REVIEW ARTICLE
Association between Atopic Dermatitis and Dementia: Evidence from Systematic Review, Meta-analysis, and Mendelian Randomization
Yeon-Su GWAK1#, Seo-Yeon KIM1#, Chae-Eon WOO1#, Kihyuk SHIN2–4, Eunjeong SON5, Jin-Woo KIM6, Sung-Jin KIM7, Tae-Jin SONG8, Hae Ryoun PARK9–11, Kihun KIM12,13, Dai Sik KO14 and Yun Hak KIM12,13
1School of Medicine, Pusan National University, Yangsan, 2Department of Dermatology, College of Medicine, Pusan National University, Busan, 3Department of Dermatology, Pusan National University Yangsan Hospital, Yangsan, 4Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Busan, 5Division of Respiratory and Allergy, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, 6Department of Oral and Maxillofacial Surgery, Research Institute for Intractable Osteonecrosis of the Jaw, Ewha Womans University College of Medicine, Seoul, 7Department of Oral Histology and Developmental Biology, School of Dentistry and Dental Research Institute, Seoul National University, Seoul, 8Department of Neurology, Seoul Hospital, Ewha Womans University College of Medicine, Seoul, 9Department of Periodontology and Dental Research Institute, Pusan National University Dental Hospital, Yangsan, 10Periodontal Disease Signaling Network Research Center, School of Dentistry, Pusan National University, Yangsan, 11Department of Oral Pathology, Dental and Life Science Institute, Pusan National University, Yangsan, 12Department of Biomedical Informatics, School of Medicine, Pusan National University, Yangsan, 13Department of Anatomy, School of Medicine, Pusan National University, Yangsan, and 14Division of Vascular Surgery, Department of General Surgery, Gachon University College of Medicine, Gil Medical Center, Incheon, Republic of Korea
#These authors contributed equally to this work as first authors.
Abstract
Recent cohort studies suggest a potential association between atopic dermatitis and dementia, though the evidence remains conflicting. This study aims to elucidate the association between atopic dermatitis and dementia employing systematic review, meta-analysis, and Mendelian randomization (MR). A comprehensive search was performed to select eligible cohort studies using Medline, Embase, Scopus, ScienceDirect, and the Web of Science database. In MR analysis, genomic data from the Genome Wide Association Study (GWAS) (864,982 European individuals) for atopic dermatitis cases and dementia cases were obtained from the MRBase. Statistical analyses included the inverse-variance weighted (IVW) method, sensitivity tests, and MR-PRESSO for outliers. The adjustment accounted for various factors, including sex, age, smoking status, and other medical comorbidities, along with several additional variables. In the systematic review and meta-analysis, 5 longitudinal cohort studies (12,576,235 participants) indicated a significant association between atopic dermatitis and all-cause dementia (adjusted hazard ratio: 1.15, 95% CI: 1.07–1.23). Subgroup analyses revealed an adjusted hazard ratio of 1.18 (95% CI: 1.08–1.27) for Alzheimer’s disease in patients with atopic dermatitis, and an adjusted hazard ratio of 1.37 (95% CI: 1.21–1.55) for all-cause dementia in patients with moderate-to-severe atopic dermatitis. However, MR analysis showed no significant causal link between atopic dermatitis and dementia, Alzheimer’s disease, vascular dementia, or cognitive performance. While the meta-analysis revealed a significant association, MR analysis did not substantiate a significant causal link. Future research should consider demographic variables and medication influences in unravelling the intricate atopic dermatitis–dementia interplay.
SIGNIFICANCE
This study investigates a potential link between atopic dermatitis (a chronic skin condition) and dementia using large-scale data and genetic research. We found that while people with atopic dermatitis may have a higher risk of dementia, especially Alzheimer’s disease, our genetic analysis did not confirm a direct cause. This highlights the need for further research into factors like age, lifestyle, and medications. Our findings could help shape future healthcare strategies, improving early detection and prevention of dementia for those with atopic dermatitis, ultimately benefiting patients and their families.
Key words: atopic dermatitis; dementia; Alzheimer’s disease; systematic review; meta-analysis; Mendelian randomization.
Citation: Acta Derm Venereol 2025; 105: adv41321. DOI: https://doi.org/10.2340/actadv.v105.41321.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Aug 11, 2024; Accepted after revision: Nov 13, 2024. Published: Jan 3, 2025.
Corr: Kihun Kim, MD, Department of Anatomy and Department of Biomedical Informatics, Pusan National University, Yangsan 50612, Republic of Korea, Dai Sik Ko, MD, PhD, Division of Vascular Surgery, Department of General Surgery, Gachon University College of Medicine, Gil Medical Center, Incheon 21565, Republic of Korea, Prof. Yun Hak Kim, MD, PhD, Department of Anatomy and Department of Biomedical Informatics, Pusan National University, Yangsan 50612, Republic of Korea. E-mails: kihun7603@naver.com; daisik.ko@gilhospital.com; yunhak10510@pusan.ac.kr
Competing interests and funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00207946). This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. RS-2024-00439078).
The authors have no conflicts of interest to declare.
INTRODUCTION
Dementia, encompassing various disorders affecting memory, cognitive functions, and behaviour, significantly hinders an individual’s capacity to maintain their activities of daily living (1). It is broadly classified into Alzheimer’s disease, vascular dementia, frontotemporal dementia, dementia with Lewy bodies, and other forms (2). According to the World Health Organization (WHO), approximately 55 million people worldwide suffer from dementia, with nearly 10 million new cases reported annually (3). In 2019, the global societal costs of dementia were estimated at US$1313.4 billion for the 55.2 million affected individuals, translating to an average of US$23,796 per person with dementia (4). There is a consensus that cognitive impairment is a pivotal risk factor for functional disabilities in dementia patients. Functional status, encompassing self-care, self-maintenance, and physical activity, deteriorates with cognitive and functional decline (5). As these impairments escalate, the complexity of care practices and associated themes also increases (6). Functional loss in older individuals leads to adverse outcomes, including heightened utilization of hospital services, institutionalization, and an elevated risk of mortality (7).
Several well-established modifiable risk factors, such as lower education, hearing loss, traumatic brain injury, hypertension, diabetes, obesity, alcohol consumption, smoking, exposure to air pollution, physical inactivity, social isolation, and depression, contribute to the development of dementia (8). Recent studies suggest that atopic dermatitis, a chronic and highly pruritic inflammatory skin disease adversely affecting quality of life (9), may serve as a potential risk factor for heightened dementia risk, possibly attributed to immunologic dysregulation and disturbance (10–13). However, a systematic review and meta-analysis comprehensively synthesizing the published cohort studies have not been published. In addition, there is a lack of recent literature employing Mendelian randomization (MR) – widely utilized for inferring causal associations – to evaluate the relationship between atopic dermatitis and dementia. Therefore, our aim is to analyse the association between atopic dermatitis and dementia through a comprehensive approach, encompassing systematic review, meta-analysis, and MR.
METHODS
Systematic review and meta-analysis
Eligibility criteria. Our review exclusively considered longitudinal cohort studies that provided clear information on atopic dermatitis and dementia. We limited our selection to articles published in English but imposed no restrictions on publication year. We included only studies involving humans, while excluding conference papers and review articles from our analysis. In this study, the term “dementia” encompassed various forms of dementia, including Alzheimer’s disease, vascular dementia, and all-cause dementia. Our “atopic dermatitis group” was identified in the included studies under various terms such as “atopic dermatitis”, “atopic eczema”, or “atopy”. The diagnostic criteria for atopic dermatitis and dementia were determined according to the guidelines specified in each study, which may have included methods such as self-reporting or review of medical records. For self-reporting, participants were mailed a questionnaire with specific questions concerning their history of atopic dermatitis. For medical records, the criteria included diagnosis codes from the national registry based on ICD codes or prescription data.
Information sources and search strategy
This study was conducted in strict accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (14). We registered our initial plan in PROSPERO, with the registration number CRD42023410582. Initially, we conducted a comprehensive search for relevant articles in Medline, Embase, Scopus, ScienceDirect, and Web of Science, covering publications up to 30 March 2024. In our search, we prioritized Medical Subject Headings (MeSH) terms related to atopic dermatitis and dementia. The MeSH terms used are as follows: Dermatitis, Atopic; Dementia; Cognitive Dysfunction; Alzheimer Disease; Frontotemporal Dementia; Dementia, Vascular; Lewy Body Disease; Huntington Disease. However, since relying solely on MeSH terms proved insufficient for a comprehensive literature search, we supplemented our search strategy with additional related terms. A detailed search strategy for each database is provided in Table SI. We confined our search to the titles and abstracts of pertinent papers. Additionally, we conducted manual searches in Google Scholar to include grey literature sources.
Selection process
Three authors (CW, SK, and YG) independently reviewed the abstracts and titles of the identified studies. Subsequently, these same 3 authors conducted a full-text review to assess the eligibility of the papers. Any differences in evaluations were resolved through discussion.
Data collection process and data items. During the initial phase, we gathered the following data: title, abstract, journal, author names, publication year, and publication type. To provide a comprehensive depiction of the included papers, we also compiled additional information, including authors, study design, study location, study period, age range, the number of participants, definitions of atopic dermatitis and dementia, and the data source. Our inclusion criteria were studies that provided either the number of samples or hazard ratios relating to atopic dermatitis and dementia.
Assessment of risk of bias. The Newcastle-Ottawa scale, a widely recognized tool, is extensively employed for assessing bias risk in observational studies across various disciplines. In this study, the Newcastle-Ottawa quality-assessment scale was utilized to evaluate the risk of bias in a cohort study (15). The assessment criteria encompassed 3 key categories: selection, comparability, and outcome. Based on the scores within each category, the risk of bias for the cohort study was categorized into 3 levels: “good”, “fair”, and “poor”, following the standards set by the Agency of Healthcare Research and Quality. Three authors (CW, SK, and YG) conducted an independent quality assessment of the included studies, with any disagreements in their assessments resolved through discussion.
Statistical analysis. Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were employed to assess the risk of atopic dermatitis in patients with dementia. Unadjusted and adjusted pooled HR were extracted from within the papers. The heterogeneity was assessed using the I2 statistic as proposed by Higgins et al. (16). We considered heterogeneity as moderate (I2: 50–75%) or substantial (I2 > 75%) to indicate significant heterogeneity. In cases of substantial heterogeneity, we applied the random-effects model; otherwise, the fixed-effects model was employed for meta-analysis (17, 18). RevMan version 5.4.1 (supported by Cochrane in London, UK; https://test-training.cochrane.org/online-learning/core-software-cochrane-reviews/review-manager-revman/download-revman-5) to create forest plots for visual representation of the results. We conducted subgroup analysis for 2 subtypes of dementia: Alzheimer’s disease and vascular dementia. Additionally, we conducted subgroup analysis based on the severity of atopic dermatitis. For this classification, “mild” referred to patients without a history of treatment such as corticosteroids, phototherapy, and immunosuppressants. On the other hand, “moderate-to-severe” encompassed patients with a history of systematic treatment.
Mendelian randomization
Study design. This study adopts an MR methodology to investigate the potential causal link between atopic dermatitis and dementia, including its subtypes such as Alzheimer’s disease, vascular dementia, and cognitive performance as an intermediate outcome. A two-sample MR analysis is the core of this research, with atopic dermatitis designated as the exposure and various forms of dementia as the outcome. This research relies solely on existing publications and publicly accessible databases, thereby obviating the need for additional ethical approval or consent.
Data resources. Data for this MR analysis are meticulously curated from the most comprehensive genome-wide association study (GWAS) to date on atopic dermatitis, encompassing a vast European meta-analysis with an impressive sample size of 864,982 individuals, which includes 60,653 cases and 804,329 controls spanning 40 cohorts (19). The outcome data were sought from the MRBase database (20). The dataset for all-cause dementia was derived from FinnGen with a sample size of 216,771 individuals. Alzheimer’s disease data were taken from the ADGC, EADI, CHARGE, and GERAD/PERADES consortia, encompassing 63,926 individuals. Vascular dementia data were also sourced from FinnGen (https://r5.risteys.finngen.fi/phenocode/F5_VASCDEM) with 212,389 participants. Cognitive performance data were based on 257,841 individuals (Table SII). This comprehensive collection of data allowed for a thorough examination of the association between atopic dermatitis and various cognitive outcomes.
Selection of instrumental variables. The selection of instrumental variables in this study is guided by stringent criteria, ensuring robustness and validity in the analysis. Single nucleotide polymorphisms (SNPs) associated with atopic dermatitis are chosen based on a genome-wide significance threshold of p < 5 x 10–8 with a minor allele frequency of more than 0.01 to select our genetic instruments. To mitigate the confounding effects of linkage disequilibrium, a clumping process with an R2 cutoff of 0.01 within a 500-KB window is employed. This study also adopts a refined approach to handling palindromic SNPs, inferring positive strand alleles using allele frequencies. In instances where a specific SNP was absent from the outcome summary data, we utilized the LDlink (https://ldlink.nci.nih.gov/) API10 to identify substitute proxy SNPs, with a minimum requirement of a linkage disequilibrium (LD) value of r2 = 0.8.
Statistical analysis
Statistical analyses are conducted using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria), leveraging the capabilities of the TwoSampleMR (20) and MR-PRESSO packages (21). The primary analysis utilizes the inverse-variance weighted (IVW) method, a standard approach in MR studies for estimating causal effects. To enhance the robustness of findings and address potential methodological concerns, a comprehensive suite of sensitivity analyses is conducted. These include the MR Egger method for evaluating pleiotropy, the weighted median method for ensuring validity despite potential invalid instrumental variables, and the MR-PRESSO for identifying and adjusting for outliers. This multifaceted analytical approach ensures a thorough and nuanced exploration of the hypothesized causal pathways.
RESULTS
Systematic review and meta-analysis
Study selection and characteristics. Our comprehensive search strategy initially yielded 432 records, with an automated tool removing 44 duplicates. Among the 388 remaining papers, review articles, animal research, and non-research papers were filtered out, reducing the initial set of 240 records. After reviewing titles and abstracts, 225 papers were excluded. From the remaining 15 papers, 11 were eliminated for reasons such as data unavailability or failure to report relevant outcomes, such as cognitive decline or pemphigoid. Two papers from the grey literature were initially under consideration, but 1 was excluded because it duplicated data from the other source. Finally, 5 papers met the criteria for inclusion in the analysis. For a visual representation of this process, please consult Fig. 1, which is presented as a PRISMA flow diagram. The analysis comprised 12,576,235 individuals and encompassed cohort studies that utilized national databases or registries published between 2008 and 2023. The characteristics of the included studies are summarized in Table I.
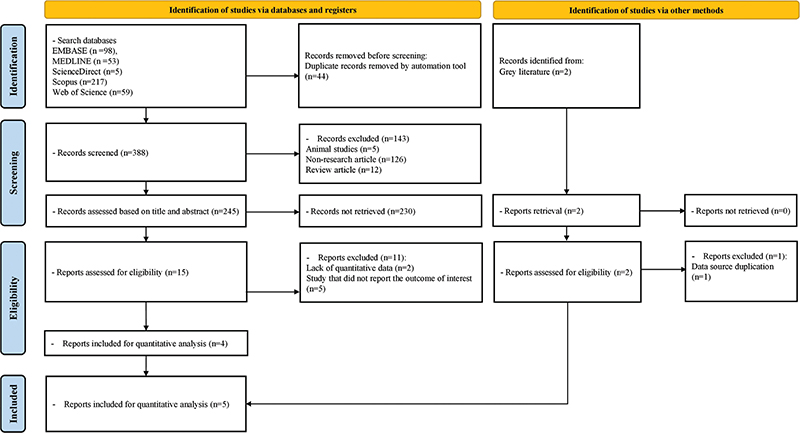
Fig. 1. PRISMA flowchart depicting the process of study selection.
| First author, year | Place and time of study | Study design | Number of samples | Age, year | Data source | Severity | Criteria of atopic dermatitis | Criteria of dementia | Adjustments |
| Eriksson, 2008 (22) | Sweden, Jan 1974–Dec 2001 | Cohort | 22,188 | Not specified | Population-based Swedish Twin Registry | Not specified | Self-report (questionnaire), a physician’s diagnosis | International classification -8, -9, -10, clinical diagnoses of dementia followed the DSM-IV criteria | Sex, age, history of smoking, level of education, and myocardial infarction |
| Pan, 2021 (10) | Taiwan, 1996–2013 | Cohort | 11,649 | > 45 | Taiwan National Health Insurance Research Database (NHIRD) | Mild/moderate-to-severe | International Classification of Diseases (ICD-9-CM) code: 691.8 | International Classification of Diseases (ICD-9-CM) code: 290.0~4, 294.1~2, 331.0~2,331. 82 | Demographic data, Charlson comorbidity index score, and all-cause clinical visits |
| Magyari, 2022 (11) | UK, mean (SD): no eczema 6.8 (5.1), eczema 5.8 (4.5) | Cohort | 1,767,667 | 60–100 | Health Improvement Network; representative of the general population in the UK | Mild/moderate/severe | Medical code for atopic eczema, at least 2 prescriptions for atopic eczema therapy | Previously validated medical codes recorded in the practice records via Mendeley | Sex, calendar period, practice, Townsend score, history of smoking, history of alcohol use, and body mass index, depression, anxiety, diabetes, hypertension, major adverse cardiovascular events, asthma, rhinitis, and antihistamine prescriptions |
| Joh, 2023 (12) | Korea, 2009–2017 | Cohort | 6,785,948 | > 40 | National Health Information Database on national health screening and healthcare utilization of all Koreans | Outpatient visit: no, low (1–3), high ( > 3) | International Disease Code, at least 3 times outpatient visit for atopic dermatitis in a year | Prescribed any of the anti-dementia medications, at least twice using the International Classification of Diseases 10th Revision diagnosis codes | Sex, body mass index, smoking status, alcohol use, regular physical activity, income, area of residence, history of hypertension, dyslipidaemia, diabetes, stroke, and myocardial infarction, systolic blood pressure, glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and estimated glomerular filtration rate |
| Vingeliene, 2023 (23) | Sweden, 1990–2018 | Cohort | 3,988,783 | 50–85.9 | Swedish linked register data | Not specified | National Patient Register inpatient and outpatient records | National Patient Register and Cause of Death Register | Sex, year of birth, geographical region at age 30, and socioeconomic index at age 30 |
Synthesis of the results
Overall impact of atopic dermatitis on all-cause dementia. Five studies were incorporated to assess the risk of all-cause dementia in patients with atopic dermatitis vs those without it. The pooled unadjusted HR (uHR) for all-cause dementia was 1.06 (95% CI: 0.95–1.17; p = 0.31, I² = 94%), and pooled adjusted HR (aHR) for all-cause dementia was 1.15 (95% CI: 1.07–1.23; p < 0.001, I² = 75%) (Fig. 2).
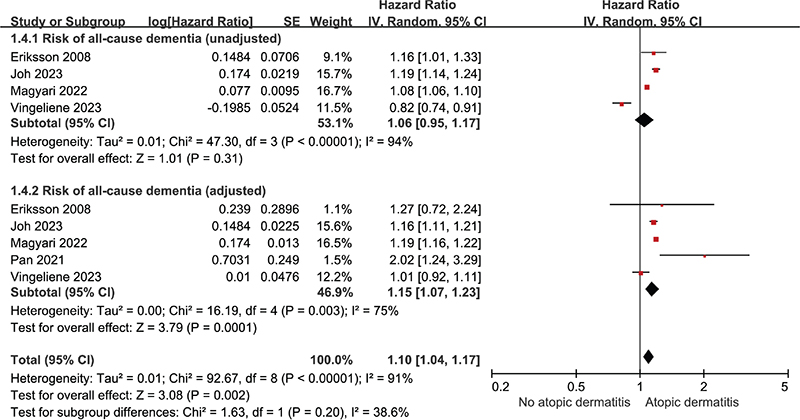
Fig. 2. Forest plot illustrating hazard ratio for all-cause dementia in patients with atopic dermatitis categorized as unadjusted and adjusted.
Subgroup analysis: dementia type
Five studies were included to evaluate the risk of Alzheimer’s dementia, while 3 studies were included to assess the risk of vascular dementia in patients with atopic dermatitis compared with those without the condition. The pooled uHR for Alzheimer’s dementia was 1.12 (95% CI: 1.04–1.20; p = 0.003, I² = 70%), and pooled aHR for Alzheimer’s dementia was 1.18 (95% CI: 1.08–1.27; p < 0.001, I² = 64%) (Fig. 3). The pooled uHR for vascular dementia was 0.93 (95% CI: 0.55–1.60; p = 0.80, I² = 94%), and pooled aHR for vascular dementia was 1.13 (95% CI: 0.90–1.42; p = 0.29, I² = 56%) (Fig. 4).
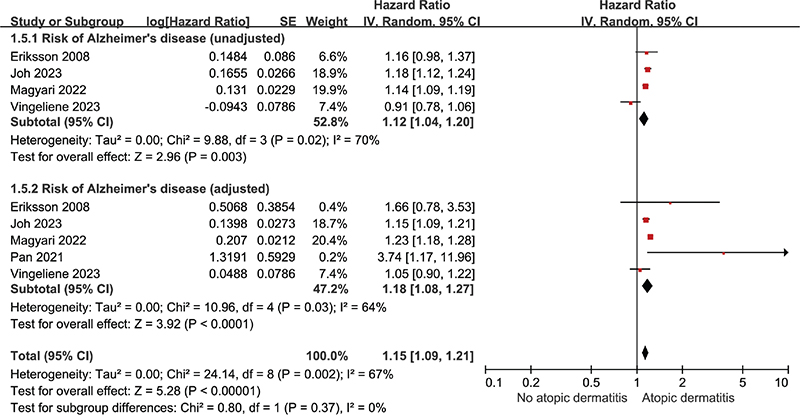
Fig. 3. Forest plot illustrating hazard ratio for Alzheimer’s dementia in patients with atopic dermatitis categorized as unadjusted and adjusted.
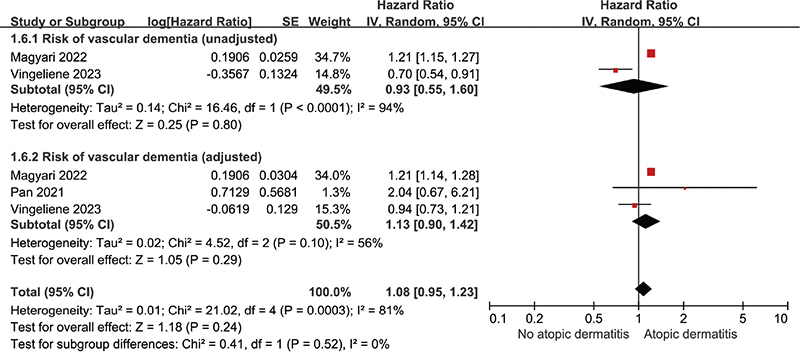
Fig. 4. Forest plot illustrating hazard ratio for vascular dementia in patients with atopic dermatitis categorized as unadjusted and adjusted.
Subgroup analysis: atopic dermatitis severity
Of the 5 papers, 2 articles underwent subgroup analysis because 2 papers did not stratify atopic dermatitis by severity (22, 23), and the other paper did not align with our defined criteria for severity (12). The pooled uHR for all-cause dementia in patients with mild atopic dermatitis was 1.57 (95% CI: 0.66–3.72; p = 0.31, I² = 89%), and pooled aHR for all-cause dementia in patients with mild atopic dermatitis was 1.53 (95% CI: 0.77–3.03; p = 0.23, I² = 81%) (Fig. S1). The pooled uHR for all-cause dementia in patients with moderate-to-severe atopic dermatitis was 2.23 (95% CI: 0.56–8.81; p = 0.25, I² = 95%), and pooled aHR for all-cause dementia in patients with moderate-to-severe atopic dermatitis was 1.37 (95% CI: 1.21–1.55; p < 0.001, I² = 0%) (Fig. S2).
Risk of bias in studies
We assessed the risk of bias using the Newcastle-Ottawa quality-assessment scale. All 5 cohort studies included in this study were rated as “Good” quality. Detailed evaluation results are given in Table SIII.
Mendelian randomization
In the MR analysis evaluating the association between atopic dermatitis and dementia and its subtypes, 81 SNPs were identified as instrumental variables with a significance level of p < 5 × 10–8 (Table SIV). For any-cause dementia, an analysis of 78 single nucleotide polymorphisms (SNPs) using the IVW method showed an odds ratio (OR) of 1.01 (95% CI: 0.83–1.25) with a p-value of 0.90. In the context of Alzheimer’s disease, examining 61 SNPs revealed an OR of 1.01 (95% CI: 0.86–1.19) with a p-value of 0.89. For vascular dementia, the analysis included 78 SNPs and resulted in an OR of 0.97 (95% CI: 0.62–1.53) with a p-value of 0.90. In evaluating cognitive performance using 79 SNPs, the IVW method yielded an OR of 1.01 (95% CI: 0.98–1.05) with a p-value of 0.40 (Table II). In the MR-PRESSO analysis, outliers were identified in the outcomes of any-cause dementia, Alzheimer’s disease, and cognitive dementia. However, the MR-Egger regression test did not indicate the presence of pleiotropy (Table SV).
DISCUSSION
This paper represents the first comprehensive analysis investigating the association and causation between atopic dermatitis and dementia through a systematic review, meta-analysis, and MR. The meta-analysis involved 12,576,235 participants from 5 cohort studies, with participants aged 39 and older. The systematic review and meta-analysis indicated significant results for all-cause dementia (aHR: 1.15 [1.07–1.23]). Notably, within specific subgroups of dementia types, Alzheimer’s disease stood out with an uHR of 1.12 (1.04–1.20), and an aHR of 1.18 (1.08–1.27). Likewise, in the severity subgroup of atopic dermatitis, moderate-to-severe cases (aHR: 1.37; 95% CI: 1.21–1.55) were particularly prominent. To overcome biases and confounding issues inherent in cohort studies, MR analysis was conducted (24). However, the MR analysis, a valuable tool for assessing causation when randomized controlled trials are impractical, did not reveal any significant causal relationship between the 2 variables based on 864,982 European individuals (25, 26). Considering the limitations, including a restricted number of included studies, heterogeneity between studies, and discrepancies in adjusted variables, it is crucial to approach the interpretation of these findings with caution. Moreover, the apparent absence of a significant effect size indicates that the potential causal link between atopic dermatitis and dementia might not be notably robust, underscoring the need for thoughtful consideration during interpretation.
Several hypotheses have been proposed to explain the association between atopic dermatitis and dementia. Chiefly, the proposed mechanisms revolve around the idea that chronic systemic inflammation and immune alterations associated with allergic diseases may impact neuroinflammation and immune regulation in the brain. The activation of T-helper type 2 (TH2) inflammation and the release of proinflammatory cytokines during allergic responses in atopic dermatitis are believed to play a pivotal role in the pathophysiology of dementia (27–31).
Dysregulated proinflammatory cytokines can breach the blood–brain barrier (BBB) during allergic responses, triggering abnormal neuroimmune processes within specific neural circuits related to cognitive modulation (32, 33). These alterations gradually impede specific brain functions and neural circuitry involved in memory and cognition (10). Additionally, excess circulating proinflammatory cytokines, upon penetrating the BBB, may induce neuroinflammation (34). Subsequent elevated levels of immune mediators, such as cytokines, can sustain microglia activation, ultimately culminating in chronic neuroinflammation and neurodegeneration (35–37). Furthermore, interleukin (IL)-6, a type of cytokine, is implicated in promoting the expression of the amyloid-beta (Aβ) precursor protein (38, 39). The presence of circulating proinflammatory mediators and immune alterations might expedite Aβ accumulation by impairing the Aβ clearance function of microglia in the brain (36, 37). Additionally, IL-33, a member of the IL-1 family, is considered a crucial mediator of glial cell response to neuropathological lesions (30, 31). Xiong et al. (30) found that IL-33/ST2-positive cells were also significantly increased in Alzheimer’s disease brains when compared with non-Alzheimer’s disease brains.
Second, mast cells are posited to function as primary intermediaries linking the peripheral system to neuroinflammatory processes (40, 41). These cells release a variety of preformed mediators, including histamine, serotonin, cytokines, leukotrienes, and tumour necrosis factor-α. This leads to the subsequent synthesis of additional cytokines, chemokines, and lipid mediators, which can adversely impact glial cells, neurons, and endothelial cells, ultimately affecting BBB permeability (40–42). In a phase II randomized placebo-controlled trial, the addition of a mast cell inhibitor to standard care demonstrated a delay in cognitive decline in individuals with mild-to-moderate Alzheimer’s disease (42).
Third, allergic reactions may instigate vascular pathology and cerebrovascular dysfunction. Numerous previous studies have associated allergic diseases with cerebrovascular diseases (43). Cerebrovascular pathology can contribute to endothelial dysfunction and BBB breakdown, resulting in diminished and dysregulated cerebral blood flow, chronic cerebral hypoxia, and the accumulation of neurotoxic substances. These events can ultimately lead to neurovascular dysfunction (44, 45).
Moreover, medications used to manage allergies may impact the risk of dementia. Longitudinal data suggest that cumulative anticholinergic use is associated with an increased risk of dementia, with first-generation antihistamines possessing anticholinergic properties (46). However, it is noteworthy that both medication non-users and users of topical corticosteroids also exhibited an elevated risk of dementia, a phenomenon not entirely attributable to medication effects alone (12).
Limitations and strengths
There are some limitations to our study. In the systematic literature review and meta-analysis, we aimed to investigate the association between atopic dermatitis and both mild and severe forms of dementia. However, due to the lack of data on dementia severity, we were unable to perform a subgroup analysis. There are variations in the classification of atopic dermatitis severity among the included studies. The definition of dementia relied on clinical assessments, potentially leading to under-detection of early stages of dementia and thereby attenuating risk estimates toward the null (47). Furthermore, some studies in our analysis did not specifically control for the use of antidepressants or antipsychotics, both of which have been associated with an increased risk of dementia. Meta-analyses of observational studies often face issues with bias, confounding, and publication bias, which can introduce bias into the summary effect (48). In MR studies, selection bias and misclassification can occur when there are inaccuracies in the selection or classification of participants or variables, potentially leading to bias in the results in either direction (49). Furthermore, the applicability of MR analyses to populations of different racial backgrounds may be limited, as the study participants were exclusively European. This raises concerns regarding the external validity of the results when extrapolating them to other racial groups. There might be variants in LD with independent predictive power; however, they were removed to ensure the robustness of the analysis.
Despite these limitations, our study boasts several strengths that enhance the meaningfulness of its findings. The statistical power of our results is derived from a substantial number of participants, and the mean follow-up time in the included cohort studies is 18.75 years (ranging up to 28 years, with a minimum of 9 years). Additionally, the use of MR analysis allowed us to evaluate causal associations within the scope of our investigation.
Conclusion
In summary, the systematic review and meta-analysis uncovered a notable correlation between atopic dermatitis and dementia, particularly Alzheimer’s disease. However, Mendelian randomization analysis failed to substantiate a significant causal relationship. Given the limitations of the study, the results require meticulous interpretation. Further research is imperative to unravel the intricate interplay between atopic dermatitis and dementia, considering potential demographic variables such as racial differences and medication influences.
ACKNOWLEDGEMENTS
Protocol registration number: CRD42023410582.
REFERENCES
- Duong S, Patel T, Chang F. Dementia: what pharmacists need to know. Can Pharm J 2017; 150: 118–129. https://doi.org/10.1177/1715163517690745
- Wu Y-T, Clare L, Hindle JV, Nelis SM, Martyr A, Matthews FE. Dementia subtype and living well: results from the Improving the experience of Dementia and Enhancing Active Life (IDEAL) study. BMC Med 2018; 16: 1–9. https://doi.org/10.1186/s12916-018-1135-2
- World Health Organization. Geneva, Switzerland: World Health Organization. Fact sheets of dementia [Internet]. 2023 [cited 2024 Jun 19]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
- Wimo A, Seeher K, Cataldi R, Cyhlarova E, Dielemann JL, Frisell O, et al. The worldwide costs of dementia in 2019. Alzheimers Dement 2023; 10.1002/alz.12901. https://doi.org/10.1002/alz.12901
- Cipriani G, Danti S, Picchi L, Nuti A, Fiorino MD. Daily functioning and dementia. Dement Neuropsycho 2020; 14: 93–102. https://doi.org/10.1590/1980-57642020dn14-020001
- Prizer LP, Zimmerman S. Progressive support for activities of daily living for persons living with dementia. Gerontol 2018; 58: S74–S87. https://doi.org/10.1093/geront/gnx103
- Brown RT, Diaz-Ramirez LG, Boscardin WJ, Lee SJ, Williams BA, Steinman MA. Association of functional impairment in middle age with hospitalization, nursing home admission, and death. JAMA Intern Med 2019; 179: 668–675. https://doi.org/10.1001/jamainternmed.2019.0008
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Tsakok T, Woolf R, Smith C, Weidinger S, Flohr C. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol 2019; 180: 464–474. https://doi.org/10.1111/bjd.16934
- Pan TL, Bai YM, Cheng CM, Tsai SJ, Tsai CF, Su TP, et al. Atopic dermatitis and dementia risk: a nationwide longitudinal study. Ann Allergy Asthma Immunol 2021; 127: 200–205. https://doi.org/10.1016/j.anai.2021.03.001
- Magyari A, Ye M, Margolis DJ, McCulloch CE, Cummings SR, Yaffe K, et al. Adult atopic eczema and the risk of dementia: a population-based cohort study. J Am Acad Dermatol 2022; 87: 314–322. https://doi.org/10.1016/j.jaad.2022.03.049
- Joh HK, Kwon H, Son KY, Yun JM, Cho SH, Han K, et al. Allergic diseases and risk of incident dementia and Alzheimer’s disease. Ann Neurol 2023; 93: 384–397. https://doi.org/10.1002/ana.26506
- Yoon S, Kim K, Shin K, Kim HS, Kim B, Kim MB, et al. The safety of systemic Janus kinase inhibitors in atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol 2024; 38: 52–61. https://doi.org/10.1111/jdv.19426
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. [The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Declaracion PRISMA 2020: una guia actualizada para la publicacion de revisiones sistematicas]. Rev Panam Salud Publica 2022; 46: e112. https://doi.org/10.26633/RPSP.2022.112
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford, 2000. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. https://doi.org/10.1136/bmj.327.7414.557
- Ahn D, Kim J, Kang J, Kim YH, Kim K. Congenital anomalies and maternal age: a systematic review and meta-analysis of observational studies. Acta Obstetric Gynecolog Scand 2022; 101: 484–498. https://doi.org/10.1111/aogs.14339
- Kim CM, Lee S, Hwang W, Son E, Kim TW, Kim K, et al. Obesity and periodontitis: a systematic review and updated meta-analysis. Front Endocrinol (Lausanne) 2022; 13: 999455. https://doi.org/10.3389/fendo.2022.999455
- Budu-Aggrey A, Kilanowski A, Sobczyk MK, Shringarpure SS, Mitchell R, Reis K, et al. European and multi-ancestry genome-wide association meta-analysis of atopic dermatitis highlights importance of systemic immune regulation. Nat Commun 2023; 14: 6172. https://doi.org/10.1038/s41467-023-41180-2
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018; 7: e34408. https://doi.org/10.7554/eLife.34408
- Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018; 50: 693–698. https://doi.org/10.1038/s41588-018-0099-7
- Eriksson UK, Gatz M, Dickman PW, Fratiglioni L, Pedersen NL. Asthma, eczema, rhinitis and the risk for dementia. Dement Geriatr Cogn Disord 2008; 25: 148–156. https://doi.org/10.1159/000112729
- Vingeliene S, Hiyoshi A, Carlberg M, Garcia-Argibay M, Lentjes M, Fall K, et al. Atopic dermatitis, systemic inflammation and subsequent dementia risk. JEADV Clin Pract 2023; 10.1002/jvc2.249. https://doi.org/10.1002/jvc2.249
- Ramirez-Santana M. Limitations and biases in cohort studies. IntechOpen London, UK, 2018. https://www.intechopen.com/chapters/59393
- Lee YH. Overview of Mendelian randomization analysis. J Rheum Dis 2020; 27: 241–246.
- Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Primers 2022; 2: 6.
- Ferretti MT, Cuello AC. Does a pro-inflammatory process precede Alzheimer’s disease and mild cognitive impairment? Curr Alzheimer Res 2011; 8: 164–174. https://doi.org/10.2174/156720511795255982
- Griffin WS. Neuroinflammatory cytokine signaling and Alzheimer’s disease. N Engl J Med 2013; 368: 770–771. https://doi.org/10.1056/NEJMcibr1214546
- Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer’s disease. Front Integr Neurosci 2013; 7: 59. https://doi.org/10.3389/fnint.2013.00059
- Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, Zaheer A. Alzheimer’s disease: evidence for the expression of interleukin-33 and its receptor ST2 in the brain. J Alzheimers Dis 2014; 40: 297–308. https://doi.org/10.3233/JAD-132081
- Abd Rachman Isnadi MF, Chin VK, Abd Majid R, Lee TY, Atmadini Abdullah M, Bello Omenesa R, et al. Critical roles of IL-33/ST2 pathway in neurological disorders. Mediators Inflamm 2018; 2018: 5346413. https://doi.org/10.1155/2018/5346413
- Pfab F, Valet M, Napadow V, Tölle TR, Behrendt H, Ring J, et al. Itch and the brain. Chem Immunol Allergy 2012; 98: 253–265. https://doi.org/10.1159/00033652913
- Schut C, Mochizuki H, Grossman SK, Lin AC, Conklin CJ, Mohamed FB, et al. Brain processing of contagious itch in patients with atopic dermatitis. Front Psychol 2017; 8: 1267. https://doi.org/10.3389/fpsyg.2017.01267
- Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol 2015; 16: 229–236. https://doi.org/10.1038/ni.3102
- Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol 2018; 18: 759–772. https://doi.org/10.1038/s41577-018-0051-1
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 2015; 16: 358–372. https://doi.org/10.1038/nrn3880
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14: 388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
- Griffin WST, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A 1989; 86: 7611–7615. https://doi.org/10.1073/pnas.86.19.7611
- Griffin WST, Barger SW. Neuroinflammatory cytokines: the common thread in Alzheimer pathogenesis. Eur Neurol Rev 2011; 6: 89–96. https://doi.org/10.17925/enr.2011.06.02.89
- Jones MK, Nair A, Gupta M. Mast cells in neurodegenerative disease. Front Cell Neurosci 2019; 13: 171. https://doi.org/10.3389/fncel.2019.00171
- Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA. Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev 2017; 79: 119–133. https://doi.org/10.1016/j.neubiorev.2017.05.001
- Piette F, Belmin J, Vincent H, Schmidt N, Pariel S, Verny M, et al. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimers Res Ther 2011; 3: 16. https://doi.org/10.1186/alzrt75
- Wang L, Gao S, Yu M, Sheng Z, Tan W. Association of asthma with coronary heart disease: a meta analysis of 11 trials. PLoS ONE 2017; 12: e0179335. https://doi.org/10.1371/journal.pone.0179335
- Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018; 14: 133–150. https://doi.org/10.1038/nrneurol.2017.188
- Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet 2021; 397: 1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175: 401–407. https://doi.org/10.1001/jamainternmed.2014.7663
- Wacholder S, Hartge P, Lubin JH, Dosemeci M. Non-differential misclassification and bias towards the null: a clarification. Occup Environ Med 1995; 52: 557–558. https://doi.org/10.1136/oem.52.8.557
- Metelli S, Chaimani A. Challenges in meta-analyses with observational studies. BMJ Ment Health 2020; 23: 83–87. https://doi.org/10.1136/ebmental-2019-300129
- Clayton GL, Gonçalves A, Goulding N, Borges MC, Holmes MV, Davey G, et al. A framework for assessing selection and misclassification bias in mendelian randomisation studies: an illustrative example between body mass index and covid-19. BMJ 2023; 381: e072148. https://doi.org/10.1136/bmj-2022-072148