RESEARCH LETTER
Effectiveness and Safety of Spesolimab in Patients with Generalized Pustular Psoriasis: A Single-centre Retrospective Study
Yoshiki OKADA1, Masahiro KAMATA1*, Kotaro HAYASHI1, Kazumitsu SUGIURA2 and Yayoi TADA1
1Department of Dermatology, Teikyo University School of Medicine, 2-11-1 Kaga, Itabashi-ku, Tokyo, and 2Department of Dermatology, Fujita Health University, Toyoake, Japan.
*E-mail: mkamata-tky@umin.ac.jp
Citation: Acta Derm Venereol 2025; 105: adv42879. DOI: https://doi.org/10.2340/actadv.v105.42879.
Copyright: © 2025 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Jan 7, 2025. Accepted after revision: Jan 15, 2025. Published: Feb 5, 2025
Dear Editor,
Spesolimab, an anti-interleukin (IL)-36 receptor antibody, showed efficacy for generalized pustular psoriasis (GPP) in a clinical trial (1). However, as it had many exclusion criteria, it did not necessarily reflect the effectiveness and safety of spesolimab in a real-world setting. Furthermore, because GPP is a rare disease, real-world evidence is limited. We retrospectively investigated the effectiveness and safety of spesolimab treatment in GPP patients in our department.
GPP patients treated with spesolimab in our department until August 2024 were included. Effectiveness was evaluated, including generalized pustular psoriasis area and severity index (GPPASI); Japanese Dermatological Association (JDA) severity score (2); percentages of patients who achieved generalized pustular psoriasis physician global assessment (GPPGA) 0/1, a GPPGA pustulation subscore of 0, an erythema subscore of 0/1, or scales/desquamation subscore 0/1; and safety. The diagnosis of GPP was made based on clinical and pathological manifestations by experienced board-certified dermatologists following the Japanese guidelines (2).
Seven patients (4 males and 3 females) were analysed, including 2 patients previously reported (3, 4). The median age of the patients was 49 (1st quartile, 43; 3rd quartile, 55) years. Four patients received etretinate, one cyclosporine, one bimekizumab, and one brodalumab immediately before initiating spesolimab. Gene analysis was performed in 3 patients; the IL36RN mutation was observed in 1 patient (4). The median GPPASI and JDA severity scores at the beginning of treatment with spesolimab were 18.8 (14.1, 41.3) and 10 (7, 12), respectively. Five patients had a GPPGA score of 3, and 2 patients had a score of 2. GPPASI significantly decreased at days 1–3 (median, 63.6%; 1st quartile, 60.9%; 3rd quartile, 73.8%; p = 0.0018), week 1 (36.9%, 24.0%, 41.9%; p < 0.0001), and week 2 (24.8%, 13.9%, 36.7%; p < 0.0001) in percentage compared with baseline values, and did so in absolute values at week 1 (9, 4.1, 12.4; p = 0.0167) and week 2 (6.1, 4.5, 7.2; p = 0.0076; Fig. 1A). The JDA severity score significantly decreased at week 1 (71.4%, 41.7%, 82.5%; p = 0.0385) and week 2 (40%, 25%, 50%; p = 0.0014) in percentage compared with baseline values, and did so in absolute values at week 2 (3, 2, 4; p = 0.0163; Fig. 1B). All patients achieved a GPPGA subscore of 0/1 at day 7 (Fig. 1C). Although all patients achieved a GPPGA pustulation subscore of 0 on day 7, the percentage of patients who achieved a GPPGA ery-thema or scales/desquamation subscore of 0/1 was 57% and 43%, respectively (Fig. 1D). Two patients received ixekizumab (for further improvement) on days 2 or 4. During treatment with spesolimab, erythema multiforme was observed in 1 patient, as previously described (4).
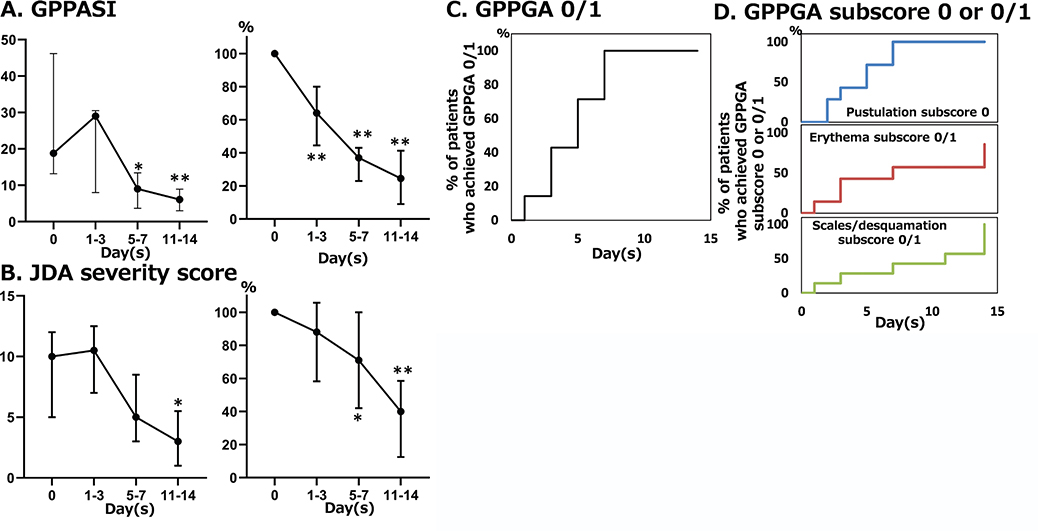
Fig. 1. Effectiveness of spesolimab. (A) Change in generalized pustular psoriasis area and severity index (GPPASI) in absolute values (left panel) and in percentage relative to baseline values (right panel). (B) Change in Japanese Dermatological Association (JDA) severity scores in absolute values (left panel) and in percentage relative to baseline values (right panel). (C) Cumulative percentage of patients who achieved generalized pustular psoriasis physician global assessment (GPPGA) 0 or 1. (D) Cumulative percentage of patients who achieved GPPGA pustulation subscore 0, erythema subscore 0 or 1, or scales/desquamation subscore 0 or 1. The values are shown as medians ±1st and 3rd quartiles. Normality was assessed using the Shapiro–Wilk test. Ordinary one-way ANOVA then Dunnett’s multiple comparison test were utilized for multiple comparison. *p < 0.05, **p < 0.01.
In this study, patients receiving spesolimab treatment showed rapid improvement in clinical manifestations, especially pustules, with tolerable safety. Erythema and scales took longer to improve than pustules. Spesolimab showed effectiveness regardless of IL36RN mutation, as did ixekizumab (5). As the JDA severity score includes serum levels of albumin and C-reactive protein, it might have taken more time for the score to decrease significantly compared with GPPASI. The small number of patients with GPP (due to its rarity) and the concomitant treatment are study limitations. Our data indicate a rapid onset of the effectiveness of spesolimab with tolerable safety in a real-world setting.
ACKNOWLEDGEMENTS
Potential conflicts of interest: MK received honoraria for lectures from Boehringer Ingelheim. YT received honoraria for lectures and grants for research irrelevant to this study from Boehringer Ingelheim.
Data availability: Data available on reasonable request, subject to privacy/ethical restrictions.
Author contributions: MK and YT designed the study. YO and MK wrote the manuscript. All authors contributed to data collection. YO and MK interpreted the results. YT supervised this study. All authors read and approved the final manuscript.
Previous presentations: Parts of our results will be presented at the 2025 AAD annual meeting held in Orlando, USA, on 7–11 March 2025.
Patient consent statement: Informed consent was obtained in the form of opt-out on the website. Patients who rejected were excluded.
Approval of the research protocol: This study was approved by the ethics committee of Teikyo University (24-087), and was carried out under the principles of the Declaration of Helsinki.
REFERENCES
- Bachelez H, Choon SE, Marrakchi S, Burden AD, Tsai TF, Morita A, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med 2021; 385: 2431–2440. https://doi.org/10.1056/NEJMoa2111563
- Fujita H, Terui T, Hayama K, Akiyama M, Ikeda S, Mabuchi T, et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol 2018; 45: 1235–1270. https://doi.org/10.1111/1346-8138.14523
- Suzuki S, Kamata M, Ito M, Watanabe A, Uchida H, Chijiwa C, et al. Spesolimab improved pustules on the palms and soles that were refractory to tumor necrosis factor inhibitors and interleukin-17 inhibitors in a patient with generalized pustular psoriasis: a case report. J Dermatol 2024; 51: e416–e418. https://doi.org/10.1111/1346-8138.17337
- Nakajima H, Kamata M, Okada Y, Ito M, Watanabe A, Azuma S, et al. Erythema multiforme after initiating spesolimab in a patient with generalized pustular psoriasis with IL36RN mutation. Eur J Dermatol 2024; 34: 324–325. https://doi.org/10.1684/ejd.2024.4711
- Nagata M, Kamata M, Fukaya S, Hayashi K, Fukuyasu A, Tanaka T, et al. Real-world single-center experience with 10 cases of generalized pustular psoriasis successfully treated with ixekizumab. J Am Acad Dermatol 2020; 82: 758–761. https://doi.org/10.1016/j.jaad.2019.09.040