RESEARCH LETTER
Multiple Acantholytic Acanthomas in Junctional Epidermolysis Bullosa
Sota ITAMOTO, Ken NATSUGA*, Takashi SEO, Shota TAKASHIMA and Hideyuki UJIIE
Department of Dermatology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, N15W7, Sapporo 0608638, Japan. *E-mail: natsuga@med.hokudai.ac.jp
Citation: Acta Derm Venereol 2024; 104: adv42258. DOI: https://doi.org/10.2340/actadv.v104.42258.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Oct 14, 2024; Accepted after revision: Oct 21, 2024; Published: Nov 19, 2024
To the Editor,
Acantholytic acanthoma (AA) is a rare benign keratosis characterized by epidermal thickening with acantholysis. Clinically, it is typically seen as a solitary nodular lesion, with a predilection for the trunk (1), although multiple lesions have been reported in immunocompromised patients (2, 3). Epidermolysis bullosa (EB) is a group of disorders characterized by congenital skin fragility. Classical EB shows skin separation at the dermo-epidermal junction (DEJ) due to disease-causing variants in the genes encoding epidermal basement membrane zone proteins. AA and EB are generally considered mutually exclusive because their histological skin split levels differ. We encountered a junctional EB (JEB) patient with multiple AAs.
The patient was a 38-year-old Japanese man who had been diagnosed with JEB based on his compound heterozygous COL17A1 variants (NM_000494.4:c.1179del (p.Ala394Leufs*9) and c.4159C>T (p.Gln1387*)) (4–6). The same patient had developed cherry haemangiomas on his scalp, as reported previously (6). He had noticed multiple keratotic nodules on his scalp for several years. On examination, nodular lesions with marked keratinization and crusts on the left auricle and occiput were observed (Fig. 1A, B). We originally removed the occipital lesion and performed transplantation of an autologous epidermal sheet (7) on the lesion, but the keratotic lesion recurred. We then performed histological examinations of the 2 lesions. Both specimens showed marked hyperkeratosis, acanthosis, and acantholysis. Intraepidermal skin splits were observed, with acantholytic keratinocytes and the epidermal basal layers present with the underlying dermis (tombstone appearance; Fig. 1C–F). Dyskeratosis and cytological atypia were not observed in the epidermis. Subepidermal skin clefts, a hallmark of JEB, were not seen. A direct immunofluorescence study of the patient’s non-keratotic skin showed no immunoglobulin deposits in epidermal intercellular spaces, excluding pemphigus diseases. The diagnosis of multiple AAs was made.
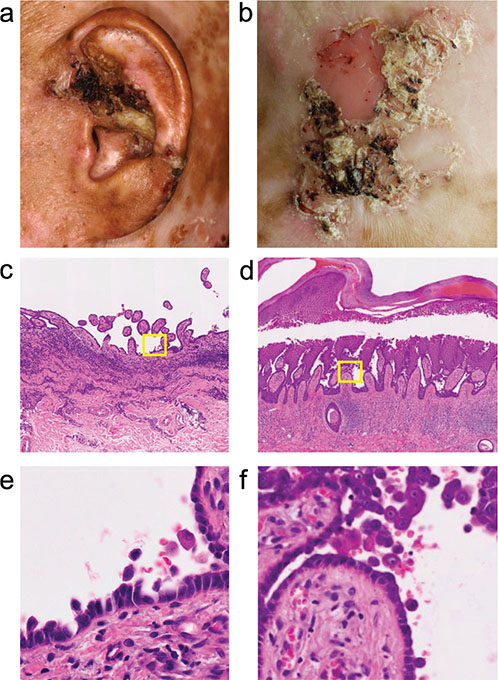
Fig. 1. Clinical manifestations and histopathology. (A, B) Hyperkeratotic lesions on the (A) left auricle and (B) occiput. (C–F) Haematoxylin and eosin staining. Low- and high-magnification images of the (C, E) left auricle and (D, F) occiput specimens. Original magnification: (C, D) x100 and (E, F) x400.
The patient had multiple normal-looking skin spots on his whole body, previously confirmed as revertant mosaicism (RM) (5). RM refers to a spontaneous correction of genetic abnormalities at the somatic cell level in patients with congenital disorders, such as congenital ichthyosis and EB (8). We previously showed that his RM skin was positive for collagen XVII (COL17), encoded by the COL17A1 gene, whereas his diseased skin was COL17-negative (5). We wondered whether AAs might have developed on his RM skin because the AA lesions showed skin splits above the epidermal basal layers, not at the DEJ. To investigate this, we compared the patient’s non-RM skin, RM skin, and AA skin using formalin-fixed paraffin-embedded sections. The institutional review board of the Hokkaido University Graduate School of Medicine approved this study (ID: 13-043). The study was conducted following the principles of the Declaration of Helsinki. The patient provided written informed consent.
Double staining of keratin 14 (K14) and keratin 10 (K10) confirmed that the AA skin split developed between K10-positive suprabasal and K14-positive basal layers (Fig. 2A–C). COL17 was present at the DEJ of RM skin, but its labelling was markedly weak in non-RM skin and AA skin (Fig. 2D–F). This indicated that AA developed from his non-RM skin but not from RM skin.
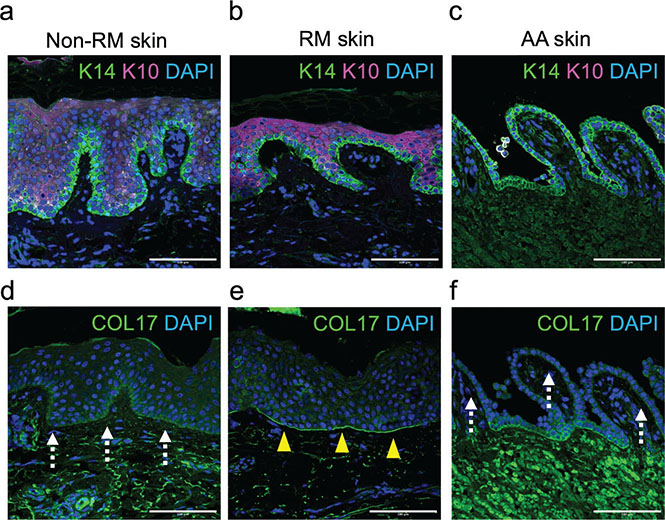
Fig. 2. Immunofluorescence studies. (A–C) K10 and K14 labelling of (A) non-RM, (B) RM, and (C) AA lesions. Scale bar: 100 μm. (D–F) COL17 labelling (D) of non-RM, (E) RM, and (F) AA lesions. The COL17-positive area is indicated by arrowheads. COL17-weak areas are indicated by dotted arrows. Scale bar: 100 μm.
The aetiology of AA is unknown. To our knowledge, this is the first case of a patient with EB developing AA. Our case highlights that multiple AAs can occur in non-immunocompromised hosts and that skin fragility at the DEJ, such as COL17 deficiency in our case, does not preclude the development of AA.
REFERENCES
- Brownstein MH. Acantholytic acanthoma. J Am Acad Dermatol 1988; 19: 783–786. https://doi.org/10.1016/S0190-9622(88)70234-0
- Ramos-Caro FA, Sexton FM, Browder JF, Flowers FP. Acantholytic acanthomas in an immunosuppressed patient. J Am Acad Dermatol 1992; 27: 452–453. https://doi.org/10.1016/S0190-9622(08)80880-8
- Bürgler C, Schlapbach C, Feldmeyer L, Haneke E. Multiple acantholytic dyskeratotic acanthomas in an organ transplant recipient. JAAD Case Rep 2018; 4: 695–697. https://doi.org/10.1016/j.jdcr.2018.04.009
- Nakamura H, Sawamura D, Goto M, Nakamura H, Kida M, Ariga T, et al. Analysis of the COL17A1 in non-Herlitz junctional epidermolysis bullosa and amelogenesis imperfecta. Int J Mol Med 2006; 18: 333–337. https://doi.org/10.3892/ijmm.18.2.333
- Wang Y, Kitahata H, Kosumi H, Watanabe M, Fujimura Y, Takashima S, et al. Collagen XVII deficiency alters epidermal patterning. Lab Invest 2022; 102: 581–588. https://doi.org/10.1038/s41374-022-00738-2
- Itamoto S, Natsuga K, Takashima S, Ujiie H. Atypical manifestations of hemangiomas in epidermolysis bullosa. J Dermatol 2024 Jun 14. [Online ahead of print] https://doi.org/10.1111/1346-8138.17341
- Matsumura W, Fujita Y, Shinkuma S, Suzuki S, Yokoshiki S, Goto H, et al. Cultured epidermal autografts from clinically revertant skin as a potential wound treatment for recessive dystrophic epidermolysis bullosa. J Invest Dermatol 2019; 139: 2115–24.e11. https://doi.org/10.1016/j.jid.2019.03.1155
- Nomura T. Recombination-induced revertant mosaicism in ichthyosis with confetti and loricrin keratoderma. J Dermatol Sci 2020; 97: 94–100. https://doi.org/10.1016/j.jdermsci.2019.12.013