmune disease (n = 1), blood clotting disorder (n = 1), and hyperimmunoglobulin E syndrome (n = 1). AD: atopic dermatitis; BSA: body surface area; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; IGA: Investigator Global Assessment; SCORAD: Scoring Atopic Dermatitis; SD: standard deviation. |
|
Characteristics of the pregnancies
There were 115 pregnancies exposed to dupilumab. No studies reported concomitant use of teratogenic treatments during pregnancy.
More than one-third of women with available data (9/24; 37.5%) continued using dupilumab throughout their pregnancy. Biological therapy was discontinued at some point during pregnancy in 78.6% of pregnant women (44/56). The mean duration of exposure to dupilumab during pregnancy in the 52 women with available data was 27.52 (SD 11.16) weeks (Table III).
| Variables | n (%)a |
| Pregnancies exposed to biologicalsb | 115 (100) |
| Biologicals used during pregnancy (n = 81) | |
| Dupilumab | 115 (100) |
| Tralokinumab | 0 (0) |
| Classic concomitant treatments during pregnancy (n = 51) | |
| Systemic corticosteroids (n = 24) | 3 (5.9) |
| Phototherapy | 1 (2) |
| Intravenous immunoglobulin | 1 (2) |
| Cyclosporine | 0 (0) |
| Azathioprine | 0 (0) |
| Exposure 3 months before conception or previously (n = 24) | 21 (87.5) |
| Trimester of exposure (n = 24) | |
| 1st trimester | 2 (8.3) |
| 2nd trimester | 3 (12.5) |
| 3rd trimester | 2 (8.3) |
| 1st and 2nd trimester | 2 (8.3) |
| 2nd and 3rd trimester | 1 (4.2) |
| 1st and 3rd trimester | 0 (0) |
| 1st, 2nd, and 3rd trimester | 5 (20.8) |
| Throughout pregnancy | 9 (37.5) |
| Maintained during breastfeeding (n = 52) | 15 (28.9) |
| Discontinuation of biological therapy (n = 56) | 44 (78.6) |
| Exposure duration during pregnancy in weeks, mean (SD) (n = 52) | 27.52 (11.16) |
| aUnless otherwise specified. bTwin pregnancies were counted as one event. SD: standard deviation. |
|
The main maternal complication was AD exacerbation during pregnancy (36.5%), followed by gestational diabetes (5.8%). The type of delivery was vaginal in 17 of the 20 women with available data (Table IV).
| Variablesa | n (%) |
| Maternal complications during pregnancy and postpartum period | |
| AD flare during pregnancy (n = 52) | 19 (36.5) |
| Gestational diabetes (n = 52) | 3 (5.8) |
| Twin pregnancy (n = 47) | 2 (4.3) |
| Ectopic pregnancy (n = 57) | 2 (3.5) |
| Postpartum AD flare (n = 52) | 1 (1.9) |
| Gestational hypertension (n = 52) | 1 (1.9) |
| Preeclampsia (n = 53) | 1 (1.9) |
| Heterotopic pregnancy (n = 53) | 1 (1.9) |
| Infectious complications (n = 52) | 0 (0) |
| Eclampsia (n = 52) | 0 (0) |
| Type of delivery (n = 20) | |
| Vaginal | 17 (85) |
| Instrumental | 1 (5.9) |
| Elective Caesarean | 2 (10) |
| Emergency Caesarean | 1 (5) |
| aTwin pregnancies were counted as one event. AD: atopic dermatitis. |
|
Pregnancy and live birth outcomes
Table V summarises the results for our primary and secondary outcomes. Fourteen studies (all except Khamisy-Farah et al. [24]) recorded data on spontaneous abortions and live births, but reporting was less consistent across studies for other outcomes, such as small for gestational age (SGA), premature birth, congenital malformations, low birthweight (LBW), neonatal complications, and long-term growth and development. Among the 73 women with known results, there were 58 live births, 13 spontaneous abortions, and 2 elective abortions.
| Outcomesa | No. of participants (studies) | Observations |
| Pregnancy outcomes | ||
| Live births | 58/73 (14 studies) | All articles except one (24) reported spontaneous abortions and live births.b In addition, there were 5 ongoing pregnancies, 3 losses to follow-up, and 2 twin pregnancies |
| Spontaneous abortion | 13/73 (14 studies) | |
| Elective abortion | 2/73 (14 studies) | Only the pharmacovigilance report of the phase 2 and phase 3 clinical trials of dupilumab reported elective abortions (of which there were 2) (17). No articles focused on the frequency of elective abortions in women exposed to biologicals |
| SGA | 4/44 (12 studies) | 2 of the SGA foetuses were from a twin pregnancy |
| Live birth outcomes | ||
| Congenital malformations | 0/58 (14 studies) | — |
| Preterm birth | 9/52 (11 studies) | 2 of the premature newborns were from a twin pregnancy 11 observational studies, involving 42 newborns, reported gestational age at birth (mean 37.4 weeks, SD 2.26) |
| Low birthweight | 4/44 (12 studies) | 2 of the newborns with low birthweight were from a twin pregnancy with preterm delivery 10 observational studies, involving 32 neonates, reported birthweight (mean 2.93 kg, SD 0.56) |
| Neonatal complications | 2/54 (12 studies) | There were 2 reported cases of neonatal complications: 1 case of neonatal jaundice (24) and 1 case of respiratory distress (27) |
| Long-term growth and development | 42 (8 studies) | 8 observational studies, involving 42 neonates, reported long-term outcomes and physiological development of the offspring. The studies described 42 healthy children with completely normal growth. The mean follow-up time was 46.4 week (SD 26.81) |
| aThe newborns from the twin pregnancy were counted as 2 events. bThe study by Khamisy-Farah et al. (24) reported only pregnancy, puerperium, and perinatal adverse drug reactions (n = 36). SD: standard deviation; SGA: small for gestational age. |
||
Weighted prevalence and sensitivity analysis
Table VI shows the main findings of our prevalence meta-analyses and sensitivity analyses for the outcomes of spontaneous abortion, live births, and congenital malformations.
We meta-analysed the results of studies that included more than 1 pregnant woman, excluding isolated clinical case reports (Figs 2 and 3). We were unable to include 1 study in the meta-analyses of spontaneous abortions and live births because it provided no usable data (24). We then performed a sensitivity analysis, including all studies, to check whether this resulted in any substantial change in the weighted prevalences (15, 16).
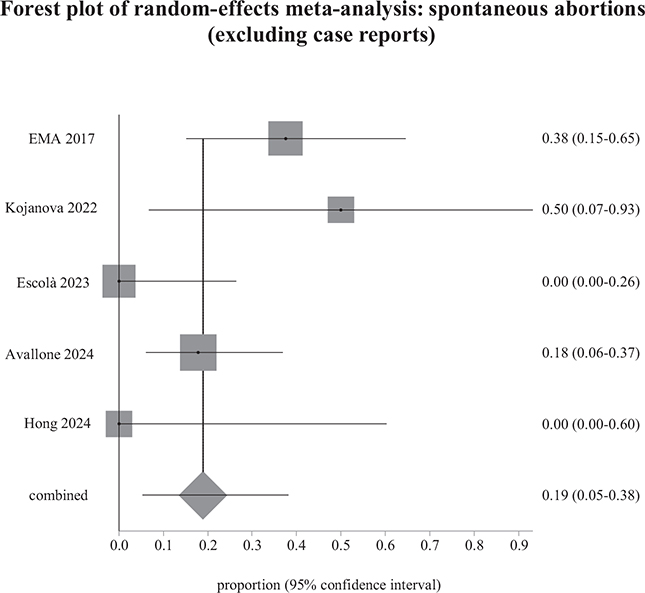
Fig. 2. Weighted prevalence of spontaneous abortions relative to the total number of pregnancies exposed to biological therapy, after excluding case reports. EMA: European Medicines Agency.
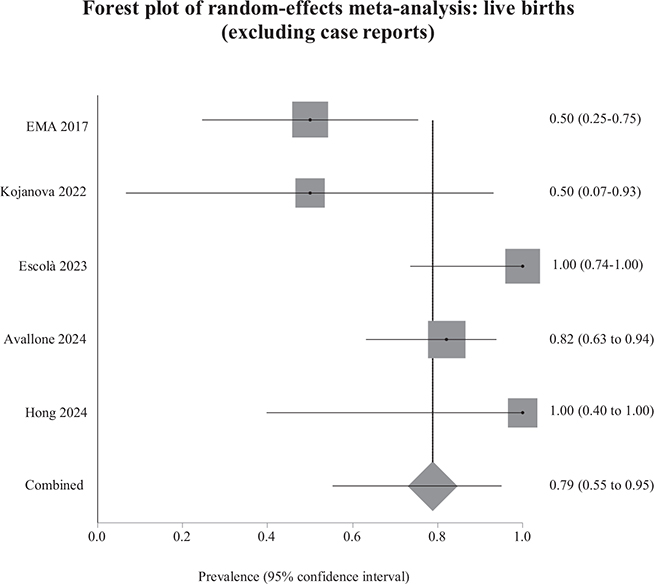
Fig. 3. Weighted prevalence of live births relative to the total number of pregnancies exposed to biological therapy, after excluding case reports. EMA: European Medicines Agency.
The results of the sensitivity analyses were similar to those of the main analyses. The weighted prevalence of spontaneous abortions reduced from 18.9% to 18.3% (Fig. 4), and the weighted prevalence of live births increased from 78.8% to 80.2% (Fig. 5). Between-study heterogeneity was low for both sensitivity analyses. The weighted prevalence of congenital malformations was 0% in the main analysis and the sensitivity analysis. We detected no funnel plot asymmetry (Fig. S1A–B), which we corroborated using Egger’s test (p = 0.83 for spontaneous abortions; p = 0.88 for live births).
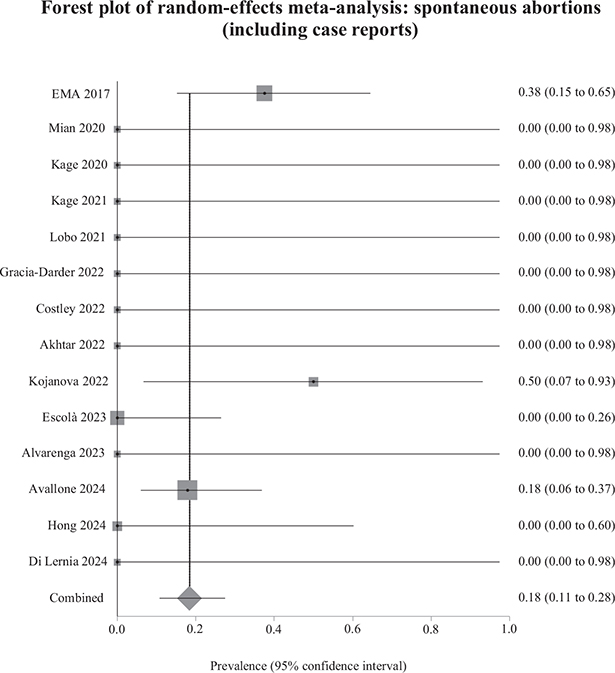
Fig. 4. Weighted prevalence of spontaneous abortions relative to total pregnancies exposed to biological therapy (including case reports). EMA: European Medicines Agency.
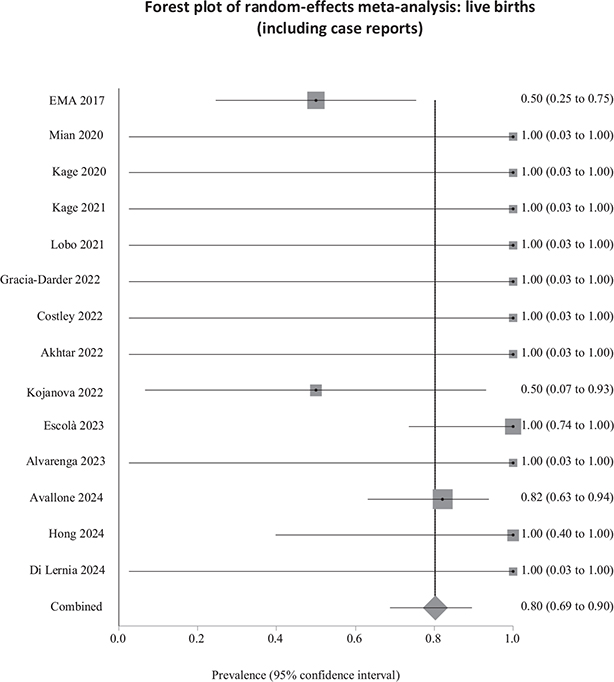
Fig. 5. Weighted prevalence of live births relative to total pregnancies exposed to biological therapy (including case reports). EMA: European Medicines Agency.
DISCUSSION
We performed a systematic review and prevalence meta-analysis to synthesize the available evidence on the impact of biological therapy during pregnancy in women with AD. To the best of our knowledge, ours is the first meta-analysis to estimate the weighted prevalence of spontaneous abortions in women with AD exposed to biological drugs during pregnancy, and the weighted prevalence of congenital malformations in their newborns. Our review included 15 observational studies in 115 women.
Although AD is one of the most common diseases among women of reproductive age, there is a lack of research on the pharmacokinetics and safety of biological therapy during pregnancy (31). Controlled clinical trials of biologicals exclude pregnant women for ethical reasons, and participants must have frequent pregnancy tests after the trial begins (32). For this reason, when women who use biological drugs become pregnant or plan to conceive, their treating physician has little evidence on which to base a clinical decision (32).
This scientific need explains the recent contributions to the literature on this topic: most of the studies included in our meta-analysis (93.3%) were published in the last 5 years in journals of high scientific impact. However, most publications were case reports or case series, with low methodological quality (33). In addition, we found no studies of pregnant women exposed to tralokinumab, which reveals a persisting gap in the current evidence.
In recent years, various international societies and organizations have highlighted the need to include pregnant and breastfeeding women in clinical trials and pharmacovigilance studies (31, 32, 34, 35). In addition, researchers have performed observational studies to help establish practical recommendations for pregnant and breastfeeding women (36). Regarding treatment for AD during pregnancy, there are 2 ongoing observational studies of dupilumab (NCT04173442 (37) and NCT03936335 (38)), both of which are currently recruiting; the estimated completion dates are in 2026 and 2027, respectively (31).
Maternal age and comorbidities are important risk factors for congenital abnormalities and other adverse pregnancy events. The mean age in our review (33.46 years) was similar to that of the Xolair Pregnancy Registry (EXPECT) cohort (31 years) (36, 39). The main comorbidities in our population were other atopic diseases (asthma, conjunctivitis, and food allergies, all of which are commonly associated with AD). These results are comparable to the baseline clinical characteristics of the treatment groups in clinical trials of dupilumab performed in adults and adolescents with AD (40–43). The main non-allergic comorbidities in our population were mental health disorders, in line with previous research. Seeger and colleagues reported a greater consumption of psychotherapeutic drugs in pregnant women with psoriasis and AD than in the control group; this result may reflect an association between dermatitis flares during pregnancy and stress (44).
The women in our review had an average baseline Eczema Area and Severity Index (EASI) score of 36.3 (SD 18.7) and received dupilumab during pregnancy for 27.5 (SD 11.1) weeks. However, 78.6% of women with available data stopped using dupilumab at some point during pregnancy, and 36.5% had AD exacerbations during the pregnancy.
Although AD tends to worsen during pregnancy, 2 recent studies showed that many women discontinue use of topical and systemic medications once they are aware of their pregnancy, and gradually taper off over the 3 trimesters (45,46). Specifically, 1 study showed that the rates of dupilumab reduced from 2.0% before pregnancy to 0.7% during the first trimester and 0.3% during the second and third trimesters (46). This could reflect a tendency for women to endure more AD flares during pregnancy, combined with a more cautious and restrictive treatment approach (45).
During pregnancy, foetal exposure to biological drugs depends on the maternal IgG concentration. At term, foetal IgG can be 20% to 30% higher than maternal levels (47). Because IgG antibodies are large molecules (> 100 kDa), placental IgG transfer depends on the neonatal Fc receptor (FcRn) in the syncytiotrophoblast, and the order of transport efficacy is IgG1, IgG4, IgG3, then IgG2. Both dupilumab and tralokinumab are IgG4 monoclonal antibodies (48). Although IgG4 is the second most transported IgG across the placenta, experts think there is no exposure during early embryogenesis owing to the absence of FcRn in the first trimester, meaning the risk of teratogenicity is low (48). In our review, as most women with available data used dupilumab for some period of time in all 3 trimesters, we consider their outcomes provide relevant safety evidence.
Regarding pregnancy outcomes, our meta-analysis shows that the weighted prevalence of spontaneous abortions and congenital malformations in newborns exposed to dupilumab during pregnancy did not differ from rates estimated in the general population, which are 11–22% for spontaneous abortion, and 2–5.5% for congenital malformations (49–51). In our review, among the 73 pregnancies with known results, the weighted prevalence of spontaneous abortions was 18.9% (18.3% with the case reports). There were no reports of congenital malformations in the live newborns.
The prevalence of spontaneous abortions in our study is very similar to the prevalence reported in women with AD. Seeger and colleagues observed a total prevalence of spontaneous abortions of 20.3% in the group of pregnant women with AD, and 19.3% in the group of pregnant women with psoriasis (44). In addition, they showed that the age-adjusted incidence rate of spontaneous abortions in women with AD were similar to that estimated for the control group and the psoriasis group (44, 52).
Animal studies of dupilumab have indicated no increased risk of malformation (17, 53, 54), and there is no evidence that dupilumab causes other pregnancy complications, such as preterm birth or spontaneous abortion. Nevertheless, dupilumab works by blocking Th2 immune response. Th2 cytokines play a role in the maintenance of pregnancy (3, 55, 56). In this sense, Piccinni and colleagues demonstrated reduced production of IL4 and IL10 by T cell clones generated in the placenta of women who had unexplained recurrent spontaneous abortions, compared with women who had elective abortions (57). Despite this, there is no explanation in the current literature for the improvement in AD after blockade of the Th2 response with dupilumab without an increase in gestational complications. Therefore, we cannot rule out that this contradictory lack of increase in the prevalence of spontaneous abortions in pregnant women exposed to dupilumab in our study may not be due to the fact that the total of 115 cases is too low to make a reliable statement.
Regarding the use of dupilumab for other indications, a European Medicines Agency assessment report on the extension of marketing authorization of dupilumab to treatment of asthma, published in 2019, presented a summary of the pregnancy outcomes of 1 phase 2b study and 2 phase 3 studies, concluding that the rate of spontaneous abortions was no higher than the general rate (53). However, comparing the 2 atopic diseases seems inappropriate, in view of the differences in foetal outcomes (36, 58–62).
Although the evidence is scarce, dupilumab appears to have no impact on human fertility (4). There is more evidence from animal studies, which also suggest no effect on fertility (17, 53, 54). However, in theory, dupilumab could be present in the seminal fluid of men who receive the drug (4). Bosma and colleagues presented a case series of 2 men and 2 women who conceived during or after dupilumab therapy and who experienced no complications related to their ability to conceive, the course of the pregnancy, or foetal outcomes (63). The two women stopped dupilumab before conceiving because of the planned pregnancy; both experienced aggravation of AD symptoms (63). In our study, 21 women were receiving dupilumab in the preconception period, and there were no reported problems with conceiving.
In our review, there were no reported complications in the newborns of women who continued dupilumab therapy during breastfeeding. It is unknown whether dupilumab is excreted in breast milk or enters the newborn’s bloodstream after ingestion (64). Since dupilumab is a large protein molecule, with high molecular weight, the amount present in breast milk is expected to be low, except during the first 3 days after birth, when the large spaces between the alveolar cells in the breast allow the passage of immunoglobulins. Furthermore, systemic absorption is improbable because the molecule is almost certainty destroyed in the infant’s gastrointestinal tract (64–66).
Although no studies included in our review reported developmental abnormalities in the long term (growth, psychomotor development, infectious complications, or risk of atopy), several studies did not record these results or did not specify the follow-up time. The scientific community has yet to uncover the effects of exposure to dupilumab during pregnancy on the newborn’s immune system. In 1 study of women with IBD, intrauterine exposure to tumour necrosis factor (TNF)-alpha showed no negative impact on long-term development of the children, compared with non-exposure in children born to mothers without IBD (67).
We found no published clinical data on the use of tralokinumab in pregnant or breastfeeding women. Tralokinumab is an IgG4 monoclonal antibody against IL-13, and should be transported across the placenta similarly to dupilumab. However, while animal studies of tralokinumab have shown no direct or indirect harmful effects, there are no clinical data in humans to support any conclusions (68, 69).
Our review has some limitations. First and foremost, 13 of the 15 studies we included were descriptive. The case series were at risk of selection bias because the clinician selected the cases, which may be atypical in clinical practice (70). Owing to the type of studies included, we were only able to perform prevalence meta-analyses, without effect estimates (risk ratio or odds ratio), which means our findings cannot show a causal relationship. However, we have summarized the available evidence in the absence of higher-quality studies, which can take several years to complete (15, 16, 70). Second, we found no studies of pregnant women exposed to tralokinumab. Third, there was substantial clinical heterogeneity among the studies due to inconsistent reporting of some outcomes of interest and a lack of standard definitions for the outcomes evaluated (or, in some cases, no definition at all). In addition, most studies had a limited or unclear follow-up period after birth, meaning they may have missed relevant late adverse events, such as neonatal infection.
In conclusion, our findings are relatively reassuring: data published in a small number of pregnancies suggest that dupilumab is probably safe during pregnancy and breastfeeding. Most studies found no significant increase in the risk of adverse maternal outcomes, pregnancy outcomes, or foetal outcomes. We are unable to draw any conclusions concerning tralokinumab due to lack of data, although we expect both biologicals behave similarly during pregnancy and breastfeeding due to their comparable pharmacology and molecular weight. The findings of this review are inconclusive owing to the limited number of large, well-designed clinical studies. There is a need for appropriate pharmacological trials in women of reproductive age (8).
ACKNOWLEDGEMENTS
The authors would like to thank Julia Turner for her help in translating this manuscript.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akuffo-Addo E, Nicholas MN, Lansang P. Reported pregnancy outcomes in women with severe atopic dermatitis treated with dupilumab: a systematic review. J Cutan Med Surg 2023; 27: 177–178. https://doi.org/10.1177/12034754231152223
- Ambros-Rudolph CM, Müllegger RR, Vaughan-Jones SA, Kerl H, Black MM. The specific dermatoses of pregnancy revisited and reclassified: results of a retrospective two-center study on 505 pregnant patients. J Am Acad Dermatol 2006; 54: 395–404. https://doi.org/10.1016/j.jaad.2005.12.012
- Horiuchi Y. Th2 shift in pregnancy and labor: an immunological perspective to elucidate the pathogenesis onset of atopy. Am J Reprod Immunol 2023; 90: e13716. https://doi.org/10.1111/aji.13716
- Vestergaard C, Wollenberg A, Barbarot S, Christen-Zaech S, Deleuran M, Spuls P, et al. European Task Force on Atopic Dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol 2019; 33: 1644–1659. https://doi.org/10.1111/jdv.15709
- Avgil M, Ornoy A. Herpes simplex virus and Epstein-Barr virus infections in pregnancy: consequences of neonatal or intrauterine infection. Reprod Toxicol 2006; 21: 436–445. https://doi.org/10.1016/j.reprotox.2004.11.014
- De Caux D, Mariappa G, Perera G, Girling J. Prescribing for pregnancy: chronic skin diseases. Drug Ther Bull 2023; 61: 55–60. https://doi.org/10.1136/dtb.2022.000036
- Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020; 34: 2717–2744. https://doi.org/10.1111/jdv.16892
- Pfaller B, José Yepes-Nuñez J, Agache I, Akdis CA, Alsalamah M, Bavbek S, et al. Biologicals in atopic disease in pregnancy: an EAACI position paper. Allergy 2021; 76: 71–89. https://doi.org/10.1111/all.14282
- Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema – part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol 2022; 36: 1904–1926. https://doi.org/10.1111/jdv.18429
- Pfaller B, Bendien S, Ditisheim A, Eiwegger T. Management of allergic diseases in pregnancy. Allergy 2022; 77: 798–811. https://doi.org/10.1111/all.15063
- Escolà H, Figueras-Nart I, Bonfill-Orti M, Coll Puigserver N, Martin-Santiago A, Rodríguez Serna M, et al. Dupilumab for atopic dermatitis during pregnancy and breastfeeding: clinical experience in 13 patients. J Eur Acad Dermatol Venereol 2023; 37: e1156–1160. https://doi.org/10.1111/jdv.19165
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. https://doi.org/10.1136/bmj.n71
- Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. https://doi.org/10.1371/journal.pmed.1000097
- Sterne JAC, Higgins JPT, Elbers RG, Reeves BC, and the development group for ROBINS-I. Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I): detailed guidance, updated 12 October 2016. Available from: https://www.riskofbias.info/ (accessed 4 May 2024).
- Sampayo-Cordero M, Miguel-Huguet B, Malfettone A, Pérez-García JM, Llombart-Cussac A, Cortés J, et al. The value of case reports in systematic reviews from rare diseases: the example of Enzyme Replacement Therapy (ERT) in patients with Mucopolysaccharidosis Type II (MPS-II). Int J Environ Res Public Health 2020; 17: 6590. https://doi.org/10.3390/ijerph17186590
- Sampayo-Cordero M, Miguel-Huguet B, Pardo-Mateos A, Moltó-Abad M, Muñoz-Delgado C, Pérez-López J. Agreement between the results of meta-analyses from case reports and from clinical studies regarding the efficacy of laronidase therapy in patients with mucopolysaccharidosis type I who initiated enzyme replacement therapy in adult age: an example of case reports meta-analyses as an useful tool for evidence-based medicine in rare diseases. Mol Genet Metab 2018; 123: 69–75. https://doi.org/10.1016/j.ymgme.2018.01.002
- European Medicines Agency (EMA) Committee for Medicinal Products for Human Use. (CHMP) [Internet]. Dupixent Assessment Report July 2017. 2017. Available from: https://www.ema.europa.eu/en/documents/assessment-report/dupixent-epar-public-assessment-report_en.pdf (accessed 5 December 2023).
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep 2020; 6: 1051–1052. https://doi.org/10.1016/j.jdcr.2020.08.001
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol 2020; 34: e256–257. https://doi.org/10.1111/jdv.16235
- Kage P, Simon J-C, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol 2021; 48: E484–485. https://doi.org/10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol 2021; 13: 248–256. https://doi.org/10.1159/000515246
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, Martín-Santiago A. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther 2022; 35: e15237. https://doi.org/10.1111/dth.15237
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol 2022; 47: 960–961. https://doi.org/10.1111/ced.15049
- Khamisy-Farah R, Damiani G, Kong JD, Wu J, Bragazzi NL. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBaseTM). Eur Rev Med Pharmacol Sci 2021; 25: 5448–5451.
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, Dhadwal G, Kanani A. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol 2022; 18: 9. https://doi.org/10.1186/s13223-022-00650-w
- Kojanova M, Tanczosova M, Strosova D, Cetkovska P, Fialova J, Dolezal T, et al. Dupilumab for the treatment of atopic dermatitis: real-world data from the Czech Republic BI-OREP registry. J Dermatolog Treat 2022; 33: 2578–2586. https://doi.org/10.1080/09546634.2022.2043545
- Avallone G, Cavallo F, Tancredi A, Maronese CA, Bertello M, Fraghì A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol 2024. https://doi.org/10.1111/jdv.19794
- Hong N, Park SY, Kook HD, Lee DH, Jung HJ, Park MY, et al. Atopic dermatitis treated safely with dupilumab during pregnancy and lactation: a case series of four patients. Australas J Dermatol 2024; 65: e100–e103. https://doi.org/10.1111/ajd.14255
- Di Lernia V, Peccerillo F. Long-term follow-up of dupilumab treatment during conception, pregnancy and lactation. Indian J Dermatol 2024; 69: 193–195. https://doi.org/10.4103/ijd.ijd_447_23
- Alvarenga JM, Maria Lé A, Torres T. Dupilumab for atopic dermatitis during pregnancy and breastfeeding: a case report. Actas Dermosifiliogr 2023. https://doi.org/10.1016/j.ad.2023.10.005
- Carnovale C, Parisi F, Battini V, Zavatta A, Cheli S, Cattaneo D, et al. The use of biological agents in pregnant women affected by autoimmune disorders: why we need more research of this neglected area. Pharmacol Res 2021; 171: 105786. https://doi.org/10.1016/j.phrs.2021.105786
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol 2019; 33: e276–277. https://doi.org/10.1111/jdv.15552
- Lambert JLW, Segaert S, Ghislain PD, Hillary T, Nikkels A, Willaert F, et al. Practical recommendations for systemic treatment in psoriasis according to age, pregnancy, metabolic syndrome, mental health, psoriasis subtype and treatment history (BETA-PSO: Belgian Evidence-based Treatment Advice in Psoriasis; part 1). J Eur Acad Dermatol Venereol 2020; 34: 1654–1665. https://doi.org/10.1111/jdv.16684
- List of Pregnancy Exposure Registries. Available from: https://www.fda.gov/consumers/pregnancy-exposure-registries/list-pregnancy-exposure-registries (accessed 30 March 2024).
- MotherToBaby. Available from: https://mothertobaby.org/ongoing-study/ (accessed 31 March 2024).
- L. Ramos C, Namazy J. Monoclonal antibodies (biologics) for allergic rhinitis, asthma, and atopic dermatitis during pregnancy and lactation. Immunol Allergy Clin North Am 2023; 43: 187–197. https://doi.org/10.1016/j.iac.2022.07.001
- ClinicalTrial.gov. Post-authorization safety study to monitor pregnancy and infant outcomes following administration of dupilumab during planned or unexpected pregnancy in North America 2018. Available from: https://clinicaltrials.gov/study/NCT04173442 (accessed 1 April 2024).
- ClinicalTrial.gov. Dupilumab and pregnancy outcomes: a retrospective cohort study using administrative healthcare databases (Dupi PODS) 2019. Available from: https://clinicaltrials.gov/study/NCT03936335 (accessed 1 April 2024).
- Namazy J, Cabana MD, Scheuerle AE, Thorp JM, Chen H, Carrigan G, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135: 407–412. https://doi.org/10.1016/j.jaci.2014.08.025
- Blauvelt A, Guttman-Yassky E, Paller AS, Simpson EL, Cork MJ, Weisman J, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopic dermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol 2022; 23: 365–383. https://doi.org/10.1007/s40257-022-00683-2
- Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287-2303. https://doi.org/10.1016/S0140-6736(17)31191-1
- Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156: 44–56. https://doi.org/10.1001/jamadermatol.2019.3336
- de Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178: 1083–1101. https://doi.org/10.1111/bjd.16156
- Seeger JD, Lanza LL, West WA, Fernandez C, Rivero E. Pregnancy and pregnancy outcome among women with inflammatory skin diseases. Dermatology 2007; 214: 32–39. https://doi.org/10.1159/000096910
- Hamann CR, Egeberg A, Wollenberg A, Gislason G, Skov L, Thyssen JP. Pregnancy complications, treatment characteristics and birth outcomes in women with atopic dermatitis in Denmark. J Eur Acad Dermatol Venereol 2019; 33: 577–587. https://doi.org/10.1111/jdv.15256
- Schoder K, Zhu Y, Schneeweiss S, Merola JF, Savage TJ, Gibbs LR, et al. Use of systemic immunomodulating medications in pregnant women with atopic dermatitis: a nation-wide US study. J Am Acad Dermatol 2023; 89: 178–181. https://doi.org/10.1016/j.jaad.2023.02.047
- Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012: 985646. https://doi.org/10.1155/2012/985646
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol 2017; 3: 21–25. https://doi.org/10.1016/j.ijwd.2016.12.003
- Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000; 320: 1708–1712. https://doi.org/10.1136/bmj.320.7251.1708
- Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol 2012; 94: 417–423. https://doi.org/10.1002/bdra.23014
- Baldacci S, Gorini F, Santoro M, Pierini A, Minichilli F, Bianchi F. Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence. Epidemiol Prev 2018; 42: 1–34.
- Sánchez-García V, Hernández-Quiles R, de-Miguel-Balsa E, Giménez-Richarte, Ramos-Rincón JM, Belinchón-Romero I. Exposure to biologic therapy before and during pregnancy in patients with psoriasis: systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2023; 37: 1971–1990. https://doi.org/10.1111/jdv.19238
- European Medicines Agency. Dupixent CHMP assessment report on extension of marketing authorisation and an extension of indication variation. 2019. [accessed 30 March 2024] Available from: https://www.ema.europa.eu/en/documents/variation-report/dupixent-h-c-4390-x-0004-g-epar-assessment-report-extension_en.pdf
- Sanofi-aventis U.S. LLC. Dupixent (Dupilumab) injection. Prescribing information. 2022 [accessed 30 March 2024]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
- Piccinni MP, Maggi E, Romagnani S. Role of hormone-controlled T-cell cytokines in the maintenance of pregnancy. Biochem Soc Trans 2000; 28: 212–215. https://doi.org/10.1042/bst0280212
- Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol 2000; 109: 30–33. https://doi.org/10.1016/S0165-5728(00)00299-X
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in un-explained recurrent abortions. Nat Med 1998; 4: 1020–1024. https://doi.org/10.1038/2006
- Murphy VE, Wang G, Namazy JA, Powell H, Gibson PG, Chambers C, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013; 120: 812–822. https://doi.org/10.1111/1471-0528.12224
- Trønnes H, Wilcox AJ, Markestad T, Tollånes MC, Lie RT, Moster D. Associations of maternal atopic diseases with adverse pregnancy outcomes: a national cohort study. Paediatr Perinat Epidemiol 2014; 28: 489–497. https://doi.org/10.1111/ppe.12154
- Kojima R, Yokomichi H, Akiyama Y, Ooka T, Miyake K, Horiuchi S, et al. Association between preterm birth and maternal allergy considering IgE level. Pediatr Int 2021; 63: 1026–1032. https://doi.org/10.1111/ped.14635
- Pali-Schöll I, Namazy J, Jensen-Jarolim E. Allergic diseases and asthma in pregnancy, a secondary publication. World Allergy Organ J 2017; 10: 10. https://doi.org/10.1186/s40413-017-0141-8
- Mawhirt SL, Fonacier L. Atopic dermatitis and allergic contact dermatitis in pregnancy. In: Namazy J, Schatz M, editors. Asthma, allergic and immunologic diseases during pregnancy. Cham: Springer, 2019: p. 101–121. https://doi.org/10.1007/978-3-030-03395-8_7
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, Spuls PI. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol 2021; 46: 1089–1092. https://doi.org/10.1111/ced.14725
- Napolitano M, Ruggiero A, Fontanella G, Fabbrocini G, Patruno C. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther 2021; 34: e14475. https://doi.org/10.1111/dth.14475
- Witzel SJ. Lactation and the use of biologic immunosuppressive medications. Breastfeed Med 2014; 9: 543–546. https://doi.org/10.1089/bfm.2014.0107
- Ingrasci G, Lipman ZM, Yosipovitch G. When topical therapy of atopic dermatitis fails: a guide for the clinician. Expert Rev Clin Immunol 2021; 17: 1245–1256. https://doi.org/10.1080/1744666X.2021.2000390
- Duricova D, Dvorakova E, Hradsky O, Mitrova K, Durilova M, Kozeluhova J, et al. Safety of anti-Tnf-Alpha therapy during pregnancy on long-term outcome of exposed children: a controlled, multicenter observation. Inflamm Bowel Dis 2019; 25: 789–796. https://doi.org/10.1093/ibd/izy294
- European Medicines Agency. Adtralza (Tralokinumab). European Public Assessment Report. 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/adtralza (accessed 30 March 2024).
- LEO Pharma Inc. Adtralza (Tralokinumab) injection. Product monograph. 2021. [accessed 30 March 2024] Available from: https://pdf.hres.ca/dpd_pm/00063207.PDF
- Nambiema A, Sembajwe G, Lam J, Woodruff T, Mandrioli D, Chartres N, et al. A protocol for the use of case reports/studies and case series in systematic reviews for clinical toxicology. Front Med (Lausanne) 2021; 8: 708380. https://doi.org/10.3389/fmed.2021.708380