SHORT COMMUNICATION
Clinical Improvement of Bullous Pemphigoid with Hyperkeratosis and Palmoplantar Keratoderma in Two Patients Treated with Dupilumab
Giulia PASCOLINI1, Feliciana MARIOTTI2, Anna PIRA2, Biagio DIDONA1 and Giovanni DI ZENZO2*
1Rare Skin Diseases Center, Istituto Dermopatico dell’Immacolata, IDI-IRCCS, Rome, 2Laboratory of Molecular and Cell Biology, Istituto Dermopatico dell’Immacolata, IDI-IRCCS, Via dei Monti di Creta 104, IT-00167 Rome, Italy. *E-mail: g.dizenzo@idi.it
Citation: Acta Derm Venereol 2024; 104: adv41984. DOI: https://doi.org/10.2340/actadv.v104.41984.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Sep 11, 2024. Accepted: Sep 17, 2024. Published: Oct 10, 2024
Competing interests and funding: The authors have no conflicts of interest to declare.
This article was partially supported by the “Progetto Ricerca Corrente” of the Italian Ministry of Health.
INTRODUCTION
Bullous pemphigoid (BP) is a rare chronic autoimmune disease, characterized by sub-epithelial blister formation, resulting in vesicles, bullae, and pruritic eruptions (1). We have previously identified in 3 patients a subtype of BP associated with hyperkeratotic lesions and palmoplantar (P-P) keratoderma (2).
Topical and/or systemic corticosteroids represent the main initial treatment of BP while immunomodulatory therapies can be an option to minimize the adverse effects of chronic corticosteroid therapy or to augment improvement in the disease (3).
Dupilumab, binding to the α-subunit of the interleukin (IL)-4 receptor, is considered an effective drug for several autoimmune diseases, comprising dermatologic and bronchopulmonary disorders (4, 5). A recent application in BP has been provided (6, 7); however, to the best of our knowledge, there is no literature documenting the use of dupilumab in hyperkeratotic BP with P-P keratoderma.
Following our earlier study, we describe the successful treatment with dupilumab in 2 of our former patients manifesting this atypical BP.
CASE REPORTS
The patients were enrolled at the Rare Skin Diseases Center of the Istituto Dermopatico dell’Immacolata, IDI-IRCCS, of Rome and the study was conducted according to the Helsinki declaration.
The present report includes patients 1 and 2 of our prior research (2).
The 2 atypical BP patients manifested vesicles, blisters, pruritic eruption, diffuse palmoplantar hyperkeratosis (Fig. 1: patient 1, panels A and C; patient 2, panels G and I) and nodular lesions on the forearms and legs (Fig. 1: patient 1, panels E and M). They also displayed P-P keratoderma with features of nigricans-like acanthosis (1). Direct immunofluorescence (DIF) examination was diagnostic for BP in patients 1 and 2, who also showed eosinophilia, high total IgE levels, and positivity for anti-BP180 IgG and anti-BP230 IgE (this latter only in patient 1) (1).
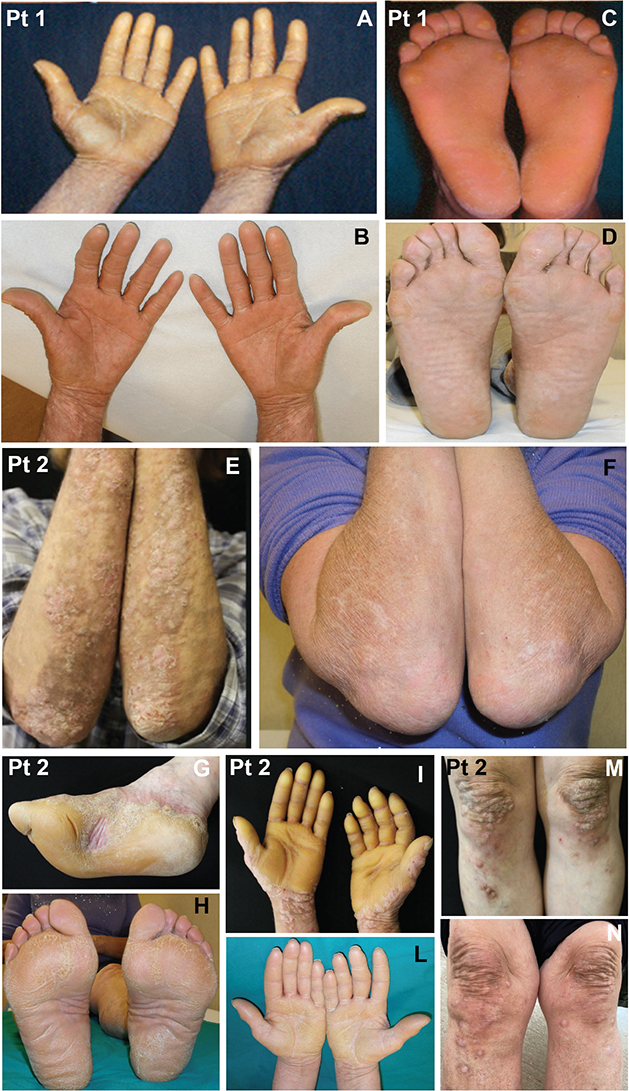
Fig. 1. Dupilumab treatment in the present patients presenting with bullous pemphigoid and P-P keratoderma. The skin lesions are shown before (patient 1: panels A, C; patient 2: panels E, G, I, M) and after (patient 1: panels B and D; patient 2: panels F, H, L, N) dupilumab treatment. Note the marked clinical improvement in both two cases
Multiple drugs therapy, including corticosteroids, and steroid-sparing agents, was attempted in the past in all 3 patients, without an appreciable improvement in signs and symptoms. Rituximab has been administered off-label in cases 1 and 2, leading to the resolution of the disease only in case 2 that relapsed 2 years ago. All these findings indicated that this variant of hyperkeratotic BP represented a resistant BP.
Thus, treatment with dupilumab was considered as alternative therapy and administered in both subjects. The patients underwent a standardized dosage protocol approved for atopic dermatitis. Specifically, an initial dose of 600 mg, succeeded by a maintenance dose of 300 mg, was administered biweekly via subcutaneous injection.
The efficacy of the treatment was evaluated after 6 months in patient 1 and 14 months in patient 2 (Fig. 1: patient 1, panels B and D; patient 2, panels F, H, L, and N). In addition to the control of lesions an evident reduction of hyperkeratosis and P-P keratoderma was identifiable for both patients (Fig. 1: patient 1, panels B and D; patient 2, panels H and L). The hyperkeratotic cutaneous lesions combined with urticarial plaques in patient 1 and pruritic nodular and bullous eruption with hyperkeratotic lesions on the limbs in patient 2 were significantly improved (Fig. 1, F, N). They also informed us of a marked reduction of pruritus. In both patients BP180 and, when present, BP230 IgG reactivity reached negative values.
DISCUSSION
Pathophysiological bases of hyperkeratosis and P-P keratoderma are represented by a compensatory hyperproliferation of the epidermis with hyperproduction of stratum corneum in response to altered cornification of the skin. Blistering disorders caused by mutations in adhesion molecules may show abnormal keratinizing skin lesions (8) and palmoplantar hyperkeratosis that could also be present in autoimmune bullous diseases (9, 10). In fact, hereditary keratinizing and blistering diseases are closely related and often show overlapping genetic backgrounds. In this context, the effects of mutations or specific autoantibodies targeting adhesion proteins may alter the keratinization process, causing hyperkeratosis. A possible explanation lies in the alteration of cell adhesion causing an increased turnover of the keratinocytes, which could provoke retention of the cornified layer (8).
Thus, in the present cases, possibly in the context of a genetic predisposition, the impairment of cell adhesion due to anti-BP180 autoantibodies may have led not only to the formation of BP lesions but also to abnormal keratinization.
Dupilumab is a monoclonal antibody (mAb), binding to the α-subunit of the IL-4 receptor, part of both the IL-4 and IL-13 receptor complex, which are crucial cytokines in the Th2 response. This drug is employed mostly in the treatment of autoimmune disorders, including skin and other systemic diseases, such as atopic dermatitis (AD), eczema, prurigo nodularis, nummular eczema, and spontaneous chronic urticaria as well as allergic respiratory diseases (asthma, bronchopulmonary aspergillosis, chronic eosinophilic pneumonia, rhinitis). Recently, dupilumab has been proposed as alternative BP treatment, due to the evidence of therapeutic efficacy, and reduction of hospitalization time as well as of clinical side effects, which can more frequently be associated with chronic corticosteroid therapy (4, 5). On the other hand, dupilumab was also successfully given to patients affected by genetic skin disorders characterized by abnormal keratinization and pruritus (11–15).
Thus, considering the BP, important pruritus, and diffuse hyperkeratosis and P-P keratoderma of our 2 patients, which had been unresponsive to conventional treatments, we decided to administer dupilumab as off-label tentative therapy.
After some months we have noted a marked improvement of BP together with hyperkeratosis and P-P keratoderma, which were also significantly reduced (Fig. 1: patient 1, panels B and D; patient 2, panels H and L). This was applicable also to pruritic nodular and bullous eruption on the limbs of patient 2 (Fig. 1: panels F, and N). Moreover, both individuals reported a drastic amelioration of itchy symptoms.
In BP, Th2 cells mediate inflammation through the production of cytokines such as IL-4, IL-5, and IL-13, which are also implicated in the recruitment of mast cells, eosinophils, B-cell differentiation, and the switch to production of IgE. The inhibition of Th2-mediated inflammation through the blockage of IL-4/IL-13 signalling may explain the activity of dupilumab regarding BP, abnormal keratinization, and chronic itch in these patients.
In conclusion, we report 2 patients affected by BP with hyperkeratosis and P-P keratoderma, who were resistant to conventional therapies but responded successfully to dupilumab treatment, with an appreciable regression of both hyperkeratosis and P-P keratoderma as well as pruritus. These data confirm and extend the therapeutic value of dupilumab in diseases with abnormal keratinization not only of genetic origin, as previously reported, but also in those associated with an autoimmune response. Thus, dupilumab can also represent a potential promising choice for intractable BP when associated with hyperkeratosis and P-P keratoderma. The biological mechanisms of these encouraging results must be further clarified and explored in depth, representing an interesting future research field.
ACKNOWLEDGEMENTS
IDI-IRCCS is a healthcare provider of the European Reference Network (ERN)-Skin.
REFERENCES
- Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 1: clinical manifestations. J Dtsch Dermatol Ges 2011; 9: 844–856. https://doi.org/10.1111/j.1610-0387.2011.07793.x
- Fania L, Didona D, Pacifico V, Mariotti F, De Luca N, Abeni D, et al. Bullous pemphigoid with hyperkeratosis and palmoplantar keratoderma: three cases. J Dermatol 2018; 45: 1135–1140. https://doi.org/10.1111/1346-8138.14529
- Borradori L, Van Beek N, Feliciani C, Tedbirt B, Antiga E, Bergman R, et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol 2022; 36:1689–1704. https://doi.org/10.1111/jdv.18220
- Zhao L, Wang Q, Liang G, Zhou Y, Yiu N, Yang B, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol 2023; 159: 953–960. https://doi.org/10.1001/jamadermatol.2023.2428
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol 2022; 13: 928621. https://doi.org/10.3389/fimmu.2022.928621
- Huang D, Zhang Y, Yu Y, Jiang Y, Kong L, Ding Y, et al. Long-term efficacy and safety of dupilumab for severe bullous pemphigoid: a prospective cohort study. Int Immunopharmacol 2023; 125: 111157. https://doi.org/10.1016/j.intimp.2023.111157
- Miller AC, Temiz LA, Adjei S, Duran MA, Sassmannshausen J, Dominguez A, et al. Treatment of bullous pemphigoid with dupilumab: a case series of 30 patients. J Drugs Dermatol 2024; 23: e144–e148. https://doi.org/10.36849/JDD.8258
- Hamada T, Tsuruta D, Fukuda S, Ishii N, Teye K, Numata s, et al. How do keratinizing disorders and blistering disorders overlap? Exp Dermatol 2013; 22: 83–87. https://doi.org/10.1111/exd.12021
- Razack EM, Premalatha S, Rao NR, Zahra A. Acanthosis palmaris in a patient with bullous pemphigoid. J Am Acad Dermatol 1987; 16: 217–219. https://doi.org/10.1016/S0190-9622(87)80066-X
- Bolling MC, Mekkes JR, Goldschmidt WF, Van Noesel CJM, Jonkman MF, Pas HH, et al. Acquired palmoplantar keratoderma and immunobullous disease associated with antibodies to desmocollin 3. Br J Dermatol 2007; 157: 168–173. https://doi.org/10.1111/j.1365-2133.2007.07920.x
- Mi Z, Sun F, Wang Z, Zhao Q, Pan F, Yan X, et al. Severe atopic eczema treated by dupilumab in a child with keratitis-ichthyosis-deafness syndrome. Australas J Dermatol 2022; 63: 527–529. https://doi.org/10.1111/ajd.13913
- Yan S, Wu X, Jiang J, Yu S, Fang X, Yang H, et al. Dupilumab improves clinical symptoms in children with Netherton syndrome by suppressing Th2-mediated inflammation. Front Immunol 2022; 13: 1054422. https://doi.org/10.3389/fimmu.2022.1054422
- Odorici G, Schenetti C, Marzola E, Monti A, Borghi A, Corazza M. Treatment of Netherton syndrome with dupilumab. J Dtsch Dermatol Ges 2022; 20: 1636–1640. https://doi.org/10.1111/ddg.14914
- Wu PC, Dai YX, Li CL, Chen CC, Chang YT, Ma SH. Dupilumab in the treatment of genodermatosis: a systematic review. J Dtsch Dermatol Ges 2023; 21: 7–17. https://doi.org/10.1111/ddg.14924
- Galdo G, Fania L. A Netherton syndrome case report: response to dupilumab treatment. Dermatol Ther 2022; 35: e15862. https://doi.org/10.1111/dth.15862