Basal cell carcinoma is the most prevalent cancer in Caucasians worldwide. The aim of this study was to examine the overall risk of melanoma among patients diagnosed with basal cell carcinoma. This population-based retrospective cohort study included data from January 2010 to December 2018 from the databases of the Clalit Health Maintenance Organization and 2 major pathology laboratories in North District, Israel. The incidence and hazard ratio of melanoma in patients with a diagnosis of basal cell carcinoma were determined. Of 466,700 participants, 51% were women and the mean (standard deviation) follow-up was 6.7 (2.9; range 1–9) years. A total of 3,338 patients were diagnosed with basal cell carcinoma during the study period, 82 of whom subsequently developed melanoma. Patients with basal cell carcinoma had a significantly higher incidence of melanoma than patients without basal cell carcinoma (2.46% vs 0.37%; p < 0.0001). Univariate Cox regression analysis revealed a hazard ratio of 6.6 (95% confidence interval: 3.6–12.1; p < 0.0001) for melanoma in patients with a diagnosis of basal cell carcinoma. In conclusion, a diagnosis of basal cell carcinoma confers a significant risk of melanoma.
Key words: basal cell carcinoma; melanoma; malignant melanoma; skin cancer; risk factor; cohort study.
Accepted Dec 16, 2022; Published Jan 5, 2023
Acta Derm Venereol 2023; 103: adv00841.
DOI: 10.2340/actadv.v103.4402
Corr: Daniella Kushnir-Grinbaum, Dermatology Department, Emek Medical Center, Yitshak Rabin Boulevard 21, Afula 1834111, Israel. E-mail: daniellakushnir@gmail.com
SIGNIFICANCE
In this large, population-based, retrospective cohort study that included 466,700 adults, we found that patients with a basal-cell carcinoma diagnosis had a 6.6-fold increased risk of melanoma than those without a basal-cell carcinoma diagnosis. In addition to basal-cell carcinoma, male sex and age ≥ 60 years were found to be independent risk factors for melanoma. Our findings suggest that patients with basal-cell carcinoma should be considered at high risk for melanoma and undergo regular screening for melanoma, especially during the first 2–3 years after basal-cell carcinoma diagnosis.
INTRODUCTION
Basal cell carcinoma (BCC) is the most common cancer in Caucasians worldwide (1–3). The lifetime risk of BCC in Americans is estimated to be 30% (4). Limited information is available in the literature regarding the epidemiology of BCC, because BCCs are not routinely documented in most national cancer registries.
Early diagnosis of malignant melanoma is of utmost importance, as its prognosis dramatically worsens when the diagnosis is made at later stages of the disease (5). Predisposing factors for malignant melanoma include fair skin, history of sun exposure, family history of melanoma, multiple naevi (6), dysplastic naevi (7), and genetic factors (8).
Ample evidence suggests that patients with non-melanoma skin cancer are at increased risk of neoplasia (9–16), although there is limited evidence regarding the risk of melanoma associated with BCC. Several studies on this subject determined a diagnosis of BCC based solely on clinical features, and the validity of the diagnosis was not supported by pathological findings, suggesting the possibility of bias (9–12).
The aim of this study was to examine BCC as a risk factor for melanoma and to define at-risk subpopulations.
MATERIALS AND METHODS
Ethics considerations
This population-based retrospective cohort study was approved by the ethics committees of Emek Medical Center, Afula, Israel, and Clalit Health Maintenance Organization (HMO), North Israel District, Israel. Both committees waived the need for informed consent.
Participants
The study included patients 18 years or older who were insured by Clalit HMO Northern Israel District between 1 January 2010 and 31 December 2018. Exclusion criteria were: patients with a pre-existing diagnosis of melanoma and those who had been diagnosed with both melanoma and BCC if their melanoma diagnosis preceded their BCC diagnosis (n = 60). BCC and melanoma were diagnosed based on the results of pathology reports.
Data extraction
Data were extracted from the databases of the Clalit HMO and 2 major pathology laboratories in Northern District, Israel: Emek Pathology Laboratories and Patho-Lab Diagnostics. Clalit HMO is the largest health insurance provider in Israel; 70% of the population of Northern District is insured by Clalit. Data regarding the demographic characteristics of the patients and controls were extracted from medical records using a standardized data collection tool. Data regarding BCC or melanoma diagnosis, if any, were extracted from the digital database of the pathology laboratories. The dates of BCC diagnosis and/or melanoma diagnosis (if any) were obtained for each eligible patient. The year of enrolment was defined as the year in which BCC was first diagnosed in that patient.
Statistical analyses
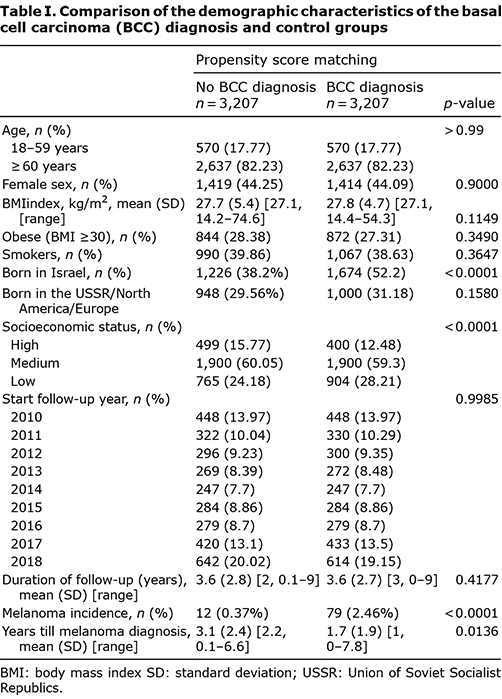
Statistical analyses and data management were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). Propensity score matching (PSM) according to age, sex, place of birth, smoking status, socioeconomic status, and religious background was performed to identify a control group. The PSM was conducted to minimize differences in the demographic characteristics between the study and control groups and to equalize the group sizes. The year of enrolment was matched between the control and BCC diagnosis groups.
Continuous variables are presented as a mean (standard deviation; SD) or a median (range), while categorical variables are presented as frequency and percentage. The groups were compared using a χ2 test or Fisher’s exact test for categorical variables and t-test or Wilcoxon rank-sum test for continuous variables. Univariate and multivariable Cox regression analyses were performed to explore the associations between potential risk factors and melanoma incidence. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated. Statistical significance was set at p < 0.05.
RESULTS
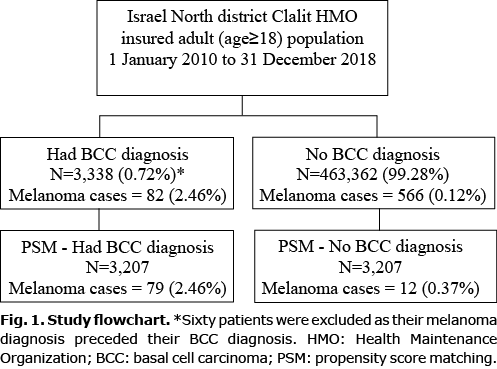
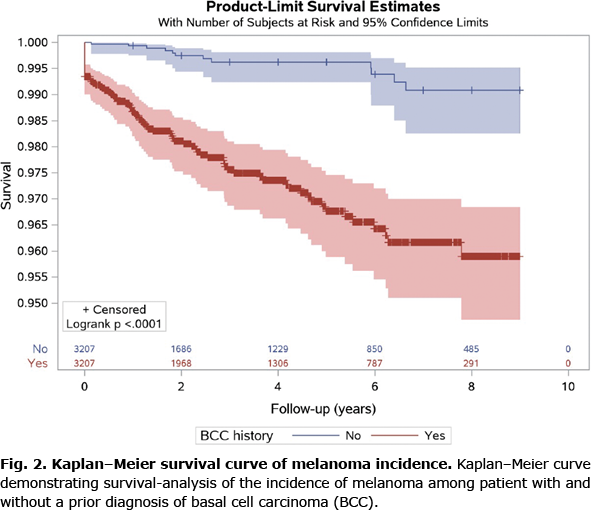
A total of 466,700 individuals were included in the study; 60 participants were excluded as their diagnosis of melanoma preceded their diagnosis of BCC. A total of 3,338 patients were diagnosed with BCC during the study period, of whom 82 (2.46%) subsequently developed melanoma (Fig. 1). The PSM was conducted with 1:1 greedy matching. There was a 96% (n = 3,207) propensity score match between the BCC diagnosis and control groups (Table I). Patients’ data for a mean (standard deviation; SD) of 6.7 (2.9) years (range 1–9 years) were available, and 51% of the participants were women. The mean (SD) times to melanoma were 1.7 (1.9) years and 3.1 (2.4) years in patients with and without a BCC diagnosis, respectively (p = 0.0136), reflecting a significantly shorter time to melanoma diagnosis in patients with a BCC diagnosis than in those without a BCC diagnosis. In addition, patients with a BCC diagnosis had a significantly higher incidence of melanoma than patients without a BCC diagnosis (2.46% vs 0.37%; p < 0.0001). The Kaplan–Meier survival curve of the comparison of melanoma incidence between the 2 groups is shown in Fig. 2. Twenty-one patients (0.7%) were simultaneously diagnosed with BCC and melanoma. The results of the log-rank test indicated that there was a significant difference between the 2 groups (p > 0.0001), reflecting a higher melanoma incidence in patients with a BCC diagnosis than in patients without a BCC diagnosis.
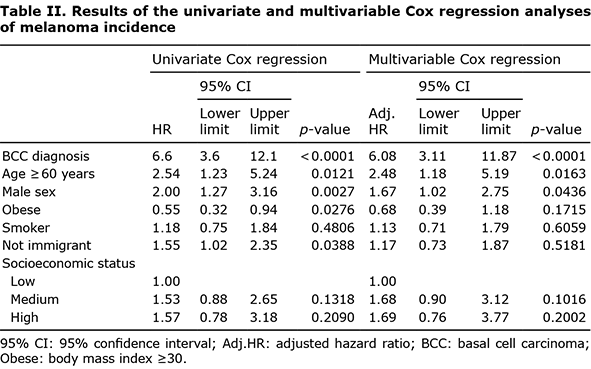
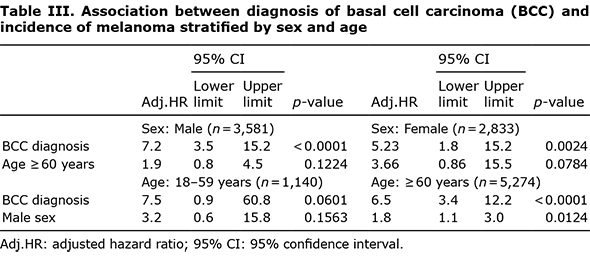
Univariate Cox regression analysis revealed a HR of 6.6 (95% CI 3.6–12.1; p < 0.0001) for melanoma after BCC diagnosis, indicating that, in patients with a BCC diagnosis, the risk of melanoma is 6.6 times higher than that in patients without a BCC diagnosis. Although we created similar groups in terms of demographic characteristics using PSM, a multivariable Cox regression model was also applied (Table II). After adjusting for potential risk factors, only age ≥ 60 years, male sex, and BCC diagnosis were found to be independent risk factors for melanoma. The interactions between these 3 variables were not significant (data not shown). The results of the stratified analyses are shown in Table III. After adjusting for age and sex, strong effect sizes were observed between BCC diagnosis and melanoma incidence among all stratified groups. The results were not significant (p = 0.0601) for the age group 18–59 years, as this group only included 8 patients with melanoma, of whom 7 belonged to the BCC diagnosis group.
DISCUSSION
A higher incidence of melanoma was observed in patients diagnosed with BCC than in those who had not been diagnosed with BCC (2.46% vs 0.37%; p < 0.0001). Furthermore, patients with BCC had a 6.6-fold higher risk of melanoma than those without BCC. This was consistent with the findings of Rees et al., who reported an HR of 5.24 for melanoma after BCC diagnosis in patients aged ≥ 60 years (15). In addition to BCC diagnosis, age ≥ 60 years and male sex were found to be independent risk factors for melanoma. The incidence of melanoma is higher in men than in women (17–19); Ries et al. (19) reported that men are 1.5 times more likely than women to develop melanoma. The current study found that the risk of melanoma in men was twice that in women.
The current study found that obesity may have a protective affect against melanoma. In contrast, Sergentanis et al. (20) found that obesity was associated with a slightly increased risk of melanoma in men, and another study found no association between melanoma and obesity (21). A possible explanation for the current finding might be that obese individuals tend to avoid activities that involve sun exposure.
No significant association was found between smoking and melanoma risk, which was consistent with the findings of Westerdahl et al. (22). However, other studies have reported that chronic smoking is inversely related to melanoma risk (23–25). Patients in this study developed melanoma a mean of 1.7 years after BCC diagnosis, and this relatively short time interval might be explained by the high frequency of screening in patients with a BCC diagnosis.
The strengths of this study include its size, the inclusion of a control group, and the fact that BCC and melanoma were diagnosed based on the results of pathology reports. Its limitations include the possibility of information bias due to the retrospective study design and possible underestimation of the incidence of BCC or melanoma. Although the study data included age, sex, place of birth, socioeconomic status, smoking history, and religious background, no patient data was included regarding history of exposure to ultraviolet radiation and skin phototype, which are known risk factors for both BCC and melanoma (26). However, it has been shown that BCC is an independent risk factor for melanoma even when taking these 2 confounding factors into account (15).
In conclusion, BCC is a risk factor for melanoma, and patients with BCC should be carefully screened for melanoma, especially during the first 2–3 years after diagnosis of BCC. Male sex and age over 60 years are 2 important risk factors for melanoma.
ACKNOWLEDGEMENTS
We wish to extend special thanks to Naama Schwartz for her contribution to the study design and statistical analyses. We thank Mark Oisgold for his contribution to data extraction and organization in this study.
The authors have no conflicts of interest to declare.
REFERENCES
- Lomas AL, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080.
- Hoban PR, Ramachandran S, Strange RC. Environment, phenotype and genetics: risk factors associated with BCC of the skin. Expert Rev Anticancer Ther 2002; 2: 570–579.
- Ramachandran S, Fryer AA, Smith AG, Lear JT, Bowers B, Griffiths CE, et al. Basal cell carcinoma. Cancer 2000; 89: 1012–1018.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol 1994; 30: 774–778.
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199.
- Siskind V, Hughes MC, Palmer JM, Symmons JM, Aitken JF, Martin NG, et al. Nevi, family history, and fair skin increase the risk of second primary melanoma. J Invest Dermatol 2011; 131: 461–467.
- Tucker MA, Halpern A, Holly EA, Hartge P, Elder DE, Sagebiel RW, et al. Clinically recognized dysplastic nevi: a central risk factor for cutaneous melanoma. JAMA 1997; 277: 1439–1444.
- Carr S, Smith C, Wernberg J. Epidemiology and risk factors of melanoma. Surg Clin North Am 2020; 100: 1–12.
- Green AC, O’Rourke MG. Cutaneous malignant melanoma in association with other skin cancers. J Natl Cancer Inst 1985; 74: 977–980.
- Frisch M, Hjalgrim H, Olsen JH, Melbye M. Risk for subsequent cancer after diagnosis of basal cell carcinoma: a population-based, epidemiologic study. Ann Intern Med 1996; 125: 815–821.
- Song F, Qureshi AA, Giovannucci EL, Fuchs CS, Chen WY, Stampfer MJ et al. Risk of a second primary cancer after non-melanoma skin cancer in white men and women: a prospective cohort study. PLoS Med 2013; 10: e1001433.
- Wu S, Cho E, Li WQ, Qureshi AA. History of keratinocyte carcinoma and risk of melanoma: a prospective cohort study. J Natl Cancer Inst 2017; 109: djw268.
- Flohil SC, van der Leest RJ, Arends LR, de Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: a systematic review and meta-analysis. Eur J Cancer 2013; 49: 2365–2375.
- Marghoob AA, Slade J, Salopek TG, Kopf AW, Bart RS, Rigel DS. Basal cell and squamous cell carcinomas are important risk factors for cutaneous malignant melanoma. Screening implications. Cancer 1995; 75: 707–714.
- Rees JR, Zens MS, Gui J, Celaya MO, Riddle BL, Karagas MR. Non melanoma skin cancer and subsequent cancer risk. PloS One 2014; 9: e99674.
- Levi F, Vecchia CL, Te VC, Randimbison L, Erler G. Incidence of invasive cancers following basal cell skin cancer. Am J Epidemiol 1998; 147: 722–726.
- Karimkhani C, Green AC, Nijsten T, Weinstock MA, Dellavalle RP, Naghavi M, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol 2017; 177: 134–140.
- O’Neill CH, Scoggins CR. Melanoma. J Surg Oncol 2019; 120: 873–881.
- Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer 2000; 88: 2398–2424.
- Sergentanis TN, Antoniadis AG, Gogas HJ, Antonopoulos CN, Adami HO, Ekbom A et al. Obesity and risk of malignant melanoma: a meta-analysis of cohort and case-control studies. Eur J Cancer 2013; 49: 642–657.
- Pothiawala S, Qureshi AA, Li Y, Han J. Obesity and the incidence of skin cancer in US Caucasians. Cancer Causes Control 2012; 23: 717–726.
- Westerdahl J, Olsson H, Måsbäck A, Ingvar C, Jonsson N. Risk of malignant melanoma in relation to drug intake, alcohol, smoking and hormonal factors. Br J Cancer 1996; 73: 1126–1131.
- Freedman DM, Sigurdson A, Doody MM, Rao RS, Linet MS. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control 2003; 14: 847–857.
- Sondermeijer L, Lamboo LG, de Waal AC, Galesloot TE, Kiemeney LA, van Rossum M, Aben KH. Cigarette smoking and the risk of cutaneous melanoma: a case-control study. Dermatology 2020; 236: 228–236.
- Song F, Qureshi AA, Gao X, Li T, Han J. Smoking and risk of skin cancer: a prospective analysis and a meta-analysis. Int J Epidemiol 2012; 41: 1694–1705.
- Kricker A, Armstrong BK, English DR. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control 1994; 5: 367–392.