ORIGINAL ARTICLE
Itching in Atopic Dermatitis: Patient- and Physician-reported Outcomes in the German Atopic Dermatitis Registry TREATgermany
Elke WEISSHAAR1, Philipp BENTZ1, Christian APFELBACHER2,3, Eva HAUFE4, Luise HEINRICH4, Annice HERATIZADEH5, Susanne ABRAHAM6, Inken HARDER7, Andreas KLEINHEINZ8, Andreas WOLLENBERG9, Knut SCHÄKEL10, Franca WIEMERS11, Julia ERTNER12, Matthias AUGUSTIN13, Julia WILDBERGER14, Ralph von KIEDROWSKI15, Margitta WORM16, Alexander ZINK17, Isaak EFFENDY18, Andrea ASMUSSEN19, Mario PAWLAK20, Michael STICHERLING21, Melanie HILGERS22, Christiane HANDRICK23, Sven QUIST24, Beate SCHWARZ25, Magnus BELL26, Petra STAUBACH-RENZ27, Sung-Hei HONG-WELDEMANN28, Bernhard HOMEY29, Jens-Joachim BRÜCHER30, Stephan WEIDINGER7, Thomas WERFEL5, Jochen SCHMITT4 and the TREATgermany study group
1Occupational Dermatology, Department of Dermatology, University of Heidelberg, 2Institute of Social Medicine and Health Systems Research, Otto von Guericke University Magdeburg, Magdeburg, 3Institute for Epidemiology and Preventive Medicine/Medical Sociology, University of Regensburg, Regensburg, 4Center of Evidence-based Healthcare, University Hospital and Medical Faculty Carl Gustav Carus, TU Dresden, Dresden, 5Division of Immunodermatology and Allergy Research, Department of Dermatology and Allergy, Hannover Medical School, Hannover, 6Department of Dermatology, University Allergy Center, Medical Faculty Carl Gustav Carus, TU Dresden, Dresden, 7Center for Inflammatory Skin Diseases, Department of Dermatology and Allergy, University Hospital Schleswig-Holstein, Campus Kiel, Kiel, 8Clinics for Dermatology, Elbe Klinikum Buxtehude, Buxtehude, 9Clinics and Outpatient Clinics for Dermatology and Allergy, LMU Munich, Munich, 10Department of Dermatology, University of Heidelberg, Heidelberg, 11Practice Dr. med. Franca Wiemers, Leipzig, 12Practice Dr. med. Konstantin Ertner, Nuernberg, 13Institute for Health Services Research in Dermatology, University Medical Center, Hamburg Eppendorf, 14Practice Dr. med. Julia Wildberger, Hautmedizin, Bad Soden, 15Focus Practice for chronic inflammatory dermatoses, skin cancer and allergology and also Study Center CMS3 (Company for Medical Study and Service), Selters/Westerwald, 16Department of Dermatology, Allergy and Venereology, Charité Berlin, Berlin, 17Department of Dermatology and Allergy, School of Medicine, Technical University of Munich, Munich, 18Department of Dermatology, OWL University Hospital of Bielefeld University, Campus Clinic Bielefeld, Bielefeld, 19Practice Dr. med. Andrea Asmussen, Dermatology at Lesum, Bremen, 20Practice Dr. med. Anika Hünermund and Mario Pawlak, Heiligenstadt, 21Department of Dermatology, German Center for Immunotherapy, University of Erlangen, Erlangen, 22Clinics for Dermatology and Allergy, University Hospital Aachen, Aachen, 23Practice Dr. med. Christiane Handrick, Berlin, 24Dermatology Clinic, Helix Medical Excellence Center, Mainz, 25Practice Dr. med. Beate Schwarz, Langenau, 26Practice Dr. med. Magnus Bell, Andernach, 27Clinic for Dermatology, University Hospital, Mainz, 28Practice Dr. med. Sung-Hei Hong-Weldemann, Freiburg, 29Department of Dermatology and Allergology, University Hospital Duesseldorf, Duesseldorf, and 30Practice Dr. med. Jens-Joachim Brücher, Hautambulatorium Magdeburg, Magdeburg, Germany
TREATgermany is an investigator-initiated prospective disease registry. It investigates physician- and patient-reported disease severity (Eczema Area and Severity Index (EASI), objective Scoring Atopic Dermatitis (oSCORAD), Investigator Global Assessment, Patient-Oriented Eczema Measure (POEM), Patient Global Assessment (PGA)), patient-reported symptoms (itch, sleep loss, depressive symptoms), therapy courses and dermatological quality of life (DLQI) in moderate-to-severe atopic dermatitis with SCORAD > 20. 1,134 atopic dermatitis patients (mean age 41.0 ± 14.7 years, 42.5% females) were enrolled by 40 German recruiting sites (dermatological clinics and practices) between June 2016 and April 2021. The current analysis focuses on itch scores obtained with a numerical rating scale (NRS)) documented for the previous 3 days prior to baseline visit. The results show that 97.2% (1,090 of 1,121) patients experienced itch. Itch severity correlated moderately with severity of atopic dermatitis oSCORAD (rho = 0.44 (0.39–0.48)) and EASI score (rho = 0.41 (0.36–0.46)). A strong correlation was found with self-reported disease severity as PGA (rho = 0.68 (0.65–0.71)), POEM sum score (rho = 0.66 (0.63–0.69)) and dermatological quality of life impairment DLQI (rho = 0.61 (0.57–0.65)). Itch as a subjective complaint is more closely correlated with patient-reported outcomes than with objective assessments by the physician.
Key words: EASI; POEM; pruritus; quality of life; registry.
SIGNIFICANCE
Most people with atopic dermatitis experience itch and, for most of them, it is very important to be free of this complaint. Using data from the TREATgermany registry, this study investigated how patients rated the severity of their itch and how it affected their dermatological quality of life. The results show that patients associated itch more strongly with self-reported outcomes than with physician’s assessments. This indicates that the reduction in itch should be a central focus of patient-centred care for patients with atopic dermatitis.
Citation: Acta Derm Venereol 2023; 103: adv00854. DOI https://doi.org/10.2340/actadv.v103.4426.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jan 4, 2023; Published: Jan 23, 2023
Corr: Elke Weisshaar, Occupational Dermatology, Department of Dermatology, University of Heidelberg, DE-69115 Heidelberg, Germany. E-mail: elke.weisshaar@med.uni-heidelberg
Competing interests and funding: JS is Co-PI of TREATgermany and reports institutional grants for investigator-initiated research from the German GBA, the BMG, BMBF, EU, Federal State of Saxony, Novartis, Sanofi, ALK, and Pfizer. He participated in advisory board meetings as a paid consultant for Sanofi, Lilly, and ALK. KS received honoraria as investigator and/or received speakers’ honoraria and/or received grants and/or has been an advisor for the following companies: AbbVie, Almirall-Hermal, Amgen, Biogen Idec, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Eli-Lilly, Galderma, Janssen, LEO-Pharma, Merck Serono, Morphosys, Novartis, Pfizer, Regeneron, Sanofi, a, und UCB Pharma. LH is research associate of TREATgermany, which is an academic, investigator-initiated clinical registry that is financially supported AbbVie GmbH & Co. KG, Galderma S.A., LEO Pharma GmbH, Lilly Deutschland GmbH, Pfizer Inc. and Sanofi-Aventis Deutschland GmbH. SA received lecture and/or consultancy fees from Novartis, LEO Pharma, Amgen, Lilly, Sanofi, Beiersdorf, Janssen, UCB and AbbVie. EW received consultancy fees from Sanofi/Genzyme and participated in clinical trials of Galderma Ltd and TREVI Ltd. The other authors have no conflicts of interests to declare
Treatment and medical care of patients with moderate-to-severe Atopic Dermatitis (TREATgermany) is an investigator-initiated prospective disease registry of patients with moderate to severe affected atopic dermatitis (AD) in Germany and it is part of the European registry family TREAT. It represents a standardized assessment of treatment and outcomes in daily routine care of patients with AD. TREATgermany includes patients 18 years or older with moderate-to-severe AD (objective Scoring Atopic Dermatitis; oSCORAD > 20) consulting a dermatology department or a dermatology practice in Germany (A separate TREATkids for patients under 18 years was started in 2020. This data is not analysed here). First analyses demonstrated a high disease burden and a great need for systemic treatments (1, 2). Dupilumab, the first biologic for AD, which was launched in Germany in December 2017, showed similar effectiveness on clinical signs, symptoms and health-related quality of life (HRQoL) as in clinical trials (3). Itch was significantly reduced 3 months after dupilumab treatment (3).
Itch is the defining symptom of AD and occurs chronically (4–6). It affects patients in severe bouts occurring for minutes to hours, as well as constantly, on a daily basis. Itch constitutes a dominant symptom that characterizes active disease, but patients with AD also report that itch often occurs when the skin looks (macroscopically) non-diseased. Often, itch may be the first symptom of an AD relapse/flair (7). Patient focus groups reported AD-related itching to be the most bothersome AD symptom (8). The German Atopic Dermatitis Intervention Study (GADIS), including 823 children and adolescents, showed significant, but weak, correlations between itch intensity and AD disease severity (9). Another study showed similar relationships (10). Consequentially, studying the symptom of itch in AD remains important and challenging. TREATgermany provides an opportunity to gain more insight into itch in daily routine care. Since 89.0% of patients with AD in TREATgermany stated that it is “very important” for them to be free of itch, it was the aim of this analysis to investigate itch in relation to physician- and patient-reported outcome measures (PROMs).
MATERIAL AND METHODS
TREATgermany (www.treatgermany.org) is a nationwide multicentre academic registry for the long-term observation of patients with moderate-to-severe AD that has been formally approved by the Medical Faculty of Carl Gustav Carus University, Dresden, Germany (number EK 118032016) and the responsible local ethics committees at the other participating sites. Patients are recruited at dermatological practices, clinics and university hospitals. The registry has been described in detail elsewhere (2). Patients with moderate-to-severe AD, current or prior (in the past 2 years) systemic anti-inflammatory treatment and/or objective SCORAD (Scoring Atopic Dermatitis, oSCORAD) > 20 are prospectively followed over the course of at least 24 months. A broad set of physician- and patient-reported outcome measures are documented using validated measurement instruments (1, 2).
In SCORAD, mean itch intensity caused by AD during the past 3 days, was rated by the patients on a numerical rating scale (NRS) ranging from 0 (no itch) to 10 (maximum imaginable itch). The association between various verifiable (documented by the treating physician) and PROMs and itch of patients enrolled in the registry from June 2016 until April 2021 was examined. Investigator-reported outcomes were oSCORAD (11), Eczema Area and Severity Index (EASI) (12) and Investigator Global Assessment (IGA; levels 0 (healed) to 5 (extremely severe)) (13).
PROMs were Patient Global Assessment (PGA), Patient-Oriented Eczema Measure (POEM) (14), Dermatology Life Quality Index (DLQI) (15), Fatigue Severity Scale (FSS) (16) and the Patient Benefit Index (PBI) (17). In addition, pain and sleep loss caused by AD during the past 3 days rated on a numerical rating scale ranging from 0 (no disturbance) to 10 (unbearable disturbance) were examined. This approach is identical to the SCORAD to ensure comparability. The analysis focuses on the baseline visit. Software Package Stata 15 (StataCorp LLC, Texas, USA) was used for descriptive and explorative data analyses: Pearson’s χ2 test, Spearman’s rank sum test and rank correlation including 95% confidence interval (95% CI) were used for describing associations. The qualitative interpretation of the strength of correlation follows Landis and Koch (18).
RESULTS
Characteristics of the study population
As of April 2021, a total of 1,134 patients (mean age 41.0 ± 14.7 years, 42.5% females) were enrolled at 40 recruitment centres (Table I). At baseline registry patients had mean oSCORAD of 40.8 ± 16.0 and EASI scores of 16.2 ± 12.9. 53.5% of them (n = 603) were mildly/ moderately (mild respectively moderate erythema, papule forming and infiltrate) and 39.0% (n = 440) were (very) severely affected (severe erythema, papule forming and infiltrate (with oozing/crusting)) by AD according to the IGA (Table I).
Itch severity in the study population
A total of 1,121 patients (98.9%) provided data on their itch in the past 3 days prior to baseline visit. 97.2% (n = 1,090 ) of those patients with itch (NRS score > 0). 504 (45.0%) had itch scores of 7 and higher, indicating severe itch (19). Mean itch severity was 5.7 ± 2.7 and was significantly higher in females (6.1 ± 2.8) compared with males (5.5 ± 2.7) (rank-sum test, p < 0.001). There was no relevant (linear) correlation between itch and age (Table II). Compared with pain (3.7 ± 2.8) and sleep loss (4.6±3.4), which were gathered in the same way as itch, itch was the symptom with the highest rating (20). There was no significant association between itch severity and self-reported allergic comorbidities, asthma, allergic rhinitis or food allergy.
The relationship between disease duration of AD and itch severity was analysed according to age and sex (n = 1,101). As expected, due to the typical manifestation of AD early in life, the mean disease duration increased with increasing age, with the age group 51–60 years having the longest disease duration (n = 219, 39.9 ± 18.0 years). However, the proportion of patients who reported having only had AD for a relatively short time (2 years or less) was higher in the group of patients over 60 years of age than in younger patients (16.1% vs 1.5%). The mean itch according to the disease duration stratified by age and sex is shown in Table III. No correlation between duration of disease and itching is apparent; however, recall bias might reduce the reliability of the data.
Table IV shows the influence of itch severity on the importance of being free from itch measured by the PBI. Regardless of sex and reported itch severity, this issue was rated to be “quite” or “very important” in 93.8% and up to 100% of cases. This underlines the high importance of effectively treating this AD symptom and understanding its correlation with other outcomes.
A weak positive correlation was observed between itch severity and physician-rated severity of AD measured by oSCORAD (rho = 0.44 (0.39–0.48)), EASI score (rho = 0.41 (0.36–0.46)) and IGA (rho = 0.46 (0.42–0.51)) (Fig. 1). A strong positive correlation was found between itch and patient-reported disease severity, as assessed by means of the PGA (rho = 0.68 (0.65–0.71)) and POEM sum score (rho = 0.66 (0.63–0.69)). The results are described in detail in Fig. 2. Strong positive correlations were also found for itch and DLQI (rho = 0.61 (0.57–0.65)) (Fig. 2).
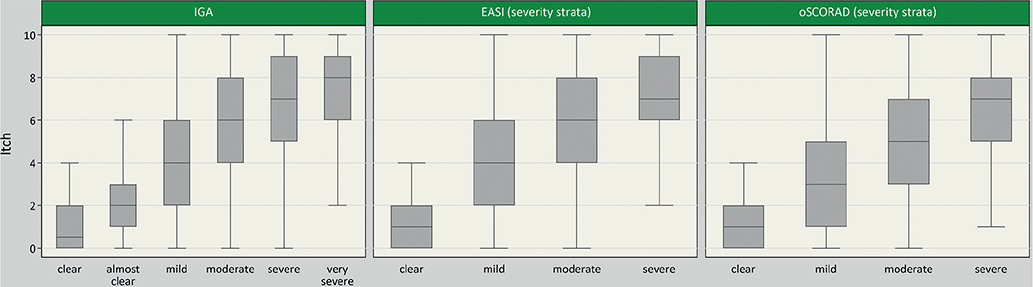
Fig. 1. Boxplots of itch severity over severity by categories of Investigator Global Assessment (IGA) (n = 1,115), Eczema Area and Severity Index (EASI) (n = 1,111) and objective Scoring Atopic Dermatitis (oSCORAD) (n = 1,115). EASI and oSCORAD were categorized as follows: oSCORAD (scale: 0–83): 0–7.9 = clear, 8–23.9 = mild, 24–37.9 = moderate, 38–83 = severe; EASI (scale: 0–72): 0 = clear, 0.1–5.9 = mild, 6–22.9 = moderate, 23–72 = severe (25). Outliers are not shown.
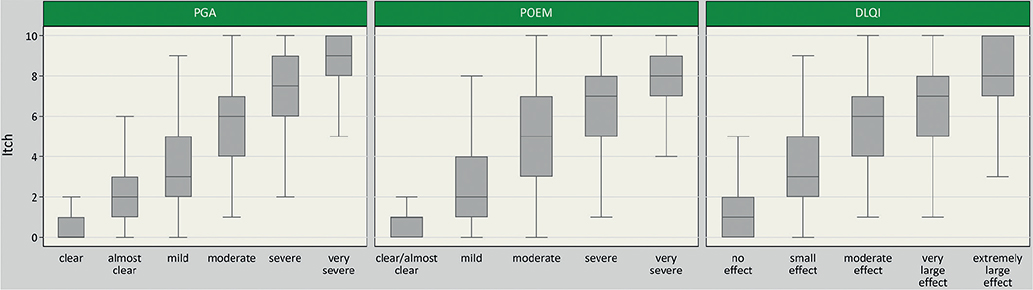
Fig. 2. Boxplots of itch severity over severity by categories of Patient Global Assessment (PGA) (n = 1,119), Dermatological Life Quality Index (DLQI) (n = 1,120) and (Patient-Oriented Eczema Measure) POEM (n = 1,121). DLQI and POEM were categorized as follows: POEM (scale: 0–28): 0–2 = clear/almost clear, 3–7 = mild, 8–16 = moderate, 17–24 = severe, 25–28 = very severe (26). DLQI (scale: 0–30): 0–1 = no effect at all on patient’s life, 2–5 = small effect, 6–10 = moderate effect, 11–20 = very large effect, 21–30 = extremely large effect.
In total, registry patients had mean total FSS scores of 3.7 ± 1.6 (Table I). 23.7% of patients showed high fatigue (FSS > 5). There was a weak correlation of itch and FSS (rho = 0.32 (0.27–0.38), n = 1,118). Itch was higher in AD patients with higher FSS scores (6.9 ± 2.4 in FSS > 5 compared with 5.2 ± 2.7 in FSS < 4).
In addition, this study examined the association between sleep loss caused by AD during the past 3 days and itch. There was a strong correlation between itch and sleep loss (rho = 0.75 (0.72–0.78), n = 1,121).
DISCUSSION
This study found strong correlations between itch severity and PROMs, such as POEM, DLQI, and PGA. This indicates that itch places substantial burden on patients with AD. On the other hand, physician-rated disease severity was not as closely correlated with itch. This suggests that it is important for clinicians who treat patients with AD to regularly assess itch. If itch and other patient-reported outcomes are not assessed, physicians may underestimate the burden of disease and patient needs.
In a recent analysis in TREATgermany, patients with AD who smoke cigarettes showed a higher disease burden, including higher itch scores compared with non-smokers (21).
The frequency of itch and the impairment of dermatological quality of life in patients with AD have been described previously; however, the current study results demonstrates that itch as a subjective complaint is more closely associated with patient-reported outcomes than with objective assessments by the physician. This reflects the difficulty of assessing AD with its range of phenotypes, clinical pictures and symptoms. HOME (Harmonizing Outcome Measures in Eczema) is an initiative of experts who defined a core outcome set containing clinical signs, patient-reported symptoms, long-term control and dermatological quality of life (www.homeforeczema.org). The peak itch numerical rating scale (NRS) of the past 24 h is the recommended instrument for measuring the intensity of itch in the symptom domain in trials of older children and adults (20). Since the TREATgermany registry was started before this recommendation, this method of measuring itch was only implemented in the data collection in 2021, so it could not yet be used for this analysis. Recap of AD (RECAP) was developed recently and introduced to capture the experience of eczema control over the past week. Two of the 7 questions address itch explicitly within 5 answer options, which are “no days”, “1–2 days”, “3–4 days”, “5–6 days” and “every day”, respectively (22).
In a study with 410 patients with AD, changes from the baseline in NRS, VRS and frequency of itch were weakly to moderately correlated with other patient-reported outcomes (POEM, SCORAD-itch, DLQI) (23). Another study showed a significant decrease in DLQI scores for patients with itch improvement after 16 weeks of treatment (24). Mediation models also showed an indirect effect of AD treatment on DLQI scores via reduction in the severity of pruritus (25).
It is known that patient-reported AD severity appears to be sufficiently valid for assessing AD severity in the clinical and epidemiological setting (26); however, physician-reported outcome measures show low or moderate correlation to itch.
The FSS has been shown to be a valuable tool to assess and quantify fatigue (27). The awareness and assessment of fatigue has also been underestimated previously and remains an under-recognized problem in AD. In addition to being a disease-related symptom, fatigue can be induced by systemic medications. Fatigue may be caused in AD by sedating antihistamines.
The growing list of comorbidities was also confirmed in TREATgermany and reflects the increasing appreciation of its high burden (28). More knowledge about this is necessary to improve the possibilities of medical and psychological interventions.
Pathophysiology and pathogenesis of itching in AD are still not fully understood. A crosstalk between the nervous system, the cutaneous immune system and keratinocyte population explain the origin and persistence of itch in AD (29). Itching in AD is influenced by various factors, such as triggers (e.g. sweat, clothing, allergens, such as dust mites, pollens, food allergens, as well as infections and psychological factors) (4, 5, 30). This explains why treating atopic itch remains a great challenge. Novel approaches have been described, however, with variable effects on itch (29). It is most likely that treating itch sufficiently means targeting inflammation, neural and neuroimmune pathways. Elucidating the roles of peripheral and central sensitization and hypersensitization in AD is a future task (29); however, investigating patient-reported outcomes of atopic itch supports this task.
The strength of these analyses can be summarized as follows: this study investigated itch in a large cohort of patients with AD. As TREATgermany is a registry study the data are obtained in a very similar and uniform pattern in AD clinics. The results attain special importance because the impact of measuring patient-reported outcomes in dermatology has been underestimated in the past, but has also gained increasing significance for drug development (31). As itch is a subjective symptom, which is not easy to score, it is not surprising that itch is more closely associated with patient-reported outcomes than with objective physician assessment.
A limitation of this analysis is measuring itch in only the previous 3 days, as is done in SCORAD, because itch in AD is chronic and long-lasting. The data were obtained in a single country not resembling cultural and ethnic aspects of itch (32). It remains to be seen if these results will be confirmed, for example in TARGET-DERM AD study, a longitudinal cohort study that plans to include 4,000 patients with AD in up to 100 clinical centres in the USA (33). The representativeness of the data is limited by the fact that participation in the registry is voluntary (on the part of both centres and patients).
In the future, TREATgermany may improve our understanding of how AD can be treated more effectively over time to reduce its impact, especially the impact of itch, on all domains of life, including cumulative life impairment.
ACKNOWLEDGEMENTS
We thank all the patients with AD for participating in this study, for their confidence in the study centres and for their great support in providing the data for TREATgermany.
TREATgermany is an academic, investigator-initiated clinical registry financially supported by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., LEO Pharma GmbH, Lilly Deutschland GmbH, Pfizer Inc. and Sanofi-Aventis Deutschland GmbH.
TREATgermany has formally been approved by the Medical Faculty of the Carl Gustav Carus University, Dresden, Germany (No. EK 118032016) and the responsible local ethics committees at the other participating sites.
REFERENCES
- Heratizadeh A, Haufe E, Stolzl D, Abraham S, Heinrich L, Kleinheinz A, et al. Baseline characteristics, disease severity and treatment history of patients with atopic dermatitis included in the German AD Registry TREATgermany. J Eur Acad Dermatol Venereol 2020; 34: 1263–1272.
- Schmitt J, Abraham S, Trautmann F, Stephan V, Folster-Holst R, Homey B, et al. Usage and effectiveness of systemic treatments in adults with severe atopic eczema: First results of the German Atopic Eczema Registry TREATgermany. J Dtsch Dermatol Ges 2017; 15: 49–59.
- Abraham S, Haufe E, Harder I, Heratizadeh A, Kleinheinz A, Wollenberg A, et al. Implementation of dupilumab in routine care of atopic eczema: results from the German national registry TREATgermany. Br J Dermatol 2020; 183: 382–384.
- Bieber T. Atopic dermatitis. N Engl J Med 2008; 358: 1483–1494.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360.
- Wollenberg A, Christen-Zach S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020; 34: 2717–2744.
- Darsow U., Ripphoff E., J. R. Pathophysiology and clinical manifestation of itch in atopic eczema. In: Ring J., Przybilla B., Ruzicka T., editors. Handbook of Atopic Eczema. 2 ed. Berlin, Heidelberg, New York: Springer 2006: p. 222–227.
- Martin SA, Brown TM, Fehnel S, Deal LS, Katz EG, Chiou CF. The atopic dermatitis itch scale: development of a new measure to assess pruritus in patients with atopic dermatitis. J Dermatolog Treat 2020; 31: 484–490.
- Weisshaar E, Diepgen TL, Bruckner T, Fartasch M, Kupfer J, Lob-Corzilius T, et al. Itch intensity evaluated in the German Atopic Dermatitis Intervention Study (GADIS): correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm Venereol 2008; 88: 234–239.
- Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, Chavda R, et al. Validation of four single-item patient-reported assessments of sleep in adult atopic dermatitis patients. Ann Allergy Asthma Immunol 2020; 124: 261–266.
- Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997; 195: 10–19.
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10: 11–18.
- Meurer M, Folster-Holst R, Wozel G, Weidinger G, Junger M, Brautigam M, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology 2002; 205: 271–277.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123.
- Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schafer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res 2009; 301: 561–571.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174.
- Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92: 502–507.
- Thomas KS, Apfelbacher CA, Chalmers JR, Simpson E, Spuls PI, Gerbens LAA, et al. Recommended core outcome instruments for health-related quality of life, long-term control and itch intensity in atopic eczema trials: results of the HOME VII consensus meeting. Br J Dermatol 2021; 185: 139–146.
- Pilz AC, Schielein MC, Schuster B, Heinrich L, Haufe E, Abraham S, et al. Atopic dermatitis: disease characteristics and comorbidities in smoking and non-smoking patients from the TREATgermany registry. J Eur Acad Dermatol Venereol 2022; 36: 413–421.
- Howells LM, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: Recap of atopic eczema (RECAP). Br J Dermatol 2020; 183: 524–536.
- Silverberg JI, Lai JS, Patel KR, Singam V, Vakharia PP, Chopra R, et al. Measurement properties of the Patient-Reported Outcomes Information System (PROMIS((R)) ) Itch Questionnaire: itch severity assessments in adults with atopic dermatitis. Br J Dermatol 2020; 183: 891–898.
- Lio PA, Simpson EL, Han G, Soung J, Ball S, Sun L, et al. Improvement in sleep and itch and enhanced quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a phase 3 trial of baricitinib therapy. J Dermatolog Treat 2022; 33: 2057–2062.
- Simpson EL, Tom WL, Bushmakin AG, Cappelleri JC, Yosipovitch G, Stander S, et al. Relationship among treatment, pruritus, investigator’s static global assessment, and quality of life in patients with atopic dermatitis. Dermatol Ther (Heidelb) 2021; 11: 587–598.
- Vakharia PP, Chopra R, Sacotte R, Patel N, Immaneni S, White T, et al. Validation of patient-reported global severity of atopic dermatitis in adults. Allergy 2018; 73: 451–458.
- Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008; 31: 1601–1607.
- Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol 2017; 137: 18–25.
- Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol 2020; 34: 239–250.
- Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbe A, Nelson L, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol 2019; 181: 761–769.
- Copley-Merriman C, Zelt S, Clark M, Gnanasakthy A. Impact of measuring patient-reported outcomes in dermatology drug development. Patient 2017; 10: 203–213.
- Sutaria N, Parthasarathy V, Roh YS, Choi J, Bordeaux ZA, Trinh P, et al. Itch in skin of colour: a multicentre cross-sectional study. Br J Dermatol 2021; 185: 652–654.
- Abuabara K, Silverberg JI, Simpson EL, Paller AS, Eichenfield LF, Bissonnette R, et al. International observational atopic dermatitis cohort to follow natural history and treatment course: TARGET-DERM AD study design and rationale. BMJ Open 2020; 10: e039928.