ORIGINAL REPORT
Vascular Characteristics of Treatment-resistant and -responsive Actinic Keratosis Identified with Dynamic Optical Coherence Tomography
GABRIELLA FREDMAN1, MERETE HAEDERSDAL1,2, PETER A. PHILIPSEN1, FLEMMING ANDERSEN3,4, PETER BJERRING3,4, STINE R. WIEGELL1,2 AND GAVRIELLE R. UNTRACHT1,5
1Department of Dermatology, Copenhagen University Hospital, Bispebjerg, Copenhagen, 2Department of Clinical Medicine, Faculty of Health and Medical Science, University of Copenhagen, Copenhagen, 3Skin Center Mølholm, Private Hospital Mølholm, Vejle, 4Department of Dermatology, Aalborg University Hospital, Aalborg, and 5Department of Health Technology, Technical University of Denmark, Kongens Lyngby, Denmark
Treatment-resistant actinic keratosis (AK) is of concern in clinical practice, often requiring retreatment. Microvascular assessments might help differentiate treatment-resistant from treatment-responsive AKs, enabling targeted treatment. Using dynamic optical coherence tomography, AK vascularization was investigated following daylight photodynamic therapy, comparing treatment-resistant with cleared AKs. AKs on face/scalp were graded according to the Olsen Classification Scheme and scanned with dynamic optical coherence tomography pre-treatment, and 3- and 12-months post-treatment. Employing dynamic optical coherence tomography, total vessel length, mean vessel length, mean vessel diameter, vessel area density, and branchpoint density were quantified. Thirty-eight patients with 62 AKs were enrolled, including 37 AK I, 18 AK II, and 7 AK III. Treatment-resistant AKs displayed a trend toward intensified vascularization compared with cleared AK at baseline (AKs I, II), suggested by higher total vessel length (median 144.0, IQR 104.3–186.6) and vessel area density (median 27.7, IQR 18.4–34.2) than in cleared AK (median 120.9, IQR 86.9–143.0 and median 22.9, IQR 17.3–26.8). Additionally, vascularization in treatment-resistant AK I–II appeared disorganized, with trends toward shorter mean vessel length (median 151.0, IQR 138.5–167.5) and increased branchpoint density (median 3.2, IQR 2.3–3.8) compared with cleared AK (median 160.0, IQR 152.0–169.3 and median 2.6, IQR 2.2–3.0). These findings suggest that dynamic optical coherence tomography holds potential to identify treatment-resistant AKs.
SIGNIFICANCE
Treatment-resistant actinic keratosis remains a clinical challenge, often requiring retreatment. This study demonstrates the potential of dynamic optical coherence tomography to differentiate between treatment-resistant and cleared actinic keratosis following daylight photodynamic therapy. Our findings show that treatment-resistant lesions have increased and disorganized vascularization compared with cleared lesions, characterized by a trend of higher total vessel length, vessel area density, shorter mean vessel length, and higher branchpoint density. These results suggest that dynamic optical coherence tomography could be a valuable tool for identifying actinic keratosis that may require further intervention, ultimately enhancing targeted treatment approaches and improving long-term management of patients.
Key words: dynamic optical coherence tomography; OCTAVA; daylight photodynamic therapy; actinic keratosis; skin microvasculature; angiography.
Citation: Acta Derm Venereol 2024; 104: adv42190. DOI: https://doi.org/10.2340/actadv.v104.42190.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Oct 14, 2024; Accepted after revision: Oct 23, 2024; Published: Nov 25, 2024
Corr: Gabriella Fredman MD, Department of Dermatology, Bispebjerg University Hospital, Bispebjerg Bakke 23, DK-2400 Copenhagen NV, Denmark. E-mail: gabloufre@gmail.com
Competing interests and funding: MH has served as a consultant for Galderma and received honoraria for speaking, teaching and research. GF, PP, FA, PB, SW, and GU declare no conflicts of interest.
The work was completed within the framework of the Skin Cancer Innovation Clinical Academic Group (SCIN-CAG)/Greater Copenhagen Health Science Partners (GCHSP) and the Danish Research Center for Skin Cancer, a public–private research partnership between the Private Hospital Mølholm, Aalborg University Hospital, and Copenhagen University Hospital, Bispebjerg and Frederiksberg. GME provided the MultiLite daylight lamps.
INTRODUCTION
Actinic keratosis (AK) is a precancerous disease that develops in chronically sun-exposed and photodamaged skin (1). Lesions predominantly occur within fields of preneoplastic alterations caused by prolonged ultraviolet radiation (UV) exposure (1). Among the white population, AK is the most common neoplasm, with a global prevalence of 14% and a high incidence in the elderly (2). Lesions have a low risk of progressing to squamous cell carcinoma (SCC), while the majority remain chronic or may spontaneously regress if left untreated (1, 3).
In addition to preventing malignant transformation, AK management is tailored according to patient preferences and severity of symptoms such as itching, tenderness of the skin, and cosmetic concerns (1, 4, 5). Currently, most AK interventions are directed towards the entire cancerized field due to their convenience and ability to mitigate subclinical damage (1, 6). This management strategy not only reduces the risk of recurrence but also limits development of new lesions (1).
Common to all types of field-treatments is that some AKs may persist and exhibit therapeutic resistance (7). These therapy-resistant lesions require additional treatment, potentially affecting the patient’s quality of life due to symptoms associated with the treatment and the time spent on multiple treatment sessions. At present, the absence of clinical criteria for identifying treatment-resistant AKs (trAK) makes it difficult to recognize these lesions prior to treatment commencement. In most clinical trials, primary efficacy endpoint is reported as lesion clearance or recurrence rates, often evaluated at 3 to 6 months (8, 9). Assessing long-term efficacy is often difficult due to the potential development of new AKs in the treated field (10). Therefore, continuous and spatially mapped monitoring of treated AKs is necessary to thoroughly study trAK (8, 10).
Non-invasive imaging tools are increasingly applied alongside clinical examination and dermatoscopy to monitor response after field-directed therapies (1, 11–15). Among these, dynamic optical coherence tomography (D-OCT), in particular, holds promise because it is commercially available and provides both qualitative and quantitative measurements of blood vessels in addition to structural changes of the skin (16).
Recent advancements in automated D-OCT image analysis have enabled detailed examination of the vascular network (17, 18). In a previous study, we applied D-OCT with automated vascular analysis to explore subsurface differences between clinical AK grades I–III and photodamaged skin (PD) (19). This study revealed a quantifiable relationship between the degree of vascularization and the level of disorganization within the vascular network across all AK grades compared with PD skin. Until now, no studies have investigated vascular changes in response to field-directed treatments. Daylight photodynamic therapy (dPDT) is often preferred among treatments because it can be completed in a single session and causes less pain than conventional photodynamic therapy (PDT) (20). Although treatment efficacy is high up to 3 months post-treatment, resistant lesions may still persist, requiring additional therapeutic interventions (21). Based on our previous observations, we hypothesize that vascular assessment could provide insights into treatment response. A more in-depth characterization of trAK may help identify cases that need more aggressive treatment than standard care. Ultimately, this targeted strategy may improve outcomes and reduce the risk of recurrence. Therefore, the aim of this study was to investigate vascular features in trAK, comparing these findings with AK showing complete clearance (clAK) 12 months after treatment with dPDT.
MATERIALS AND METHODS
Design and ethical considerations
As part of a larger investigation, this exploratory, clinical study was approved by the Danish Medicine Agency (EudraCT 2021-0015860-21), the Regional Ethics Committee of Region Hovedstaden (H-78842), and the Danish Data Protection Agency. The unit for Good Clinical Practice monitored the study, which was carried out in compliance with the Declaration of Helsinki. All patients signed an informed consent agreement before enrolment.
Study population
This study recruited patients from October 2021 to November 2022 among patients referred to the Department of Dermatology, Copenhagen University Hospital, Bispebjerg and Frederiksberg in Copenhagen. Follow-up was undertaken from November 2021 to June 2023. Inclusion criteria were age above 18 in patients presenting with AKs on face or scalp. Exclusion criteria were any AK treatment inside the test areas within the previous 3 months, immunosuppression, pregnancy, and contraindications to PDT.
Daylight photodynamic therapy: treatment procedure
The treatment methodology of the larger investigation, upon which this study extends, has previously been described (22). Briefly, a board-certified dermatologist identified AKs on the face or scalp, which were marked and numbered on a transparency to enable reidentification at follow-up. Each AK was graded according to clinically evaluated thickness into I: mild (slightly palpable, more easily felt than seen), II: moderate (moderately thick, easily felt), III: severe (very thick or obvious AK) (23). For the current study, at least one AK grade I, II, III, and adjacent PD skin were scanned with D-OCT at baseline before dPDT and at each follow-up visit at 3 and 12 months. All non-complete responding AKs were graded and recorded on the original transparency created at baseline.
Following clinical grading and D-OCT at baseline, the treatment area underwent superficial curettage before application of methyl aminolevulinate (MAL) cream (Metvix® 160 mg/g, Galderma Nordica AB, Uppsala, Sweden) (22). After a 30-min incubation period, the treatment area was illuminated with daylight for 2 h. Depending on the weather conditions, illumination was carried out using either outdoor or indoor daylight. For outdoor daylight exposure, patients were positioned either inside or outside a greenhouse located in the hospital garden. Patients exposed to outdoor daylight applied an organic sunscreen (SPF 20®, P20, Riemann & Co A/S, Hillerød, Denmark) 15 min prior to application of MAL cream (22).
For indoor daylight illumination, we used a MultiLite® lamp (German Medical Engineering, Erlangen, Germany), which emits light at wavelengths of 415, 585, and 635 nm, corresponding to the absorption spectrum of protoporphyrin IX (PpIX). Indoor illumination was performed for 2 h at a skin-to-lamp distance of 50 cm, resulting in a total PpIX-weighted effective light dose of 15 J/cm². Although we aimed for a 0° angle between the incoming light and the skin, this was not always achievable due to the curved surfaces of the face and scalp. Following daylight exposure, any remaining MAL cream was removed, and patients were advised to remain indoors for the remainder of the day (22).
Treatment response assessment
In this study, clinical treatment response was assessed at 3 and 12 months’ follow-up. At each follow-up, treatment response was defined as either complete (total lesion disappearance, confirmed visually and by palpation) or non-complete, as described in our previously published paper (22). In the examination of vascular features, the treatment-resistant vs treatment-responsive AK I and AK II were combined. Furthermore, recurring AKs were classified as treatment-resistant.
Dynamic optical coherence tomography: imaging procedure
In our study, we utilized a commercially available D-OCT scanner (Vivosight Dx, Michelson Diagnostics, Kent, UK). This scanner operates with a centre wavelength of 1,305 nm, providing a lateral resolution of less than 7.5 µm and an axial resolution of less than 5 µm, with a field-of-view measuring 6 x 6 mm (16). Structural OCT images of the skin are obtained up to a depth of 1.5 mm, with the Vivosight software automatically generating the corresponding vascular D-OCT images. Each D-OCT en-face image is displayed on the structural OCT en-face image, enabling visualization of vessels up to a depth of 500 µm (16). Imaging beyond this depth was limited due to speckle and a low signal-to-noise ratio in deeper skin layers (16). In our study, we captured volumetric images using 250 B-scans in the x–z plane.
Automated image analysis and extraction of vascular measurements
We generated maximum intensity projection images (MIP) of the superficial plexus from each D-OCT scan using MATLAB 2023a (Mathworks Inc, Natick, MA, USA) (19). These images were centred at a depth of 300 µm from the skin surface with a thickness of 100 µm. Subsequently, quantitative vascular parameters were then extracted from the MIP images using OCTAVA software (v2, MATLAB App version) (17). OCTAVA segments the vessels from the background in the MIP images and extracts skeletal representations to assess the vascular network’s connectivity (17). This software identifies nodes and calculates segment lengths through graph analysis, and vessel diameters using a Euclidean distance transform. All metrics were recorded in an Excel file (Microsoft Corp, Redmond, WA, USA) for further analysis.
For this study, we processed all images in batch mode using uniform settings. We applied the Fuzzy means technique for segmentation, followed by the Frangi filter with a kernel size range from 1 to 8. These settings were previously optimized for D-OCT images of skin (24).
As specified in our previous studies, OCTAVA quantifies total vessel length and vessel area density (VAD) to evaluate the extent of the vascular network (17, 19). Additionally, the software calculates mean blood vessel measurements such as mean length and mean diameter, which are essential for assessing vascular capacity. The organization of the vascular network is assessed using additional parameters, including branchpoint density (BD), which measures vessel connectivity by indicating the frequency of vessel branches. Furthermore, the degree of twisting and turning of blood vessels, referred to as mean tortuosity, can affect tissue perfusion. We also assessed changes in fractal dimension (FD), as this parameter reflects irregular and complex blood vessel formation (25).
Statistics
Descriptive statistics were conducted for vascular measurements provided by OCTAVA, with results visualized as boxplots. Data were reported as median (interquartile range [IQR]), and normality tested with the Kolmogorov–Smirnov test. We conducted non-parametric statistics as several parameters did not align with a normal distribution. Accordingly, to investigate differences in vascular parameters between treatment-resistant vs treatment-responsive AK, we applied the Mann–Whitney U tests. Only statistically significant p-values < 0.05 for vascular differences between trAKs and cleared AKs are reported in the main text. Non-significant p-values and significant findings not central to the main conclusions are presented in Table S3. Statistical analyses were carried out using SPSS Statistics software (Version 29; IBM Corp, Armonk, NY, USA).
RESULTS
In this study, we enrolled 38 patients with Fitzpatrick skin types I–III. All patients received treatment with dPDT on either face (n = 28) or scalp (n = 10) and attended follow-up visits at 3 and 12 months. Patients presented with a total of 62 AKs, which included 37 AK I, 18 AK II, 7 AK III, as well as 35 PD skin sites. All lesions underwent longitudinal monitoring with D-OCT assessment before dPDT at baseline as well as at each follow-up visit (Fig. 1).
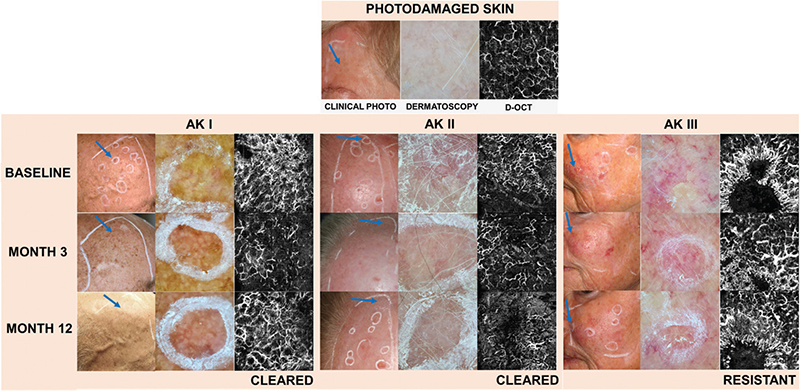
Fig. 1. Clinical photos, dermatoscopic images, and D-OCT scans of treated actinic keratosis I–III. This illustration highlights changes in vascular features of actinic keratosis (AK) lesions over time, demonstrating the effectiveness of treatments and the persistence of vascular features in resistant cases. In the clinical photographs, each imaged AK lesion is indicated with a blue arrow. At baseline, untreated AKs I–III exhibit a higher level of vascularization and disorganization of the vascular network compared with PD skin. At 3 and 12 months after dPDT, AKs I–II were effectively eliminated, with less vascularization observed during follow-up compared with baseline. In contrast, AK III exhibited treatment resistance, and displayed continued presence of radiating vessels and disorganized vessel growth.
One year after dPDT, 36 AK had completely cleared, whereas 26 AKs demonstrated treatment resistance. Among treatment-resistant lesions, 13 AKs initially cleared but reoccurred during the study period (Table I). Of all assessed AK I, 35.1% (13/37) were treatment-resistant, compared with 44.4% (8/18) in AK II, and 71.4% (5/7) in AK III.
In PD skin, vascularization remained relatively constant after dPDT throughout the study period. In contrast, longitudinal monitoring revealed differences in vascularization between trAK and clAK (Fig. 2 and Tables SI–SIII). To assess the overall trends differentiating trAK and clAK, we merged AK I and AK II due to relatively low sample size and the similarity in their vascular patterns (19, 26); AK III was excluded in this part of the analysis due to thick hyperkeratosis in some lesions, which could confound the results. As described in our previous study, hyperkeratosis can obscure vessel detection and impede reliable assessment of untreated AK III (19). We also present longitudinal data for each AK grade separately to ensure completeness. The vascular analyses split by AK grade are shown in Fig. 3 and Table SII (19).
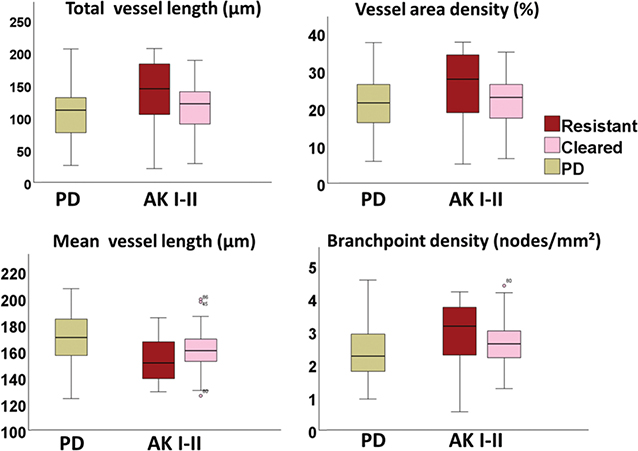
Fig. 2. Boxplots illustrating vascular differences at baseline between treatment-resistant and responsive actinic keratosis I–II and photodamaged skin. The boxplots compare vascular parameters across treatment-resistant and treatment-responsive actinic keratosis (AK) I–II and photodamaged skin (PD). For each parameter, the horizontal line in each box indicates the median value. Boxes indicate the interquartile range, encompassing the 25th and 75th percentile, while the whiskers extend to the minimum and maximum values. Outliers are marked as individual points outside of the range of the whiskers. Treatment-resistant AK I–II exhibits increased vascularization, with higher total vessel length and vessel area density compared with treatment-responsive AK. Moreover, treatment-resistant AKs appear with a disorganized vascular network, characterized by shorter mean vessel length and increased branch point density.
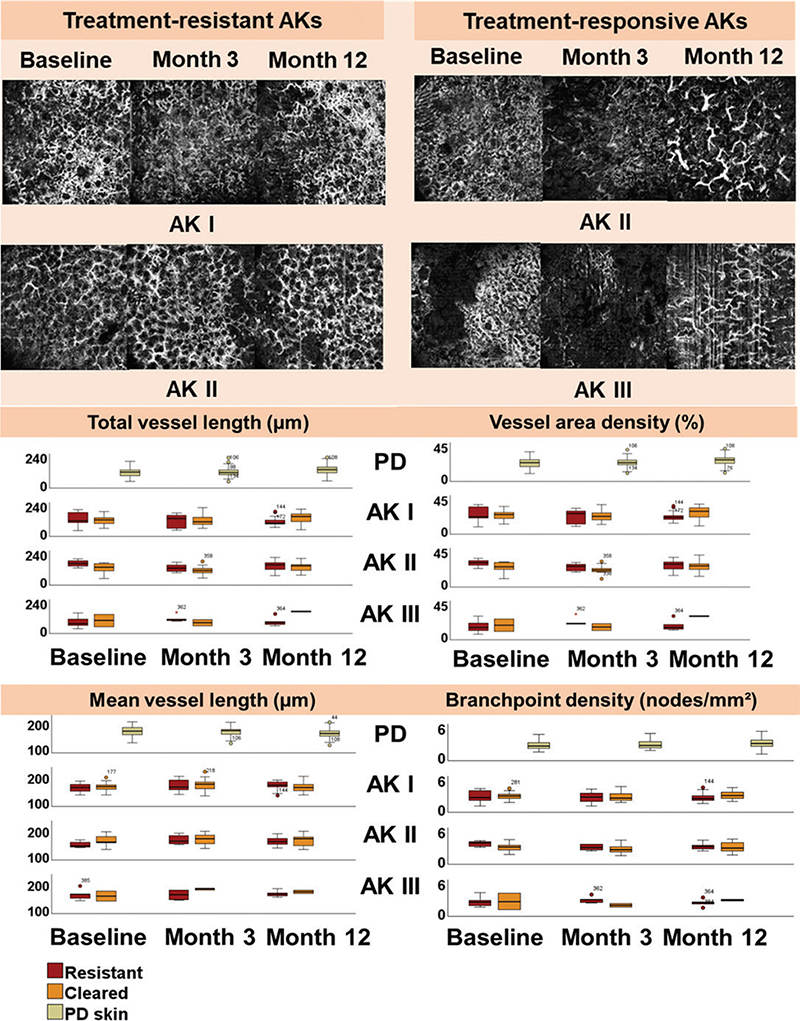
Fig. 3. D-OCT scans and boxplots illustrating vascular changes in actinic keratosis after daylight photodynamic therapy. The D-OCT scans demonstrate vascular changes in treatment-resistant and treatment-responsive actinic keratosis (AK) at baseline, and at 3 and 12 months after field-treatment with daylight photodynamic therapy. Treatment-resistant AK: Throughout the study period, a high vessel density and a disorganized vascular network are observed consistently throughout the study period. Treatment-responsive AK: At baseline, the vascular network is dense. At 3 and 12 months, the vascular network becomes more reticular with reduced vascularization. The boxplots illustrate trends in vascular differences between treatment-resistant and treatment-responsive AK I, II, and III, compared with photodamaged skin (PD) at baseline, and at 3 and 12 months post-treatment. For each parameter, the horizontal line in each box indicates the median value. Boxes indicate the interquartile range, encompassing the 25th and 75th percentile, while the whiskers extend to the minimum and maximum values. Outliers are marked as individual points outside of the range of the whiskers.
Baseline characteristics of AKs resistant to treatment versus treatment responsive AK
Assessment of vascular features in trAK and clAK showed variations in multiple parameters at baseline (Fig. 2). These parameters primarily related to the degree of vascularization and organization of the vascular network.
In our overall assessment, we observed that trAK exhibited more intense vascularization compared with treatment-responsive AK at baseline (Fig. 2 and Table SI). This observation was supported by a higher total vessel length (median 144.0, IQR 104.3–186.6) and VAD (median 27.7, IQR 18.4–34.2) in trAK than in clAK (median 120.9, IQR 86.9–143.0 and median 22.9, IQR 17.3–26.8, respectively). Additionally, vessels in trAK had a shorter mean length (median 151.0, IQR 138.5–167.5) compared witho those in clAKs (median 160.0, IQR 152.0–169.3) (Table SI).
These observations aligned with the presence of a disorganized vascular network in trAKs. In trAK, vascularization presented a more branched network. This observation was revealed through an increased BD in trAK (median 3.2, IQR 2.3–3.8) relative to clAK (median 2.6, IQR 2.2–3.0) at baseline.
Analysis split by AK grade revealed near-significantly higher total vessel length and VAD (both p = 0.055), along with a significantly higher BD (p = 0.043) in trAK II (n = 8) compared with clAK II (n = 10).
Changes in vascular features 3 months post-dPDT
Similar to the vascular differences between trAK and clAK observed at baseline, trAK displayed more extensive vascularization compared with clAKs at 3 months’ follow-up. As such, trAK appeared with a higher total vessel length (median 127.3, IQR 79.8–150.6) and VAD (median 24.5, IQR 16.0–27.9) compared with AKs that had cleared (median 99.7, IQR 75.4–138.0 and median 20.0, IQR 15.8–25.3).
In addition, organization of the vascular network also presented differently between trAK compared with clAK. In trAK, our vascular assessments indicated disorganization of vessels compared with AKs that had cleared. In trAK, vessels appeared with a lower mean vessel length (median 160.5, IQR 149.0–183.5), compared with clAK (median 172.0, IQR 151.0–182.5). However, BD in trAK (median 2.4, IQR 2.0–3.3), was similar to that in clAK (median 2.4, IQR 1.7–3.1).
Changes in vascular features 12 months post-dPDT
In contrast to our findings at baseline and 3 months’ follow-up, we observed different trends in vascularization between trAK and clAK 1 year after dPDT. At 12 months, the extent of vascularization was reduced in trAK compared with clAK. Thus, in trAK, total vessel length (median 102.6, IQR 80.5–158.1) and VAD (median 21.2, IQR 16.1–29.6) were significantly lower than in clAK (median 141.8, IQR 99.7–164.8 and median 26.0, IQR 19.3–32.7) (p = 0.035 and p = 0.046, respectively) (Table SIII).
At 12 months, vessel organization appeared similar between trAK and clAK. As such, mean vessel length (median 165.0, IQR 154.5–179.0) and BD (median 2.5, IQR 1.9–3.1) in trAK were comparable to AKs that had completely cleared after dPDT (median 166.5, IQR 146.0–172.0 and median 2.6, IQR 2.1–3.4).
Of note, vascular assessments split by AK grade revealed contradictory trends between AK I and AK II. Specifically, trAK I displayed a trend of reduced vascularization compared with clAK I, 1 year after treatment. Additionally, clAK I presented with a lower mean vessel length combined with a higher BD than trAK by the end of our study (Fig. 3 and Table SII). In contrast, assessment of trAK II followed the same trend as observed at baseline and at 3 months’ follow-up. At 12 months, trAK II appeared with an increased vascularization compared with clAK II, with a higher total vessel length and VAD than clAK II (Table SII). Moreover, we observed a shorter mean vessel length combined with an increased BD in trAK II group compared with clAK II, which aligned with our observations at baseline and 3 months (Table SII).
Finally, OCTAVA provided several additional measurements as illustrated in Tables SI–SIII. None of these parameters revealed clear trends, although they provided insights into the variability and nuances of our data.
DISCUSSION
In this study, we applied D-OCT with automated vascular analysis to investigate subsurface differences between treatment-resistant and treatment-responsive AK treated with dPDT. Our study demonstrated a trend of vascular differences between trAK and AK that showed complete clearance 1 year post-treatment. These differences were observed at baseline prior to treatment initiation, with trAK showing more intense and disorganized vascularization compared with treatment-responsive AK. At 3 months’ follow-up this trend persisted, showing a greater level of vascularization and disorganization in trAK than clAK. In contrast, by 12 months, vascularization in trAK was reduced relative to cleared skin, but with similar level of vessel organization. Based on our findings, D-OCT was able to distinguish between trAK and clAK. This observation suggests that D-OCT may be beneficial in treatment decision-making by offering a more precise method for predicting and monitoring treatment outcome.
Given the increased risk of AK development in field-cancerized skin, repeated treatments and long-term management are necessary to avoid recurrence and possible malignant transformation (1, 27, 28). At present, guidelines recommend treating all AKs without differentiating lesions based on risk assessment or the likelihood of individual lesion progression (7). Still, clinical practice demonstrates that achieving sustained clearance remains difficult in patients with severely photodamaged skin (10, 14). Our study found that nearly half of the treated AKs were resistant to dPDT, highlighting this difficulty.
The proportions of cleared AK grades I–III were lower than 12 months’ clearance rates reported in previous literature (21, 29, 30). A possible explanation might be that we focused on a relatively small number of lesions, without considering the overall disease burden and calculating the total clearance rate for the whole treated area.
Among all assessed AKs I–III, most treatment-resistant lesions were grade II or III. Our previous study demonstrated a significant increase in vascularization in thick AK III compared with thin AK I (26). These findings, along with the current study’s results, suggest that the degree of vascularization within individual lesions may be an important factor when establishing a treatment plan. Identifying lesions that are difficult to treat may aid in determining when to employ more aggressive treatment options such as combination or procedural therapies. Tailored treatment plans could potentially improve outcomes in AK patients, offering a more personalized approach to disease management.
In our study, trAKs displayed more intense and disorganized vascularization than AK that cleared after dPDT. Previous research has observed that trAKs share distinct clinical and histological characteristics compared with treatment naive lesions (7). Clinically, trAKs tend to be more painful, while histologically they show acantholysis along with considerable basal proliferation (7). A previous study has described the common presence of acantholysis and basal proliferation in AKs of organ transplant recipients (OTR) (31). The study concluded that these histological features could potentially serve as high-risk factors for malignant transformation, given that AKs in OTRs carry a high risk of developing into SCC (32). Thus, the distinct characteristics of trAK found in our study, and in previous research, suggest trAKs possess traits that may explain their reduced response to treatment. These observations highlight the need for targeted treatment strategies for trAKs, considering their unique clinical, subsurface, and histological profiles (7, 31).
Prior studies have employed non-invasive techniques to evaluate the attributes of recurring AKs following field-directed therapies (11, 33–38). Most of these studies indicate that AK characteristics present before treatment tend to persist in recurring lesions. Studies using dermatoscopy and reflectance confocal microscopy have revealed the presence of enhanced vascularization and polymorphous vessels in trAK following PDT or imiquimod treatment (35, 37). In our study, similar trends were observed at least 3 months post-treatment, with trAK exhibiting increased and disorganized vascularization compared with clAK (Fig. 3). However, at 12 months, an opposite trend emerged, with significantly increased vascularization observed in clAK compared with trAK. Given the extended duration since treatment and the relatively small sample size of this study, these findings should be interpreted with caution. Nevertheless, our observations highlight the need for further investigation in larger datasets to validate these results.
The continued presence of subsurface AK characteristics has been interpreted in various ways. Some studies suggest that this persistence indicates subclinical AK, emphasizing the need for additional therapy (11, 33–36). Other explanations propose that regeneration of epidermis and remodelling of the dermis contribute to these lingering features in skin confirmed as clear through clinical or histological examination, which could be useful for tracking the effects from different types of treatments (37, 38). Given these different interpretations, additional studies are needed to understand how to utilize imaging techniques effectively to monitor and guide treatment outcome for AK.
Strengths and limitations
Strengths of this study include the in-vivo monitoring and long-term follow up of AK treated with dPDT. As shown in our study, a subset of lesions cleared immediately after dPDT but reoccurred at 12 months. This finding highlights that long-term monitoring is important to fully assess treatment efficacy. In addition, applying automated quantified assessments improves the reliability of D-OCT evaluations compared with qualitative image analysis.
Limitations includes the small sample size, which might have compromised the reliability of our results. This constraint emphasizes the importance of performing analogous analyses on a larger dataset to expand the use of our results in a broader setting. Although most vascular differences between trAK and clAK were insignificant, likely due to the small sample size, we still identified distinct trends. Therefore, our study underscores the need for additional investigation using a larger sample size.
Conclusion
D-OCT identified trends of subsurface vascular differences between treatment-resistant AK and treatment-responsive AK post-dPDT. At baseline, AKs that were resistant to treatment showed more intense and disorganized vascularization compared with AKs that cleared after therapy. These findings emphasize the potential of D-OCT to identify AKs that may need additional treatment.
ACKNOWLEDGEMENTS
IRB approval status: The clinical study was approved by the Danish Medicine Agency (EudraCT 2021-0015860-21), the Regional Ethics Committee of Region Hovedstaden (H-78842), and the Danish Data Protection Agency.
REFERENCES
- Kandolf L, Peris K, Malvehy J, Mosterd K, Heppt MV, Fargnoli MC, et al. European consensus-based interdisciplinary guideline for diagnosis, treatment and prevention of actinic keratoses, epithelial UV-induced dysplasia and field cancerization on behalf of European Association of Dermato-Oncology, European Dermatology Forum, European Academy of Dermatology and Venereology and Union of Medical Specialists (Union Européenne des Médecins Spécialistes). J Eur Acad Dermatol Venereol 2024; 38: 1024–1047. https://doi.org/10.1111/jdv.19897
- George CD, Lee T, Hollestein LM, Asgari MM, Nijsten T. Global epidemiology of actinic keratosis in the general population: a systematic review and meta-analysis. Br J Dermatol 2024; 190: 465–476. https://doi.org/10.1093/bjd/ljad371
- Werner RN, Sammain A, Erdmann R, Hartmann V, Stockfleth E, Nast A. The natural history of actinic keratosis: a systematic review. Br J Dermatol 2013; 169: 502–518. https://doi.org/10.1111/bjd.12420
- de Berker D, McGregor J, Mohd Mustapa M, Exton L, Hughes B. British Association of Dermatologists’ guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol 2017; 176: 20–43. https://doi.org/10.1111/bjd.15107
- Morton C, Baharlou S, Basset-Seguin N, Calzavara-Pinton P, Dirschka T, Gilaberte Y, et al. Expert recommendations on facilitating personalized approaches to long-term management of actinic keratosis: the Personalizing Actinic Keratosis Treatment (PAKT) project. Acta Derm Venereol 2023; 103: adv6229. https://doi.org/10.2340/actadv.v103.6229
- Aggarwal I, Puyana C, Chandan N, Jetter N, Tsoukas M. Field cancerization therapies for the management of actinic keratosis: an updated review. Am J Clin Dermatol 2024; 25: 391–405. https://doi.org/10.1007/s40257-023-00839-8
- Schmitz L, Brehmer A, Falkenberg C, Gambichler T, Heppt MV, Steeb T, et al. Actinic keratosis grading. Ital J Dermatol Venereol 2021; 156: 213–219. https://doi.org/10.23736/S2784-8671.21.06892-9
- Reynolds KA, Schlessinger DI, Vasic J, Iyengar S, Qaseem Y, Behshad R, et al. Core outcome set for actinic keratosis clinical trials. JAMA Dermatol 2020; 156: 326–333. https://doi.org/10.1001/jamadermatol.2019.4212
- Steeb T, Wessely A, Petzold A, Schmitz L, Dirschka T, Berking C, et al. How to assess the efficacy of interventions for actinic keratosis? A review with a focus on long-term results. J Clin Med 2021; 10: 4736. https://doi.org/10.3390/jcm10204736
- Steeb T, Wessely A, Petzold A, Brinker TJ, Schmitz L, Leiter U, et al. Evaluation of long-term clearance rates of interventions for actinic keratosis: a systematic review and network meta-analysis. JAMA Dermatol 2021; 157: 1066–1077. https://doi.org/10.1001/jamadermatol.2021.2779
- Benati E, Longhitano S, Pampena R, Mirra M, Raucci M, Pellacani G, et al. Digital follow-up by means of dermatoscopy and reflectance confocal microscopy of actinic keratosis treated with Imiquimod 3.75% cream. J Eur Acad Dermatol Venereol JEADV 2020; 34: 1471–1477. https://doi.org/10.1111/jdv.16143
- Curiel-Lewandrowski C, Myrdal CN, Saboda K, Hu C, Arzberger E, Pellacani G, et al. In vivo reflectance confocal microscopy as a response monitoring tool for actinic keratoses undergoing cryotherapy and photodynamic therapy. Cancers 2021; 13: 5488. https://doi.org/10.3390/cancers13215488
- Ishioka P, Maia M, Rodrigues SB, Lellis RF, Hirata SH. In vivo confocal laser microscopy for monitoring of actinic keratosis treatment: a comparison with histopathologic assessment after treatment with topical 5% 5-fluorouracil. J Eur Acad Dermatol Venereol 2018; 32: 1155–1163. https://doi.org/10.1111/jdv.14716
- Markowitz O, Wang K, Levine A, Schwartz M, Minhas S, Feldman E, et al. Noninvasive long-term monitoring of actinic keratosis and field cancerization following treatment with ingenol mebutate gel 0.015. J Clin Aesthetic Dermatol 2017; 10: 28–33.
- Markowitz O, Schwartz M, Feldman E, Bieber A, Bienenfeld A, Nandanan N, et al. Defining field cancerization of the skin using noninvasive optical coherence tomography imaging to detect and monitor actinic keratosis in ingenol mebutate 0.015%-treated patients. J Clin Aesthetic Dermatol 2016; 9: 18–25.
- Ulrich M, Themstrup L, de Carvalho N, Manfredi M, Grana C, Ciardo S, et al. Dynamic optical coherence tomography in dermatology. Dermatology 2016; 232: 298–311. https://doi.org/10.1159/000444706
- Untracht GR, Matos RS, Dikaios N, Bapir M, Durrani AK, Butsabong T, et al. OCTAVA: an open-source toolbox for quantitative analysis of optical coherence tomography angiography images. PloS One 2021; 16: e0261052. https://doi.org/10.1371/journal.pone.0261052
- Untracht GR, Dikaios N, Durrani AK, Bapir M, Sarunic MV, Sampson DD, et al. Pilot study of optical coherence tomography angiography-derived microvascular metrics in hands and feet of healthy and diabetic people. Sci Rep 2023; 13: 1122. https://doi.org/10.1038/s41598-022-26871-y
- Fredman G, Wiegell SR, Haedersdal M, Untracht GR. Vascular feature identification in actinic keratosis grades I-III using dynamic optical coherence tomography with automated, quantitative analysis. Arch Dermatol Res 2024; 316: 391. https://doi.org/10.1007/s00403-024-03022-z
- Fargnoli MC, Piccioni A, Neri L, Tambone S, Pellegrini C, Peris K. Conventional vs. daylight methyl aminolevulinate photodynamic therapy for actinic keratosis of the face and scalp: an intra-patient, prospective, comparison study in Italy. J Eur Acad Dermatol Venereol 2015; 29: 1926–1932. https://doi.org/10.1111/jdv.13076
- Fargnoli MC, Piccioni A, Neri L, Tambone S, Pellegrini C, Peris K. Long-term efficacy and safety of daylight photodynamic therapy with methyl amninolevulinate for actinic keratosis of the face and scalp. Eur J Dermatol EJD 2017; 27: 89–91. https://doi.org/10.1684/ejd.2016.2882
- Wiegell SR, Fredman G, Andersen F, Bjerring P, Paasch U, Hædersdal M. Pre-treatment with topical 5-fluorouracil increases the efficacy of daylight photodynamic therapy for actinic keratoses: a randomized controlled trial. Photodiagnosis Photodyn Ther 2024; 46: 104069. https://doi.org/10.1016/j.pdpdt.2024.104069
- Olsen EA, Abernethy ML, Kulp-Shorten C, Callen JP, Glazer SD, Huntley A, et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol 1991; 24: 738–743. https://doi.org/10.1016/0190-9622(91)70113-G
- Untracht GR, Durkee MS, Zhao M, Kwok-Cheung Lam A, Sikorski BL, Sarunic MV, et al. Towards standardising retinal OCT angiography image analysis with open-source toolbox OCTAVA. Sci Rep 2024; 14: 5979. https://doi.org/10.1038/s41598-024-53501-6
- Reif R, Qin J, An L, Zhi Z, Dziennis S, Wang R. Quantifying optical microangiography images obtained from a spectral domain optical coherence tomography system. Int J Biomed Imaging 2012; 2012: 509783. https://doi.org/10.1155/2012/509783
- Fredman G, Fuchs CSK, Wenande E, Philipsen PA, Untracht GR, Andersen F, et al. Dynamic optical coherence tomography unveils subclinical, vascular differences across actinic keratosis grades I–III. Exp Dermatol 2024; 33: e15153. https://doi.org/10.1111/exd.15153
- Eisen DB, Asgari MM, Bennett DD, Connolly SM, Dellavalle RP, Freeman EE, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol 2021; 85: e209–233. https://doi.org/10.1016/j.jaad.2021.02.082
- Marks R, Rennie G, Selwood Thomas S. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1988; 331: 795–797. https://doi.org/10.1016/S0140-6736(88)91658-3
- Dirschka T, Ekanayake-Bohlig S, Dominicus R, Aschoff R, Herrera-Ceballos E, Botella-Estrada R, et al. A randomized, intraindividual, non-inferiority, Phase III study comparing daylight photodynamic therapy with BF-200 ALA gel and MAL cream for the treatment of actinic keratosis. J Eur Acad Dermatol Venereol 2019; 33: 288–297. https://doi.org/10.1111/jdv.15185
- Jansen MHE, Kessels JPHM, Nelemans PJ, Kouloubis N, Arits AHMM, van Pelt HPA, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med 2019; 380: 935–946. https://doi.org/10.1056/NEJMoa1811850
- Falkenberg C, Dirschka T, Gilbert G, Stockfleth E, Homey B, Schmitz L. Basal proliferation and acantholysis may represent histological high-risk factors for progression into invasive squamous cell carcinoma: a comparison study in solid organ transplant recipients and matched immunocompetent patients. Cancers 2023; 15: 1765. https://doi.org/10.3390/cancers15061765
- Szeimies RM, Ulrich C, Ferrándiz-Pulido C, Hofbauer GFL, Lear JT, Lebbé C, et al. The “Personalising Actinic Keratosis Treatment for Immunocompromised Patients” (IM-PAKT) project: an expert panel opinion. Dermatol Ther 2024; 14: 1739–1753. https://doi.org/10.1007/s13555-024-01215-y
- Ruini C, Hartmann D, Bastian M, Ruzicka T, French LE, Berking C, et al. Non-invasive monitoring of subclinical and clinical actinic keratosis of face and scalp under topical treatment with ingenol mebutate gel 150 mcg/g by means of reflectance confocal microscopy and optical coherence tomography: new perspectives and comparison of diagnostic techniques. Biophotonics 2019; 12: e201800391. https://doi.org/10.1002/jbio.201800391
- Soare C, Cozma EC, Celarel AM, Rosca AM, Lupu M, Voiculescu VM. Digitally enhanced methods for the diagnosis and monitoring of treatment responses in actinic keratoses: a new avenue in personalized skin care. Cancers 2024; 16: 484. https://doi.org/10.3390/cancers16030484
- Kaçar N, Sanli B, Zalaudek I, Yildiz N, Ergin S. Dermatoscopy for monitoring treatment of actinic keratosis with imiquimod. Clin Exp Dermatol 2012; 37: 567–569. https://doi.org/10.1111/j.1365-2230.2011.04272.x
- Mazur E, Kwiatkowska D, Reich A. Reflectance confocal microscopy and dermoscopy of facial pigmented and non-pigmented actinic keratosis features before and after photodynamic therapy treatment. Cancers 2023; 15: 5598. https://doi.org/10.3390/cancers15235598
- Lee JH, Won CY, Kim GM, Kim SY. Dermoscopic features of actinic keratosis and follow up with dermoscopy: a pilot study. J Dermatol 2014; 41: 487–493. https://doi.org/10.1111/1346-8138.12282
- Wang T, Han Q, Hu W, Ren H. Efficacy evaluation and dermoscopy predictors of photodynamic therapy with different pretreatments in the treatment of actinic keratosis. J Dermatol Treat 2022; 33: 2853–2857. https://doi.org/10.1080/09546634.2022.2089325