RESEARCH LETTER
Peripheral Blood Mean Platelet Volume and the Ratio of Mean Platelet Volume to Platelet Count as Prognostic Biomarkers in Patients with Cutaneous Angiosarcoma of the Head and Neck: A Retrospective Cohort Study
Ken HORISAKI, Tomoki TAKI*, Shoichiro MORI, Mao OKUMURA and Masashi AKIYAMA
Department of Dermatology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Aichi 466-8550, Japan.
Citation: Acta Derm Venereol 2024; 104: adv42227. DOI: https://doi.org/10.2340/actadv.v104.42227.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: October 10, 2024; Accepted: October 21, 2024. Published: Nov 7, 2024
*E-mail: white7petrolatum@gmail.com
To the Editor,
Mean platelet volume (MPV) and the ratio of MPV to platelet count (MPV/PC) have been reported to have great prognostic value in malignancies. This study addresses whether MPV and MPV/PC are useful prognostic indicators in angiosarcoma of the head and neck (ASHN).
Our retrospective study involved 45 patients who were histologically diagnosed with ASHN and underwent chemotherapy for ASHN at our hospital from February 2006 to December 2022. This study was approved by the institutional review board of the Nagoya University Graduate School of Medicine. The Mann–Whitney U test and Fisher’s exact test were used to analyse correlations between MPV and clinical features/laboratory findings. We applied receiver operating characteristic curve analysis to calculate the MPV cutoff value. Survival curves were drawn using the Kaplan–Meier method, and differences in survival were assessed using the log-rank test. The same analysis was performed for MPV/PC. We then performed multivariate analyses using the Cox proportional hazards model, including pretreatment factors and MPV/PC.
The baseline characteristics of the 45 patients with ASHN included in the present study are given in Table I and Table SI. The MPV and the MPV/PC cutoff values were defined as 10.0 (fL) and 0.06 (fL/103μL). The high-MPV group consisted of 16 patients whose MPV equalled or exceeded 10.0 (fL); the low-MPV group had 29 patients whose MPV was below 10.0 (fL). The high-MPV/PC group consisted of 9 patients whose MPV/PC equalled or exceeded 0.06 (fL/103μL); the low-MPV/PC group had 36 patients whose MPV/PC was below 0.06 (fL/103μL). There were no statistically significant differences in clinical features/laboratory findings (age, sex, Eastern Cooperative Oncology Group Performance Status [ECOG-PS] score, primary site, tumour size, number of skin lesions, lymph node metastasis, distant metastasis, first-line chemotherapy, surgery, radiation, and outcome) between the high- and low-MPV groups (Table SI). Similarly, no significant difference in clinical features was found in the MPV/PC groups (Table I). Kaplan–Meier survival analysis showed no statistically significant difference in the overall survival (OS) rate, but the high-MPV group tended to have worse prognoses by a few years (median OS time: 21.5 months vs 46.0 months, log rank-test; p-value = 0.63) (Fig. 1A). As for MPV/PC, the OS rate was significantly lower in the high-MPV/PC group than in the low-MPV/PC group (median OS time: 18.6 months vs 37.0 months, log rank-test; p-value = 0.022) (Fig. 1B). The multivariate model showed MPV/PC values exceeding 0.06 to be an independent factor for poor OS prognosis (HR: 3.90, 95% CI: 1.23–12.4, Cox proportional hazards model; p-value=0.021) (Table SII).
| Attribute | Patient group (%) | p-value* | ||
| Patients group (%) | Low MPV/PC (MPV/PC < 0.06) | High MPV/PC (MPV/PC ≥ 0.06) | ||
| Patients (%) | 45 (100%) | 36 (80) | 9 (20) | |
| Age | 0.898 | |||
| Median [IQR] | 76 [68–81] | 76 [66.8–81] | 76 [72–79] | |
| Min–max | 53–86 | 53–86 | 62–84 | |
| Sex | 1.00 | |||
| Male | 33 (73.3) | 26 (72.2) | 7 (77.8) | |
| Female | 12 (26.7) | 10 (27.8) | 2 (22.2) | |
| ECOG-PS score | 1.00 | |||
| 0–1 | 41 (91.1) | 33 (91.7) | 8 (88.9) | |
| 2–4 | 4 (8.9) | 3 (8.3) | 1 (11.1) | |
| Primary site | 1.00 | |||
| Head | 43 (95.6) | 34 (93.8) | 9 (100) | |
| Face | 2 (4.4) | 2 (6.3) | 0 (0) | |
| Tumour size | 0.482 | |||
| < 5cm | 25 (55.6) | 21 (58.3) | 4 (44.4) | |
| ≥ 5cm | 20 (44.4) | 15 (41.7) | 5 (55.6) | |
| Number of skin lesions | 0.712 | |||
| Single | 19 (42.2) | 16 (44.4) | 3 (33.3) | |
| Multiple | 26 (57.8) | 20 (55.6) | 6 (66.7) | |
| Lymph node metastasis | 0.173 | |||
| Absent | 41 (91.1) | 34 (93.8) | 7 (77.8) | |
| Present | 4 (8.9) | 2 (6.3) | 2 (22.2) | |
| Distant metastasis | 0.173 | |||
| Absent | 41 (91.1) | 34 (93.8) | 7 (77.8) | |
| Present | 4 (8.9) | 2 (6.3) | 2 (22.2) | |
| 1st line chemotherapy | 1.00 | |||
| Paclitaxel | 32 (71.1) | 25 (69.4) | 7 (77.8) | |
| Docetaxel | 2 (4.4) | 2 (5.6) | 0 (0) | |
| Interleukin-2 | 11 (24.4) | 9 (2.5) | 2 (22.2) | |
| Surgery for ASHN | 0.086 | |||
| Absent | 33 (73.3) | 24 (66.7) | 9 (100) | |
| Present | 12 (26.7) | 12 (33.3) | 0 (0) | |
| Radiation for ASHN | 1.00 | |||
| Absent | 12 (26.7) | 10 (27.8) | 2 (22.2) | |
| Present | 33 (73.3) | 26 (72.2) | 7 (77.8) | |
| Outcome | 1.00 | |||
| Alive | 21 (46.7) | 17 (47.2) | 4 (44.4) | |
| Dead | 24 (53.3) | 19 (52.8) | 5 (55.6) | |
| ASHN: angiosarcoma of the head and neck; ECOG-PS: Eastern Cooperative Oncology Group performance; IQR: interquartile range; MPV: mean platelet volume; PC: platelet count. *Statistically significant: p < 0.05. | ||||
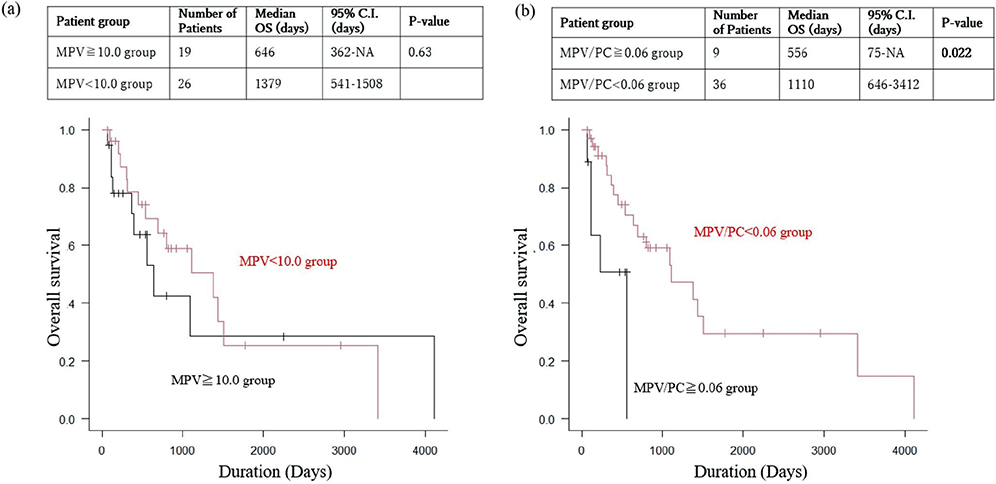
Fig. 1. Comparison of overall survival (OS) between the low-MPV/PC group and the high-MPV/PC group, and between the low-MPV group and the high-MPV Group. (A) Kaplan–Meier analysis for overall survival by MPV grouping, univariate analysis with the log-rank test. The overall survival (OS) rate is higher for the low-MPV group than for the high-MPV group (p = 0.63). (B) Kaplan–Meier analysis for overall survival by MPV/PC grouping, univariate analysis with the log-rank test. The overall survival (OS) rate is significantly higher for the low-MPV/PC group than for the high-MPV/PC group (p = 0.021).
MPV is an early marker of platelet activation (1), and higher MPV is associated with higher serum thromboembolism levels and a greater severity of systemic inflammation (2). Thrombosis, inflammation, and cancer are interrelated, and platelet activation has been shown to play a role in the development and progression of malignancies (3). A systematic review of the relationship between MPV and malignancies found that increased MPV is observed in many neoplastic diseases, with higher MPV tending to be associated with a poorer prognosis, although some malignancies show the opposite results (4). It has been proposed that the reason for the association between increased MPV and malignancy is that inflammatory cytokines such as interleukin-6 produced by tumour cells may stimulate the differentiation and proliferation of megakaryocytes, resulting in the production of abundant giant platelets. In the present study, the high-MPV group also tended to have a poorer prognosis. (Fig. 1A). With regard to MPV/PC, it has been argued that MPV and PC have an inverse relationship to maintain constant coagulability and that the MPV/PC ratio should be used as a marker of platelet activity (1, 5, 6, 7). Several studies have suggested that MPV/PC can be a useful prognostic factor for various malignancies (4, 5, 8). In the present study, the high-MPV/PC group was found to show statistically significant lower OS. (Fig. 1B). Several factors likely contribute to the association between MPV/PC and poor prognosis in ASHN. Elevated MPV, reflecting heightened inflammation as the disease progresses, and compensatory decrease in PC may be one factor. Additionally, the defining characteristic of angiosarcoma, increased bleeding events, may also play a role. This ongoing bleeding leads to platelet consumption, resulting in a decrease in PC and further contributing to the adverse outcomes observed in ASHN patients.
In conclusion, we found that increased MPV/PC is an independent factor for poor prognosis in patients with ASHN. Further large-scale investigations are needed to establish high MPV/PC as a prognostic biomarker in ASHN patients.
REFERENCES
- Thompson CB and Jakubowski JA. The pathophysiology and clinical relevance of platelet heterogeneity. Blood 1988;72: 1–8. https://doi.org/10.1182/blood.V72.1.1.1
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation. Curr Pharm Des 2011; 17: 47–58. https://doi.org/10.2174/138161211795049804
- Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015; 126: 582–588. https://doi.org/10.1182/blood-2014-08-531582
- Detopoulou P, Panoutsopoulos GI, Mantoglou M, Michailidis P, Pantazi I, Papadopoulos S, et al. Relation of mean platelet volume (MPV) with cancer: a systematic review with a focus on disease outcome on twelve types of cancer. Curr Oncol 2023; 30: 3391–3420. https://doi.org/10.3390/curroncol30030258
- Bessman JD, Williams LJ, Gilmer Jr PR. Mean platelet volume: the inverse relation of platelet size and count in normal subjects, and an artifact of other particles. Am J Clin Pathol 1981; 76: 289–293. https://doi.org/10.1093/ajcp/76.3.289
- Lin YC, Jan HC, Ou HY, Ou CH, Hu CY. Low preoperative mean platelet volume/platelet count ratio indicates worse prognosis in non-metastatic renal cell carcinoma. J Clin Med 2021; 10: 3676. https://doi.org/10.3390/jcm10163676
- Korniluk A, Koper-Lenkiewicz OM, Kamińska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm 2019; 2019: 9213074. https://doi.org/10.1155/2019/9213074
- Cho SY, Yang JJ, You E, Kim BH, Shim J, Lee HJ, et al. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets 2013; 24: 375–377. https://doi.org/10.3109/09537104.2012.701028