The chemical composition of propolis varies with geographical origin; however, it is not known whether this affects the frequency of contact allergy to propolis. In order to study the frequency of contact allergy to propolis of different geographical origins and concomitant reactions, 1,470 consecutive patients with dermatitis from Denmark, Lithuania and Spain were patch tested with propolis from China, Lithuania, North America and Sweden, and with a baseline series. Patch test reactions to any type of propolis ranged from 1.3% to 5.8%. There were no statistically significant differences in the frequency of positive reactions between the 4 types of propolis in the respective countries. Testing with a single commercially available type of propolis detects only approximately half of propolis-allergic patients. In patients allergic to propolis, concomitant reactions to Myroxylon pereirae resin, colophonium and Fragrance mix I were common, ranging from 12.5% to 50.0%.
Key words: propolis; allergic contact dermatitis; patch test; cross-reaction; colophonium; balsam of Peru.
Accepted Oct 18, 2021; Epub ahead of print Oct 18, 2021
Acta Derm Venereol 2021; 101: adv00591.
doi: 10.2340/actadv.v101.423
Corr: Gunnar Nyman, Department of Dermatology and Venereology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gröna stråket 16, SE-413 46 Göteborg, Sweden. E-mail: nyman@cutis.nu
SIGNIFICANCE
Propolis is a sticky substance produced by bees. It is used in consumer products and can cause contact allergy. The chemical composition of propolis varies depending on the geographical origin where it is produced. It is not known if this influences the tendency of propolis to cause contact allergy. This study investigated the frequencies of contact allergy to propolis of 4 different geographical origins in patients with dermatitis in Denmark Lithuania and Spain. Frequencies of contact allergy to all propolis types were similar. However, half of the patients reacting to each propolis type reacted only to that type.
INTRODUCTION
Many ingredients of cosmetics and topical remedies can cause both irritant and allergic contact dermatitis (1–3). To avoid such problems, many people use skin products of natural origin, in which propolis (bee glue) may be a component (4). Propolis is produced by honeybees from collected tree exudates, mixed with beeswax and the bee’s saliva (5).
The most important sources of plant material for propolis in Europe are different types of poplar trees (Populus spp) (5). Topical remedies and cosmetics that contain propolis are well-known causes of contact allergy among consumers (6), and propolis was therefore included in the European baseline series in 2019. Several haptens have been demonstrated in propolis, among which caffeic acid and ester derivatives thereof have been suggested as main haptens (7, 8). Due to the partly botanical origin of propolis, concomitant positive patch test reactions to propolis and fragrances or plant-derived test preparations in the baseline series are common.
Although the chemical composition of propolis differs according to geographical regions (9), it is not known how this variation influences the risk of causing contact allergy. Knowledge of the importance of the origin of propolis in diagnosis of contact allergy is scarce.
The main aim of this study was to investigate the frequencies of propolis allergy in patients with contact dermatitis in Denmark, Lithuania and Spain. Another aim was to investigate whether there were differences in the frequencies of contact allergy to propolis of different geographical origin in the respective test centres. Finally, the study also investigated the frequency of concomitant positive patch test reactions to propolis, colophonium, Myroxylon pereirae resin (MPR) and Fragrance mix I (FM I) from the baseline series. The results were compared with our previous results from a study of Swedish patients (10).
PATIENTS AND METHODS
Study population
This multicentre study was conducted at dermatology departments in Denmark (Odense), Spain (Barcelona), and 2 departments in Lithuania (Centre of Dermatovenereology and Clinic of Chest Diseases, Immunology and Allergology, both Vilnius), during a 12-month period at each centre during 2017 to 2019. Consecutive patients with dermatitis referred for patch testing with the baseline series used in the respective countries were also patch tested with propolis of 4 different origins (Table I). Of the 1,470 patients tested, 1,033 were female and 437 were male (female/male 70/30%); mean age 43.4 years.
Patch test preparations
The patch test preparations for the baseline series used in Lithuania and Spain were bought from Chemotechnique Diagnostics (Vellinge, Sweden) and Allergeaze Marti Tor Alergias (Barcelona, Spain), respectively, while the baseline series in Denmark included True Test, Smart Practice (Phoenix, AZ, USA) and petrolatum preparations from Chemotechnique Diagnostics and Smart Practice. The Dermatology department at Sahlgrenska University Hospital, Gothenburg, Sweden bought the propolis preparations of Chinese and North American origin from Chemotechnique Diagnostics and Smart Practice, respectively. The propolis originating from the west coast of Sweden and the Kaunas region in Lithuania were provided directly by 1 beekeeper in each area and prepared by Chemotechnique Diagnostics into 10% in petrolatum in the same way as the Chinese-type propolis in their regular range. All propolis preparations were then distributed from Gothenburg to the participating clinics.
Patch testing
Patch testing and reading of the patient’s results were carried out according to the European Society of Contact Dermatitis (ESCD) guideline (11). Finn chambers (8-mm diameter; Smart Practice) on Scanpor tape (Norgesplaster, Vennesla, Norway) were used for the propolis preparations in all centres. A dose of 20 mg was applied. Relevance was assessed based on time-related exposure to propolis in the patient history. According to a previously presented scoring system for multicentre studies, the current study was of high quality (12).
Statistical analyses
All data were analysed using R version 3.5.3 (The R Foundation for Statistical Computing, Vienna, Austria). Fisher’s exact test was used for comparing proportions and the exact binomial test was used for paired tests. All tests were 2-sided and p-value < 0.05 was considered statistically significant.
Confidence intervals of frequencies of contact allergy were calculated using OPENEPI (http://openepi.com).
RESULTS
In the total study population, frequencies of positive reactions to the 4 propolis types ranged from 1.2% to 1.8% and in the study populations of each country, 1.3–5.8% of the patients had positive patch test reactions to any of the propolis types (Table I). The most frequent causes of positive reactions (1.8%) were propolis originating from Lithuania (0.6–2.9%) and China (1.1–2.4%), while the least frequent cause of reactions was that originating from Sweden (1.2%, 0–2.4%). The frequencies of positive reactions to any type of propolis were significantly higher in Lithuania (p < 0.0001) and Denmark (p = 0.029) compared with Spain. Most reactions (60%) were weak (+) and 40% were strong or extreme (++ or +++).
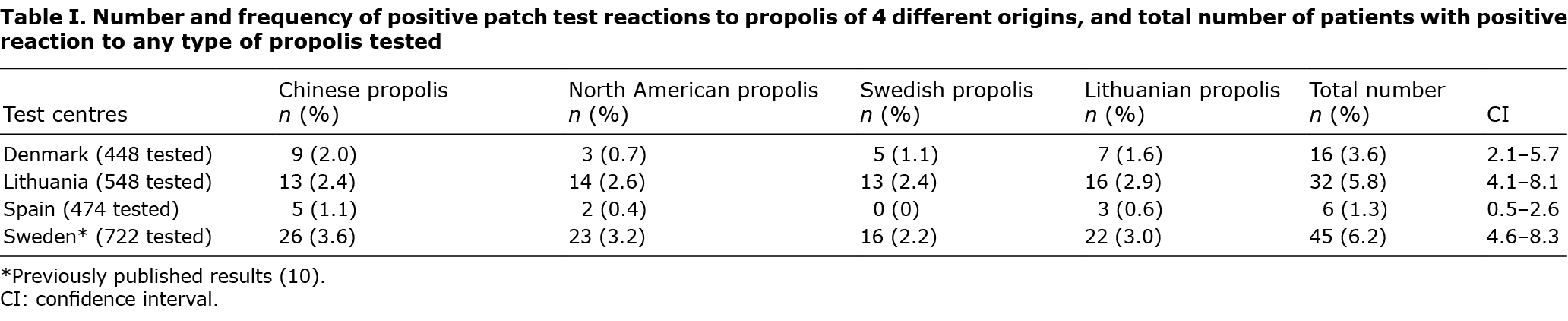
Of the 54 patients with a positive patch test to propolis, 29 (54%) reacted to only 1 of the 4 origins of propolis. A reaction only to propolis from China was found in 16 patients (30%), only to propolis from North America in 7 patients (13%), only to propolis from Lithuania in 4 patients (7%), and only to propolis from Sweden in 2 patients (4%). Sixteen patients reacted to 2 types of propolis (30%), 7 patients reacted to 3 types of propolis (13%), and 2 patients (4%) reacted to all 4 types of propolis. Concomitant reactions to 2 types of propolis were most frequent to those of Lithuanian and Swedish origin; 16 out of 26 (61%) and 16 out of 26 (61%) patients, respectively. As Swedish propolis detected very few unique reactions, it was omitted from further analysis. The pattern of concomitant reactions to propolis from China, North America and Lithuania is shown in Fig. 1. Approximately half of the positive patch test reactions to any propolis type were unique, and the rest concomitant with other propolis types.
Clinical relevance of the propolis reactions was recorded in Denmark, Spain and 1 centre in Lithuania. In Denmark, current relevance was found in 3/16 patients (19%) and past relevance in 1/16 patients (6%), while in Lithuania 3/10 patients (30%) had current and 4/10 patients (40%) had past relevance. All positive reactions to propolis in Spain 6/6 (100%) were considered as having current relevance. In total, of the patients assessed, 38% of propolis reactions were considered to have current relevance, whereas 16% of propolis reactions were considered to have past relevance.
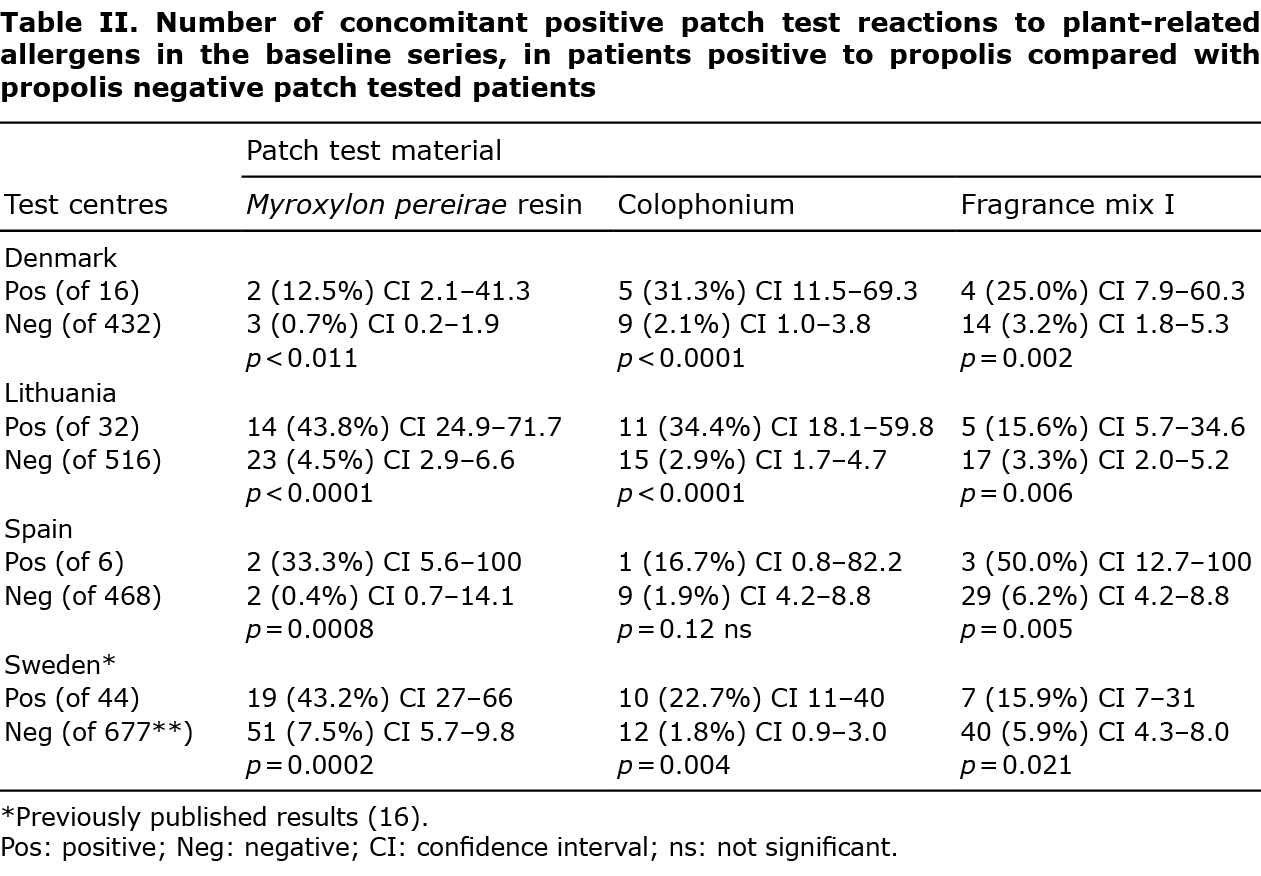
Frequent concomitant positive test reactions to propolis and plant-related allergens in the baseline series were found, mainly to MPR (12.5–43.8%), colophonium (16.7–34.4%), and FM I (15.6–50.0%) (Table II). Positive patch test reactions to fragrances and plant-derived allergens in the baseline series were significantly more frequent among patients with a positive propolis test (12.5–50.0%) than in those with a negative propolis test (0.4–6.2%) (p < 0.0001–0.011), except for colophonium in Spain (p = 0.12).
In total, there were 76 (5.2%) doubtful reactions to any type of propolis, all from 1 centre (Denmark). Eight irritant reactions (0.5%) were recorded, also all from Denmark. The majority, both of doubtful and of irritant reactions, were due to the propolis originating from China: 40 (2.7%) and 3 (0.20%), respectively. No late reactions were recorded.
DISCUSSION
Frequencies of positive reactions to all types of propolis were in the same range as those of the haptens of the baseline series (13). A higher frequency of positive patch test reactions to propolis of all 4 origins was found in Denmark and Lithuania, while the frequency was significantly lower in patients from Spain. The frequencies of reactions to all types of propolis are in line with earlier results from other European countries, 0.5–6.5% (5, 14, 15), except for 1 report from Poland, which showed 15% positive reactions to propolis in consecutive patients with dermatitis (16). In the current study of patients with dermatitis from Denmark, Lithuania and Spain, the same 4 batches of propolis from different geographical regions were used, as in our previous study of patients with dermatitis from western Sweden. The results from Denmark and Lithuania are in line with this study (Table I) (10), where positive reactions to any type of propolis were recorded in 6.2% of tested patients, ranging from 2.2% to 3.6% among the 4 types of propolis. In the Swedish patients, the frequency of positive reactions to any type of propolis was significantly higher (p < 0.0001), than that of the Spanish patients. In the Swedish patients, the propolis of Chinese origin showed the highest frequency of positive reactions, at 3.6%. It is difficult to get accurate information on the use of propolis in the general population in different countries, although the impression gained from organizations selling or producing propolis is that its use is increasing (personal contact, The Danish Beekeepers Association).
Although small differences in frequencies were found, there were no statistically significant differences in the number of positive reactions between the 4 different types of propolis in any of the 3 countries, individually or together. This result is similar to that in the Swedish patients, where no significant differences were found (16). The explanation may be that the propolis types used in this study are similar; for example, because the same type of poplar has surrounded all the beehives, or that there are several important haptens in propolis, which appear in many propolis types. However, since half of the reactions to any of the propolis types are unique, this indicates that the hapten composition in propolis of different origins might differ.
An earlier study, of contact allergy in beekeepers to propolis from different areas of the British Isles, found that 84% of beekeepers reacted, in addition to propolis from their own hives, also to propolis from other locations in the British Isles (17). The different types of propolis used in the current study originate from regions that are much more geographically separated, which could have meant greater differences in composition. This is supported by a lower proportion of concomitant reactions to the propolis types (19–56%) in the current study compared with the British beekeepers. The exception is Spain, where 83% of patients reacting to Chinese propolis also reacted to 1 or more of the others. However, in Spain the total number of positive reactions was smaller. The corresponding frequency of concomitant reactions in the study in Sweden was 58%. It is difficult to specify the origin of propolis in consumer products, since the raw substance can pass several countries before being prepared in a product available on the market. This means that individuals who handle propolis directly, such as beekeepers, are mostly exposed to propolis of known origin, but for other individuals the origin mostly remains obscure.
In screening studies of contact allergy to propolis, the most commonly used concentration of propolis in patch test preparations is 10% in petrolatum, although some investigators have used both 20% and 5% (14, 18). To our knowledge, there are no comparative studies with propolis of different concentrations. As both commercially available propolis preparations are of 10%, this concentration was used for the Swedish and Lithuanian propolis prepared for the present investigation. The investigations where propolis 5% or 20% were used for testing or where alcohol was used as a vehicle instead of petrolatum, are so few that it is difficult to draw any conclusions in the results depending on the concentration or vehicle (5, 18). One study of 1,255 consecutive children with suspected allergic contact dermatitis showed a high frequency of positive reactions to propolis 20% in petrolatum, at 5.9% (14).
There are large differences in frequencies of doubtful reactions between test centres, which might be due to local traditions of recording patch test reactions, and to the difficulties in determining if a reaction is very weak allergic or very weak irritant (19). In both the Danish cohort and in our earlier Swedish study, there are high numbers of doubtful reactions, which can be a problem if propolis is tested regularly. One explanation for this could be that the test concentration is too high; with some reactions judged as doubtful instead being weak irritant reactions. This was previously shown for patch testing with iodopropynyl butyl carbamate, for which an increased test concentration led to an increase in the number of positive test reactions, but an even greater increase in doubtful and irritant reactions (20). However, the test concentration of 10% could be too low, meaning that some weak allergic reactions are classified as doubtful. This issue has been discussed in several previous articles regarding propolis, methyldibromo glutaronitrile, limonene hydroperoxides and linalool hydroperoxides, where the increase in positive reactions when increasing the test concentration was higher than the increase in irritant and doubtful reactions (13, 21–23). The fact that the majority of positive reactions in the current study were weak could also indicate that the test concentration is too low. To evaluate the optimal test concentration of propolis, further studies are needed, including dilution series and repeated open application test. One important issue when deciding test concentration is to be aware of the risk of sensitization.
An interesting finding in the propolis-sensitized patients is the higher frequencies of concomitant reactions to colophonium, MPR and FM I compared with a general test population. This can be explained both as sharing the same haptens, for example cinnamic acid, benzyl benzoate and cinnamyl cinnamate in both propolis and MPR, and cinnamyl alcohol and eugenol in propolis and FM I (5). Propolis-positive patients react 9, 16, and 6 times as frequently to colophonium, MPR and FM I, respectively, in the Danish patients, 7, 6 and 4 times as frequently in the Lithuanian patients and 9, 80 and 8 times as frequently in the Spanish patients compared with the propolis test-negative patients (Table II). The large difference regarding MPR for the Spanish patients is probably due to the small sample size.
The clinical relevance of propolis allergy recorded here is in line with earlier studies (5), which motivated the inclusion of propolis in the European baseline series (24). As a high frequency of concomitant positive reactions to propolis and beeswax has been shown (25), contact with the latter could be relevant for patients with a positive reaction to propolis. Concomitant reactions to other plant-derived substances in the baseline series suggest concomitant exposure and might thus indicate a likelihood that the reaction to propolis is relevant. Thus, it seems important that patients with contact allergy to propolis, MPR, colophony, or FM I should be informed of the risk of concomitant reactions to other plant-related materials and fragrances.
This study, to our knowledge, is the first international one on contact allergy to propolis of different geographical origin, and together with our previous study in Sweden the first systematic studies on contact allergy to propolis in Scandinavia (16). The results from this study and the study on patients from western Sweden indicate that propolis of different geographical origin does not differ very much in pick-up rate of contact allergy. However, many patients reacted to only 1 type of propolis. There are several important haptens in propolis, and it is still to be determined which are the most important. The esters of caffeic acid have been considered as main haptens (8), but it is unclear if the esters directly, or possible oxidation products, are the most important. Similarly, the optimal test concentration of propolis needs further investigation.
The current study has some weaknesses. Information on the hapten content of propolis preparations is lacking. Another problem regards interpersonal variation in reading of the patch tests by different individuals between and in the different countries. This is a problem in most multicentre studies, and must be taken into consideration when evaluating the results.
One may conclude that contact allergy to propolis is common among patients with dermatitis in all the countries in the study, which supports the recently implemented inclusion of propolis in the European baseline series (24). There are, however, no big differences in frequencies of patch test reactions regarding the geographical origin of propolis. The situation might be different if testing special groups of patients, such as beekeepers, who might have a higher tendency to react to their local propolis. The 2 commercially available propolis test preparations using propolis originating from China and North America detect approximately half (48%) and one-third (35%), respectively, of all patients with a positive reaction to propolis in this study. Thus, although the frequencies of reactions to propolis of different geographical origin do not differ greatly, the pattern of reactivity differs. Of the preparations studied, no single preparation picks up all detected cases of propolis contact allergy. Testing with the patient’s own products is thus important and, if there is a high suspicion of propolis allergy, commercially available propolis preparations of different geographical origin can be used.
ACKNOWLEDGEMENTS
The study was supported by a grant from “Hudfonden”, Sweden. The authors thank Martin Gillstedt for statistical analysis; the apiary “Djäknegårdens honung” in County Halland; beekeeper Antanas Kliučinskas in Kaunas region, for providing raw propolis from Sweden and Lithuania, respectively; and colleague Aistė Beliauskienė for transporting the Lithuanian propolis to Sweden.
The authors have no conflicts of interest to declare.
REFERENCES
- Malhotra S DS, Singal A, Kaur M. To study the incidence of contact hypersensitivity to commonly used topical antibacterials. Internet J Dermatol 2010; 8: 1–7.
- Wolf R, Wolf D, Tüzün B, Tüzün Y. Cosmetics and contact dermatitis. Dermatol Ther 2001; 14: 181–187.
- Rastogi S, Patel KR, Singam V, Silverberg JI. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis. J Am Acad Dermatol 2018; 79: 1028–1033.e6.
- Corazza M, Borghi A, Lauriola MM, Virgili A. Use of topical herbal remedies and cosmetics: a questionnaire-based investigation in dermatology out-patients. J Eur Acad Dermatol 2009; 23: 1298–1303.
- AC dG. Propolis: a review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 2013; 24: 263–284.
- Walgrave S.E. WEM, Glesne L.A. Allergic contact dermatitis from propolis. Dermatitis 2005; 16: 209–215.
- Hausen BM, Evers P, Stüwe HT, König WA, Wollenweber E. Propolis allergy (IV) Studies with further sensitizers from propolis and constituents common to propolis, poplar buds and balsam of Peru. Contact Dermatitis 1992; 26: 34–44.
- Hausen BM. Evaluation of the main contact allergens in propolis (1995–2005). Dermatitis 2005; 16: 127–129.
- Rudzki E, Grzywa Z. Dermatitis from propolis. Contact Dermatitis 1983; 9: 40–45.
- Nyman G, Wagner S, Prystupa-Chalkidis K, Ryberg K, Hagvall L. Contact allergy in western Sweden to propolis of four different origins. Acta Derm Venereol 2020; 100: adv00256.
- Johansen JD, Aalto-Korte K, Agner T, Andersen KE, Bircher A, Bruze M, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing – recommendations on best practice. Contact Dermatitis 2015; 73: 195–221.
- Bruze M. Thoughts on how to improve the quality of multicentre patch test studies. Contact Dermatitis 2016; 74: 168–174.
- Uter W, Amario-Hita JC, Balato A, Ballmer-Weber B, Bauer A, Fortina AB, et al. European Surveillance System on Contact Allergies (ESSCA): results with the European baseline series, 2013/14. J Eur Acad Dermatol Venereol 2017; 31: 1516–1525.
- Uter W, Spiewak R, Cooper SM, Wilkinson M, Sánchez Pérez J, Schnuch A, et al. Contact allergy to ingredients of topical medications: results of the European Surveillance System on Contact Allergies (ESSCA), 2009–2012. Pharmacoepidemiol Drug Safety 2016; 25: 1305–1312.
- Giusti F, Miglietta R, Pepe P, Seidenari S. Sensitization to propolis in 1255 children undergoing patch testing. Contact Dermatitis 2004; 51: 255–258.
- Pietowska J CE, Spiewak R. The most frequent contact sensitizers and atopic diseases among consecutive patients of a Polish test clinic. Allergy 2008; 63: 320.
- Bunney MH. Contact dermatitis in beekeepees due to propolis (bee glue). Br J Dermatol 1968; 80: 17–23.
- Barile M, Cozzani E, Anonide A, Usiglio D, Burroni A, Guarrera M. Is contact allergy rare in psoriatics? Contact Dermatitis 1996; 35: 113–114.
- Svedman C, Isaksson M, Björk J, Mowitz M, Bruze M. ‘Calibration’ of our patch test reading technique is necessary. Contact Dermatitis 2012; 66: 180–187.
- Brasch J, Schnuch A, Geier J, Aberer W, Uter W. Iodopropynylbutyl carbamate 0.2% is suggested for patch testing of patients with eczema possibly related to preservatives. Br J Dermatol 2004; 151: 608–615.
- Gruvberger B, Andersen KE, Brandão FM, Bruynzeel DP, Bruze M, Frosch PJ, et al. Patch testing with methyldibromo glutaronitrile, a multicentre study within the EECDRG. Contact Dermatitis 2005; 52: 14–18.
- Wlodek C, Penfold CM, Bourke JF, Chowdhury MMU, Cooper SM, Ghaffar S, et al. Recommendation to test limonene hydroperoxides 0.3% and linalool hydroperoxides 1.0% in the British baseline patch test series. Br J Dermatol 2017; 177: 1708–1715.
- Deza G, García-Bravo B, Silvestre JF, Pastor-Nieto MA, González-Pérez R, Heras-Mendaza F, et al. Contact sensitization to limonene and linalool hydroperoxides in Spain: a GEIDACprospective study: contact allergy to limonene and linalool hydroperoxides. Contact Dermatitis 2017; 76: 74–80.
- Wilkinson M, Gallo R, Goossens A, Johansen JD, Rustemeyer T, Sánchez-Pérez J, et al. A proposal to create an extension to the European baseline series. Contact Dermatitis 2018; 78: 101–108.
- Nyman GSA, Tang M, Inerot A, Osmancevic A, Malmberg P, Hagvall L. Contact allergy to beeswax and propolis among patients with cheilitis or facial dermatitis. Contact Dermatitis 2019; 81: 110–116.