ORIGINAL REPORT
Sex Disparities of Health-related Quality of Life in Moderate to Severe Psoriasis: A Real-world Analysis from the Swiss Psoriasis Registry (SDNTT)
Ramtin LICHTENBERGER1,2#, Lara Valeska MAUL1#, Ion BIRKENMAIER1,3, Iker OYANGUREN4, Melike AK1, Kristine HEIDEMEYER5, Christoph SCHLAPBACH5, Nikhil YAWALKAR5, Alexander EGEBERG6,7, Simon Francis THOMSEN6, Jacob P. THYSSEN6,7, Christina SORBE8, Wolf-Henning BOEHNCKE9, Curdin CONRAD10, Antonio COZZIO11, Georgios KOKOLAKIS12, Raphael MICHEROLI13, Jashin J. WU14, Thomas KÜNDIG1,2, Alexander NAVARINI15 and Julia-Tatjana MAUL1,2
1Department of Dermatology, University Hospital of Zurich, University of Zurich, Zurich, Switzerland, 2Faculty of Medicine, University of Zurich, Zurich, Switzerland, 3Life Science Zurich Graduate School, Faculty of Science, University of Zurich, Zurich, Switzerland, 4Swiss4ward, Statistician and Data Analyst, Zurich, Switzerland, 5Department of Dermatology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland, 6Department of Dermato-Venereology & Wound Healing Centre, Bispebjerg Hospital, Copenhagen, Denmark, 7Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, 8Institute for Health Services Research in Dermatology and Nursing (IVDP), University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany, 9Division of Dermatology and Venereology, Geneva University Hospitals, Geneva, Switzerland, 10Department of Dermatology, University Hospital Lausanne, Lausanne, Switzerland, 11Department of Dermatology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland, 12Psoriasis Research and Treatment Center, Department of Dermatology, Venereology and Allergology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 13Department of Rheumatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland, 14Department of Dermatology, University of Miami Miller School of Medicine, Miami, USA, and 15Department of Dermatology, University Hospital Basel, University of Basel, Basel, Switzerland
#Co-shared first authorship.
Real-world data on gender differences in quality of life among psoriasis patients before and during treatment are scarce. This study analysed data of 748 adults with moderate-to-severe psoriasis enrolled in the Swiss Dermatology Network of Targeted Therapy registry between 2011 and 2023. Quality of life was assessed using the Dermatological Life Quality Index at baseline and at 3, 6, 12, 18, and 24 months. At baseline, women reported significantly lower quality of life than men, with higher Dermatological Life Quality Index scores in the IL-17 inhibitor group (15.0 vs 11.0, p = 0.027), IL-12/23 inhibitor group (7.5 vs 7.0, p = 0.049), and non-biologic therapy group (13.0 vs 9.0, p < 0.001). Although quality of life improved across all subgroups during the follow-up period, women treated with IL-12/23 inhibitors continued to report worse quality of life compared with men after 2 years (p < 0.05), while no significant differences were observed with other therapies. These findings emphasize that women with psoriasis experience lower quality of life at treatment initiation and throughout non-biologic and biologic therapies, underlining the importance of addressing gender-specific differences in the management of psoriasis.
SIGNIFICANCE
Psoriasis is a common chronic disease affecting skin, nails, and joints, and is often linked to heart disease, obesity, and depression. Both men and women with psoriasis experience a reduced quality of life. Understanding how treatments impact each gender can help physicians provide personalized care. Studies show men and women experience psoriasis differently, influenced by cultural and social factors. Over 2 years, we studied 278 women and 470 men in a Swiss national study. Initially, women felt more affected, but this difference decreased over time, except in one treatment group. Personalized treatments can improve long-term outcomes.
Key words: Dermatological Life Quality Index (DLQI); patient-reported outcome measure (PROM); psoriasis vulgaris; registry; SDNTT; sex differences.
Citation: Acta Derm Venereol 2025; 105: adv42296. DOI: https://doi.org/10.2340/actadv.v105.42296.
Copyright: © 2025 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Oct 21, 2024. Accepted after revision: Dec 19, 2024. Published: Feb 6, 2025
Corr: Prof Dr Julia-Tatjana Maul, Department of Dermatology, University Hospital Zürich, Rämistrasse 100, CH-8091 Zürich, Switzerland. E-mail: Julia-tatjana.maul@usz.ch
Competing interests and funding: Unrelated to the present manuscript, LVM has served as adviser and/or received speaking fees and/or participated in clinical trials sponsored by Almirall, Amgen, BMS, Celgene, Eli Lilly, Incyte, MSD, Novartis, Pierre Fabre, Roche, and Sanofi. BI is an employee of USZ/UZH and has declared no conflict of interest. KH has received grants or contracts from Galderma and Almirall, support for attending meetings and/or travel from AbbVie and UCB, and participated on a Data Safety Monitoring Board or Advisory Board for Amgen and Leo Pharma, all both personally and for their institution. CS has received honoraria as adviser or speaker for Abbvie, Almirall, BMS, GSK, Incyte, LEO Pharma, Lilly, Kiowa Kirin, Novartis, Pfizer, and Sanofi and has received research funding from PPM Services. NY is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, and UCB. Unrelated to the present manuscript, AE has received research funding from Pfizer, Eli Lilly, Novartis, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Zuellig Pharma Ltd, Galápagos NV, Sun Pharmaceuticals, Samsung Bioepis Co., Ltd, Pfizer, Eli Lilly and Company, Novartis, Union Therapeutics, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, and Janssen Pharmaceuticals. He is currently an employee of LEO Pharma. SFT has received research support from Abbvie, Janssen, LEO Pharma, Novartis, Sanofi, and UCB, and has been a speaker/consultant for Abbvie, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, Symphogen, UCB, and Union Therapeutics, with no relation to the present manuscript. JPT was previously an adviser for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly & Co., LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, a speaker for AbbVie, Almirall, Eli Lilly & Co., LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, and received research grants from Pfizer, Regeneron, and Sanofi-Genzyme. He is currently an employee of LEO Pharma. WHB has received honoraria as a speaker and/or adviser from Abbvie, Almirall, Janssen, Leo, Lilly, Novartis, and UCB. CC has served as a consultant, investigator and/or adviser for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, MSD, Novartis, Pfizer, Samsung, Sanofi, and UCB. AC has engaged in advisory board activity regarding psoriasis for AbbVie, Almirall, Amgen, Galderma, LEO Pharma, Janssen-Cilag, Novartis, and UCB. GK is or has acted as a speaker and/or advisory board member for honoraria from AbbVie, Abbott, Actelion Pharmaceuticals, Amgen, Basilea Pharmaceutica, Bayer, Biogen IDEC, Boehringer, Bristol Myers Squibb, Celgene, Hexal, Janssen-Cilag, LEO Pharma, Lilly, MSD, Mylan, Novartis, Parexel, Pfizer, Sanofi-Aventis, Sharpe and Dohme, STADA, Takeda, and UCB. RM has received speaker fees from Janssen, Abbvie and UCB. JJW is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, DermTech, Dr. Reddy’s Laboratories, Eli Lilly, EPI Health, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health. TK has intermittent, project focused consulting and/or advisory relationships with Leo Pharma, Janssen-Cilag, Eli Lilly, Pierre Fabre, Sanofi Genzyme, Abbvie, Biomed AG, Novartis, Almirall, Bristol-Myers Squibb, Galderma, L’Oréal/La Roche-Posay, Merck-Sharp & Dohme, Zur Rose AG, Allergy Therapeutics AG, Derma2go AG, Oncobit AG, EVAX AG, Saiba Biotechnology AG, Saiba Animal Health AG, AltiBio Corp, Encoded Corp, Mabylon AG, MannKind Corp, and XBiotech Corp. AN has received consulting fees from AbbVie, Almirall, Biomed, BMS, Boehringer Ingelheim, Eli Lilly, Galderma, GSK, InflaRx, Janssen-Cilag, LEO Pharma, MSD, Novartis, Pfizer, Pierre Fabre Pharma, Regeneron, Sandoz, Sanofi-Aventis, Takeda, and UCB-Pharma; payment/honoraria for lectures/presentations from Speakers Bureaus, manuscript writing, or educational events from these entities; payment for expert testimony by Pierre Fabre Pharma; participated on a Data Safety Monitoring Board or Advisory Board for Novartis; and has other financial or non-financial interests from Canfield Inc. Unrelated to the present manuscript, JTM is an employee of USZ and has served as adviser and/or received speaking fees and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, BMS, Celgene, Eli Lilly, LEO Pharma, Janssen-Cilag, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, and UCB. RL, IO, MAk, and CS have no conflicts of interest to declare.
The SDNTT registry is supported by AbbVie, Almirall, Bristol Myers Squibb, Janssen-Cilag, Eli Lilly, and UCB. It had previously been supported by Celgene, Amgen, Pfizer, MSD, and Novartis as the data were also collected while they were funding the SDNTT. These companies do not have any influence on the design of the register, data collection, or analyses, nor on publication decisions or manuscript constructions.
INTRODUCTION
Psoriasis vulgaris is a common, chronic, inflammatory, immune-mediated disease. It affects the skin, nails and joints (1, 2) with associated multiple comorbidities including cardiovascular disease, obesity, and depression (3). In addition, it has a significant negative impact on the psychological health of both female and male patients (4–7), profoundly affecting the health-related quality of life (HRQoL) (8). Patient-reported outcome measures (PROMs) play a crucial role in healthcare alongside clinician-reported tools, helping to assess the effectiveness of treatments from the patients’ perspective, and are often used in clinical trials and research studies to assess the efficacy of treatments, contributing to evidence-based practice (9).
Considering sex and gender as biological and social variables in research is crucial due to significant physiological differences and social constructs that can affect disease incidence, treatment responses, and drug efficacy (10). Previous studies revealed that female patients had higher treatment expectations and more specific needs than male patients (4). Moreover, women have a better clinical response to biologic and non-biologic systemic treatments than men, but also a higher HRQoL impairment across treatments (6).
Several studies have observed sex differences in psoriasis. Women have higher expectations and needs in social impairments, depression, sleep quality, and productivity, suggesting individualized therapy (4). Younger patients experience greater quality of life (QoL) impacts, with men facing more work-related stress (11). Women report higher subjective disease scores, while men have higher objective measures, indicating potential undertreatment of women (12). Symptom severity correlates with discomfort and stigmatization for both genders (13). Women rate hospital care lower and seek more privacy and pain management (14). Women report lower treatment satisfaction and more adverse events, leading to higher discontinuation rates (15). Men have more severe psoriasis and receive biologics earlier, reflecting higher disease activity (16). Dermatologists’ treatment preferences, influenced by region and phototherapy availability, contribute to biases (17). Gender differences necessitate gender-specific healthcare approaches for better outcomes (18). Women report more disability and functional limitations (19), with no significant gender effect on somatic symptoms when distress is controlled (20). Women’s lower median Psoriasis Area and Severity Index (PASI) scores may explain why men receive more systemic treatments (21). Differences in disease severity assessments between patients and physicians underscore the need to combine subjective and objective evaluations for better treatment effectiveness (22). Treatment expectations, influenced by factors such as gender, disease severity, and trust in the physician, significantly impact clinical outcomes (23).
However, data on different drug types and how they specifically impact the HRQoL over long-term treatment between the biological sexes are still scarce. This area of research is crucial due to few sex-specific recommendations in the most recent European guidelines (24, 25). Differences in QoL, drug dosing, and specific treatment types between the sexes have not yet been considered but are essential in disease management and adherence.
Previous studies on treatment-related improvement of the Dermatology Life Quality Index (DLQI) and Psoriasis Area and Severity Index (PASI) have so far been performed without considering sex (5–7).
To better understand how different treatments affect the QoL of female and male patients, our study investigated the putative sex differences in absolute DLQI scores at treatment initiation and during treatment with systemic non-biologic (forthwith simply “non-biologic”) and biologic therapies, with emphasis on the current prescription environment in Switzerland.
MATERIALS AND METHODS
Patient population and data collection
Adults with moderate-to-severe psoriasis were recruited to participate in the Swiss Dermatology Network of Targeted Therapy (SDNTT) registry from tertiary and secondary dermatological hospitals across Switzerland (4–7), including the University Hospitals of Basel, Bern, Geneva, Lausanne, and Zurich, and the Cantonal Hospitals of Aarau, Bellinzona, and St. Gallen.
The registry is electronic and patient-based, and is independently monitored by CVderm at the University Medical Center Hamburg-Eppendorf (Germany) (26), aligning with the German psoriasis registry PsoBest (NCT01848028). Treatment decisions follow the EuroGuiDerm Guidelines for systemic treatment (24, 25). Key inclusion criteria were initiation of a new systemic anti-psoriatic treatment, informed consent, and the ability to complete the questionnaires in German, Italian or French (27). Patients registered from October 2011 to June 2023, with at least 4 clinical visits and no more than 1 systemic therapy, were included. If a therapy change occurred, the observation for that patient was discontinued. Patients were grouped by treatment type for analysis: TNFα inhibitors, interleukin (IL)-17 inhibitors, IL-12/23 inhibitor, IL-23 inhibitors, and non-biologic therapies, including conventional systemic therapies (including cyclosporine, fumaric acid esters (FAE), methotrexate (MTX), retinoids) and phosphodiesterase-4 (PDE4) inhibitor (apremilast).
Several dermatology-specific ways to measure HRQoL are available and used as a PROM, among which the DLQI, developed by Finlay et al., is the most widely used assessment tool (28). According to the European consensus, the DLQI, along with the PASI, is the most important diagnostic criterion to determine disease severity and the indication for systemic treatments (24, 25). Considering its broad acceptance, we utilized the DLQI as the primary instrument for our analysis.
As for the SDNTT protocol, the interval between the first 3 registry visits was planned for every 3 months, followed by 6 months thereafter. Only DLQI measurements with no more than 2 unanswered questions were included. Patients were excluded if they lacked an initial DLQI submission or had not yet started systemic therapies during the observation time. For patients with alteration of therapy only data from the initial treatment were analysed, reducing the number of cases over time.
Statistical analysis
Baseline patient characteristics for both sexes were analysed, including DLQI completion, PASI and DLQI scores, age, duration of psoriasis, Body Surface Area (BSA), Body Mass Index (BMI), and presence of nail psoriasis and psoriatic arthritis (PsA) from the SDNTT registry. While the SDNTT registry collects various variables (DLQI, PASI, age, gender, BMI, and comorbidities including PsA and nail involvement), for this study, we followed only the DLQI as the primary outcome. Psoriatic arthritis diagnoses were always confirmed by external or internal rheumatologists. Nail involvement was assessed by the dermatologist using the Nail Psoriasis Severity Index (NAPSI), which records the number of affected nails.
The Mann–Whitney–Wilcoxon test was used for quantitative variables and Fisher’s exact test as well as the Kruskal–Wallis test for qualitative variables. Multiple linear regression assessed potential confounders or effect modifiers, considering baseline age, sex, PASI, DLQI, nail involvement, psoriatic arthritis, BMI, and duration of psoriasis as independent factors. A p-value < 0.05 was considered to be statistically significant. All statistical analyses were performed using R (version 4.1.1.) and RStudio (29) (R Foundation for Statistical Computing, Vienna, Austria).
Predictors of DLQI at baseline
A multiple linear regression was conducted to identify predictors of the DLQI at baseline, taking into account age, sex, PASI, BMI, and duration of psoriasis. Significant factors included PASI, age, and sex, with p-values of < 0.001, 0.001, and 0.005, respectively (Table SI).
Ethics
The SDNTT was registered with ClinicalTrials.gov (NCT01706692) and was approved by the Ethics Committee Northwest and Central Switzerland (EKNZ 62/11). This study was conducted in accordance with the principles of the Declaration of Helsinki (30).
RESULTS
Demographic and clinical characteristics
As of 30 June 2023, the SDNTT database included data from 1,007 patients. Of these, 748 (74.3%; female = 278, male = 470) were eligible and included in the analysis (Fig. 1). The median age for females was 44.3 years, and for males 45.8 years (p = 0.1) (Table I). At baseline, female patients had a significantly higher DLQI score compared with male patients (13.4 vs 10.7; p < 0.001). The median PASI was significantly higher for males (9.6) compared with females (8.2; p < 0.001). BSA was greater in males (12.8) than in females (11.9; p = 0.019), as was BMI, with males presenting a higher BMI (28.2 kg/m2) compared with females (26.3 kg/m2; p < 0.001). The difference in obesity rates (BMI ≥ 30 kg/m2) was not significant. Both groups showed comparable median durations of psoriasis (p = 0.5), PSA prevalence (p = 0.7), and nail involvement (p = 0.057).
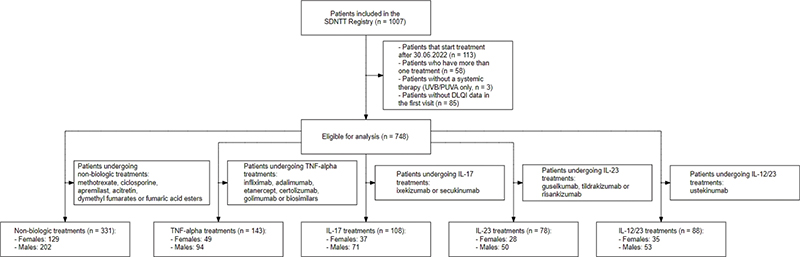
Fig. 1. Patient inclusion and exclusion flow diagram for systemic non-biologic group (methotrexate, fumarates, retinoids, cyclosporine, and apremilast) and biological groups (including interleukin (IL)-17, IL-12/23, IL-23, and TNF-α inhibitors). SDNTT: Swiss Dermatology Network for Targeted Therapies, DLQI: Dermatology Life Quality Index.
Therapy group distribution
Patients were further categorized based on their therapy at inclusion: n = 331 received non-biologic therapy, and n = 417 received biologic therapy. Male patients constituted the majority in both groups, with 64% (n = 268) in the biologic group and 61% (n = 202) in the non-biologic group. Further subdivision within the biologic therapy group was as follows: TNF-α inhibitors (n = 143), ustekinumab (n = 88), IL-17 inhibitors (n = 108), and IL-23 inhibitors (n = 78).
Prevalence of treatment types
The treatments most commonly utilized within the SDNTT registry were MTX (n = 224, 30.0%), adalimumab (n = 94, 12.6%), and ustekinumab (n = 88, 11.8%) (Table II). These treatments accounted for more than half (54.4%) of all therapies administered. From 2018 onwards, there was a significant yearly increase in the inclusion of IL-17 or IL-23 inhibitors as the most commonly administered biologic therapies in the registry, as confirmed by a two-proportion z-test (2018: p = 0.011, 2019–2022: p < 0.001) (Table SII).
DLQI differences by age group and gender
The Kruskal–Wallis test revealed a significant difference in DLQI scores between younger (18–64 years) and older patients (over 64 years) at the initial visit (Tables SIII–SIV). During the initial visit, younger patients demonstrated a significantly higher DLQI score (11.0) compared with older patients (8.0). This difference was not observed in subsequent visits (Fig. 2).
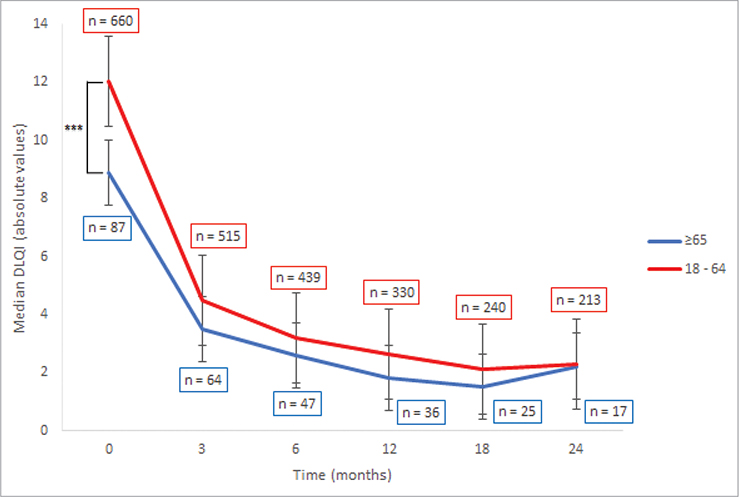
Fig. 2. Median Dermatology Life Quality Index (DLQI) of young (18–64 years) and old (≥ 65 years) patients. ***p < 0.001.
DLQI over time
In assessing DLQI over 24 months, significant differences were observed in median DLQI scores between female and male patients at the first registry visit, with females exhibiting higher scores (13 vs 10, p < 0.001) (Fig. 3, Table SV). Further subanalyses showed a consistent pattern of higher DLQI scores in females across all treatment categories, with notable significance in non-biologic treatments (13 vs 9, p < 0.001), IL-17 inhibitors (15vs 11, p = 0.027), and ustekinumab (8 vs 7, p = 0.049) (Table SVI, Fig. 4). This trend was not statistically significant for TNF-α (13 vs 10, p = 0.1) and IL-23 inhibitors (14 vs 9, p = 0.6). Subsequent follow-ups indicated significantly higher median DLQI for females under ustekinumab, whereas no significant differences emerged for the other treatment groups during follow-ups. After the baseline period, the range of median DLQI scores stayed relatively consistent for both sexes across all treatment types. For more details, refer to Tables SVII–SXII.
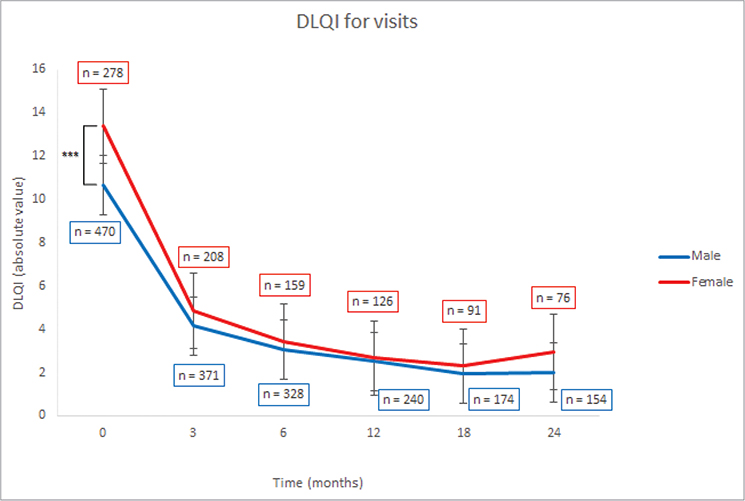
Fig. 3. Median Dermatology Life Quality Index (DLQI) of male and female patients. ***p < 0.001.
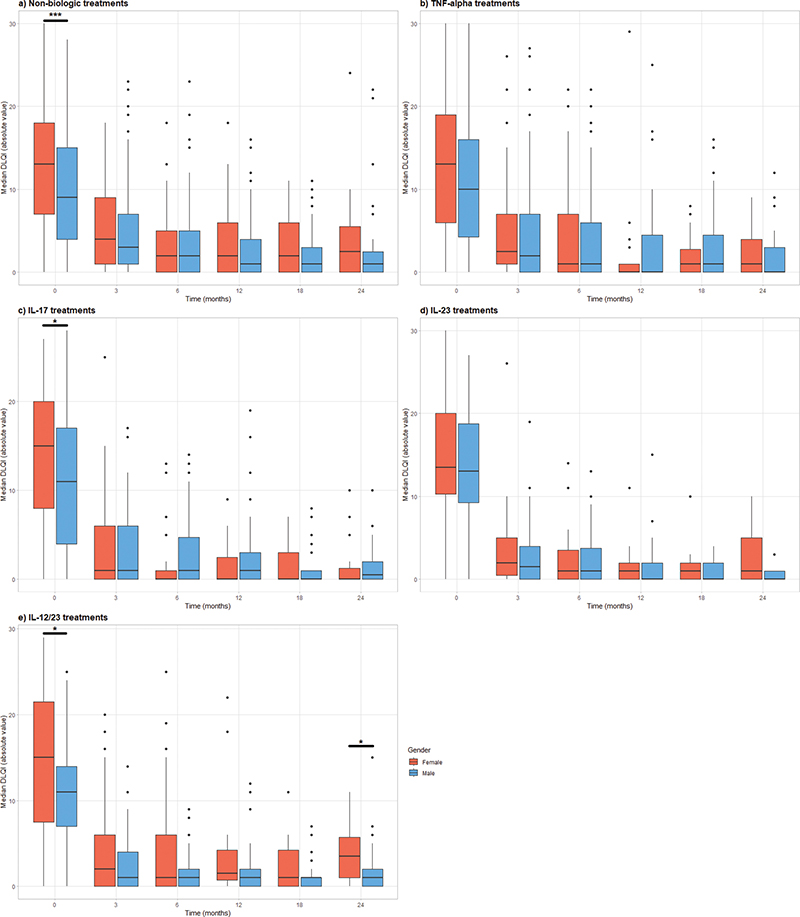
Fig. 4. Dermatology Life Quality Index (DLQI) at 0, 3, 6, 12, 18, and 24 months by sex and treatment. (A) Non-biologic treatments (methotrexate, fumarates, retinoids, cyclosporine and apremilast), (B) TNF-α inhibitors (adalimumab, certolizumab, etanercept, golimumab, infliximab), (C) IL-17 inhibitors (secukinumab, ixekizumab), (D) IL-12/23 inhibitor (ustekinumab), (E) IL 23 inhibitors (tildrakizumab, risankizumab, guselkumab). *p < 0.05 **p < 0.001.
The number of patients decreased over time, because for patients who stopped their treatment in the meantime, only measures under inclusion treatment were analysed. At month 3, n = 61 (8.2%) patients had stopped their treatment; at month 6, n = 49 (8.5%); at month 12/18/24, n = 35/19/10 (7.2/5.2/3.8%) respectively.
DISCUSSION
Despite a growing focus on sex differences in psoriasis, there is little information on individual systemic treatments stratified by sex and their effects on QoL over time.
Female patients had significantly higher DLQI scores compared with males before initiating systemic therapy, especially in non-biologic treatments and IL-17 and ustekinumab. This trend was absent in the subgroups treated with TNF-α, and IL-23 inhibitors. DLQI scores reduced across all subgroups over the 18-month study observation period, leading to comparable scores between sexes. However, after 24 months, a significant disparity re-emerged, particularly among female patients under ustekinumab who recorded higher DLQI scores than males.
Similar to other registries (16, 21, 31), we found a male predominance among patients initiating systemic treatment, thus emphasizing the importance of examining sex differences in treatment patterns. Studies have pointed to a decreased likelihood of females receiving systemic treatments, particularly biologic therapies (32), partly due to higher PASI scores in males (12) or to potential teratogenic risk in women of childbearing age (33). The observed sex differences in disease severity could be influenced by selective patient recruitment, with aspects like the DLQI not adequately considered. If women with higher DLQI scores but lower PASI scores are more likely to be enrolled, this could account for some of the sex-based severity disparities (12, 21).
Reasons why women have a higher DLQI before treatment initiation have been described previously (17, 19–21), including a higher symptomatic disease burden (12), a greater reduction in self-esteem (13), limitations in social situations, and a higher burden on mental health and QoL due to their disease (13). Female patients with psoriasis suffer more frequently from depression (34), which can have a detrimental impact on many different aspects of QoL (35) and on the progression of disease (36).
While our study identified significant sex differences in DLQI scores, potential confounding factors like socioeconomic status, employment, or healthcare access known to influence QoL (18, 32, 35), were collected but not analysed due to insufficient sample sizes. These factors are critical in shaping patient experiences and may explain disparities reported in previous research. Additionally, the DLQI, comprising 6 domains (Symptoms, Activities, Leisure, Work, Relationships, Treatment), may reveal gender-specific differences not captured in overall scores (8, 13, 15, 35). Previous findings (11, 13, 35) suggest greater limitations in personal relationships and daily activities among women, potentially linked to stigmatization. Future studies should explore these factors to provide deeper insights into sex-specific impacts of psoriasis.
Our findings align partially with those of the German and Swiss psoriasis registry study (6), which reported an increased burden of disease in women prior to therapy initiation. Our study revealed higher DLQI in women before starting therapy and significant differences during the follow-up phase. Contrary to the dual-country study, which found a significantly higher impairment in DLQI among female patients during follow-up across all treatment groups, our study found no such disparity.
The predominance of the German study population over the Swiss cohort in the referenced study could obscure country-specific differences. Different treatment options available in Germany and Switzerland at the time might have influenced the outcomes, suggesting that geographical and healthcare system differences impact results.
The significance of these findings lies in their implications to consider sex differences in the management of psoriasis to optimize patient outcomes (37). Regarding clinical trials and regulatory applications, PROMs, including the DLQI, capture patient-centric outcomes, complementing clinician-reported measures (38). The DLQI measures the impact of skin diseases on patients’ QoL, facilitates treatment evaluation and patient-centred care, and provides valuable information for regulatory and clinical decision-making (38). DLQI fluctuations and prolonged impairment can impact comorbidities, treatment satisfaction, and adherence. This is why HRQoL is a main criterion determining treatment success in the current European psoriasis guidelines (24). An increase in DLQI indicates a need to switch to or expand systemic therapies, with stricter limits for PASI and DLQI under discussion due to new therapies achieving a PASI 90 response (39).
We provide a detailed analysis of the DLQI over time for both sexes and for each individual drug class, offering a more comprehensive understanding of how QoL is affected by different treatments in female and male patients. An important finding is the absence of DLQI differences between men and women in any group during the first 18 months of follow-up. However, this changed after 24 months in the ustekinumab group, where women reported a significantly higher median DLQI compared with men, highlighting the necessity of considering sex differences in long-term psoriasis treatment planning.
Women experience more treatment related side effects than males and have higher rates of drug discontinuation (15). High expectations present for new biologic medications correspond to low tolerance for unmet expectation or perceived side effects (4). Safety concerns, especially for women of childbearing age, influence physicians’ decisions, with safer drugs potentially positively impacting QoL (17). Treatment choices rely not only on potential side effects or success but also on the treatment modality (40).
The German and Swiss dual-registry study from 2019 analysed the needs and expectations of 5,343 patients, noting age-associated differences (4). Our detailed analysis aimed to understand the DLQI–age relationship, revealing significant differences in young patients compared with older patients, raising questions about whether the DLQI should be weighted according to age.
These findings advocate for an individualized therapeutic approach sensitive to sex differences, underscoring the importance of incorporating treatment objectives, current symptoms, and QoL impairment into treatment decision. Regular re-evaluations throughout treatment are essential (4, 13), allowing healthcare providers to tailor approaches to each individual’s circumstances and needs, enhancing psoriasis management, improving QoL, and potentially reducing long-term complications.
Strengths and limitations
Our study’s major strength is the extensive collection of DLQI questionnaires, facilitating a robust analysis of patient-reported HRQoL across a diverse patient population in daily practice conditions. This dataset provides nuanced insights into the impacts of various treatments on HRQoL.
However, consistent with challenges faced in real-world registry studies, we observed a steady decline in patient participation over time due to incomplete 12-month observation periods, dropouts, loss-to-follow-up, variable visit frequencies, and deaths. These elements introduce complexity in interpreting longitudinal data and necessitate careful consideration in the analysis.
A notable disparity in the distribution of men and women in our study mirrors wider trends in real-world data, potentially influencing the applicability of our findings across different demographic groups. Future research should aim to engage a more balanced representation to enhance the generalizability of results.
The relatively low number of patients for individual therapies, reflecting Switzerland’s smaller population, limits the generalizability of our findings to larger or more diverse populations. Nonetheless, the inclusion of the latest generation of biologics provided a detailed snapshot of current prescribing trends and their implications for HRQoL.
Additionally, the use of the DLQI in assessing HRQoL among older patients revealed limitations due to the prevalence of “does not concern me” responses, particularly in areas like working life, suggesting a need for complementary HRQoL assessment tools.
Conclusion
Our investigation provides critical insights into the differential impacts of psoriasis treatments on HRQoL, underscoring the necessity of integrating sex-specific considerations into clinical practice. We found that women reported higher DLQI scores initially and during follow-up, indicating a sustained lower QoL compared with men. However, both sexes showed comparable DLQI scores over time. Although our study included extensive data collection and robust analyses, challenges such as patient dropouts and the relatively small Swiss population must be considered.
These findings highlight the complex interplay between biological and sociocultural factors in managing psoriasis and stress the importance of personalized treatment strategies to enhance patient well-being.
Our study advocates for a more nuanced approach to psoriasis treatment, one that prioritizes personalized care by addressing the specific challenges faced by women and men. By doing so, healthcare professionals can significantly improve the QoL for patients with psoriasis, paving the way for more effective and equitable healthcare practices.
ACKNOWLEDGEMENTS
Ethical approval: The SDNTT was registered with ClinicalTrials.gov (NCT01706692) and was approved by the Ethics Committee Northwest and Central Switzerland (EKNZ 62/11).
Ethics statement: This study was conducted in accordance with the principles of the Declaration of Helsinki. All participants provided informed consent before enrolment in the study.
REFERENCES
- Boehncke WH, Schön MP. Psoriasis. Lancet 2015; 386: 983–994. https://doi.org/10.1016/S0140-6736(14)61909-7
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet 2021; 397: 1301–1315. https://doi.org/10.1016/S0140-6736(20)32549-6
- Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol 2017; 76: 377–390. https://doi.org/10.1016/j.jaad.2016.07.064
- Maul JT, Navarini AA, Sommer R, Anzengruber F, Sorbe C, Mrowietz U, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. J Eur Acad Dermatol Venereol 2019; 33: 700–708. https://doi.org/10.1111/jdv.15324
- Maul JT, Anzengruber F, Conrad C, Cozzio A, Häusermann P, Jalili A, et al. Topical Treatment of Psoriasis Vulgaris: The Swiss Treatment Pathway. Dermatology 2021; 237: 166–178. https://doi.org/10.1159/000512930
- Maul JT, Augustin M, Sorbe C, Conrad C, Anzengruber F, Mrowietz U, et al. Association of sex and systemic therapy treatment outcomes in psoriasis: a two-country, multicentre, prospective, noninterventional registry study. Br J Dermatol 2021; 185: 1160–1168. https://doi.org/10.1111/bjd.20387
- Maul JT, Djamei V, Kolios AGA, Meier B, Czernielewski J, Jungo P, et al. Efficacy and Survival of Systemic Psoriasis Treatments: An Analysis of the Swiss Registry SDNTT. Dermatology 2016; 232: 640–647. https://doi.org/10.1159/000452740
- Finlay AY, Coles EC. The effect of severe psoriasis on the quality of life of 369 patients. Br J Dermatol 1995; 132: 236–244. https://doi.org/10.1111/j.1365-2133.1995.tb05019.x
- Chalmers RJ. Assessing psoriasis severity and outcomes for clinical trials and routine clinical practice. Dermatol Clin 2015; 33: 57–71. https://doi.org/10.1016/j.det.2014.09.005
- Miguel-Aliaga I. Let’s talk about (biological) sex. Nat Rev Mol Cell Biol 2022; 23: 227–228. https://doi.org/10.1038/s41580-022-00467-w
- Gupta MA, Gupta AK. Age and gender differences in the impact of psoriasis on quality of life. Int J Dermatol 1995; 34: 700–703. https://doi.org/10.1111/j.1365-4362.1995.tb04656.x
- Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med 2012; 10: 82. https://doi.org/10.1186/1741-7015-10-82
- Böhm D, Stock Gissendanner S, Bangemann K, Snitjer I, Werfel T, Weyergraf A, et al. Perceived relationships between severity of psoriasis symptoms, gender, stigmatization and quality of life. J Eur Acad Dermatol Venereol 2013; 27: 220–226. https://doi.org/10.1111/j.1468-3083.2012.04451.x
- Teunissen TAM, Rotink ME, Lagro-Janssen ALM. Gender differences in quality of care experiences during hospital stay: A contribution to patient-centered healthcare for both men and women. Patient Educ Couns 2016; 99: 631–637. https://doi.org/10.1016/j.pec.2015.10.033
- van der Schoot LS, van den Reek JMPA, Groenewoud JMM, Otero ME, Njoo MD, Ossenkoppele PM, et al. Female patients are less satisfied with biological treatment for psoriasis and experience more side-effects than male patients: results from the prospective BioCAPTURE registry. J Eur Acad Dermatol Venereol 2019; 33: 1913–1920. https://doi.org/10.1111/jdv.15733
- Hägg D, Eriksson M, Sundström A, Schmitt-Egenolf M. The higher proportion of men with psoriasis treated with biologics may be explained by more severe disease in men. PLoS One 2013; 8: e63619. https://doi.org/10.1371/journal.pone.0063619
- Wan J, Abuabara K, Troxel AB, Shin DB, Van Voorhees AS, Bebo BF Jr, et al. Dermatologist preferences for first-line therapy of moderate to severe psoriasis in healthy adult patients. J Am Acad Dermatol 2012; 66: 376–386. https://doi.org/10.1016/j.jaad.2011.03.012
- Regitz-Zagrosek V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep 2012; 13: 596–603. https://doi.org/10.1038/embor.2012.87
- Merrill SS, Seeman TE, Kasl SV, Berkman LF. Gender differences in the comparison of self-reported disability and performance measures. J Gerontol A Biol Sci Med Sci 1997; 52: M19–M26. https://doi.org/10.1093/gerona/52A.1.M19
- Piccinelli M, Simon G. Gender and cross-cultural differences in somatic symptoms associated with emotional distress. An international study in primary care. Psychol Med 1997; 27: 433–444. https://doi.org/10.1017/S0033291796004539
- Hägg D, Sundström A, Eriksson M, Schmitt-Egenolf M. Severity of Psoriasis Differs Between Men and Women: A Study of the Clinical Outcome Measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish Register Patients. Am J Clin Dermatol 2017; 18: 583–590. https://doi.org/10.1007/s40257-017-0274-0
- Tabolli S, Sampogna F, Pagliarello C, Paradisi A, Spagnoli A, Abeni D. Disease severity evaluation among dermatological out-patients: a comparison between the assessments of patients and physicians. J Eur Acad Dermatol Venereol 2012; 26: 213–218. https://doi.org/10.1111/j.1468-3083.2011.04038.x
- Clemmesen M, Jørgensen A-HR, Nielsen VW, Holgersen N, Nissen CV, Thyssen JP, et al. Disease- and treatment-related expectations, attitudes, and beliefs among adult patients initiating or switching biological therapies for psoriasis. JEADV Clin Pract 2023; 2: 973–982. https://doi.org/10.1002/jvc2.218
- Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol 2020; 34: 2461–2498. https://doi.org/10.1111/jdv.16915
- Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol 2021; 35: 281–317. https://doi.org/10.1111/jdv.16926
- Competenzzentrum Versorgungsforschung in der Dermatologie (CVderm). [Accessed December 2022] Available from: https://www.uke.de/kliniken-institute/institute/versorgungsforschung-in-der-dermatologie-und-bei-pflegeberufen/bereiche/cvderm/index.html.
- SDNTT (Swiss Dermatology Network for Targeted Therapies). [Accessed September 2022] Available from: https://www.sdntt.ch/de/.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
- World Medical Association (WMA). Declaration of Helsinki. [Accessed December 2022] Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
- Mason KJ, Barker JNWN, Smith CH, Hampton PJ, Lunt M, McElhone K, et al. Comparison of Drug Discontinuation, Effectiveness, and Safety Between Clinical Trial Eligible and Ineligible Patients in BADBIR. JAMA Dermatol 2018; 154: 581–588. https://doi.org/10.1001/jamadermatol.2018.0183
- Davison NJ, Warren RB, Mason KJ, McElhone K, Kirby B, Burden AD, et al. Identification of factors that may influence the selection of first-line biological therapy for people with psoriasis: a prospective, multicentre cohort study. Br J Dermatol 2017; 177: 828–836. https://doi.org/10.1111/bjd.15551
- Hotard RS, Feldman SR, Fleischer AB, et al. Sex-specific differences in the treatment of severe psoriasis. J Am Acad Dermatol 2000; 42: 620–623. https://doi.org/10.1067/mjd.2000.101596
- Li S, Xu Y, Zheng L, Pang H, Zhang Q, Lou L, et al. Sex Difference in Global Burden of Major Depressive Disorder: Findings From the Global Burden of Disease Study 2019. Front Psychiatry 2022; 13: 789305. https://doi.org/10.3389/fpsyt.2022.789305
- Wojtyna E, Łakuta P, Marcinkiewicz K, Bergler-Czop B, Brzezińska-Wcisło L. Gender, Body Image and Social Support: Biopsychosocial Determinants of Depression Among Patients with Psoriasis. Acta Derm Venereol 2017; 97: 91–97. https://doi.org/10.2340/00015555-2483
- Lewinson RT, Vallerand IA, Lowerison MW, Parsons LM, Frolkis AD, Kaplan GG, et al. Depression is associated with an increased risk of psoriatic arthritis among patients with psoriasis: a population-based study. J Invest Dermatol 2017; 137: 828–835. https://doi.org/10.1016/j.jid.2016.11.032
- Secrest AM, Chren MM. Incorporating patient-reported outcomes as a vital sign for dermatologic clinical care and clinical investigations. J Invest Dermatol 2022; 142: 1529–1532. https://doi.org/10.1016/j.jid.2022.01.008
- Barbieri JS, Gelfand JM. Patient-reported outcome measures as complementary information to clinician-reported outcome measures in patients with psoriasis. JAMA Dermatol 2021; 157: 1236–1237. https://doi.org/10.1001/jamadermatol.2021.3341
- Nast A, Altenburg A, Augustin M, Boehncke WH, Härle P, Klaus J, et al. German S3-Guideline on the treatment of Psoriasis vulgaris, adapted from EuroGuiDerm – Part 1: Treatment goals and treatment recommendations. J Dtsch Dermatol Ges 2021; 19: 934–950. https://doi.org/10.1111/ddg.14508
- Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res 2018; 310: 271–319. https://doi.org/10.1007/s00403-018-1808-x