REVIEW ARTICLE
Characteristics, Natural Course and Treatment of Intramuscular Capillary-type Haemangioma: A Systematic Literature Review
Jordan ORLY1,2*, Annouk BISDORFF3, Aline JOLY2,4, Afi-Emiliène EDEE2, Elsa TAVERNIER5,6, Denis HERBRETEAU7, Olivia BOCCARA8, Michel WASSEF9 and Annabel MARUANI1,2,5
1Department of Dermatology, Unit of Pediatric Dermatology, CHRU Tours, 2Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Tours), Tours, 3Reference Center for Vascular Anomalies FAVA-multi, University Hospital of Lariboisière; AP-HP, Department of Neuroradiology, Paris, 4CHRU Tours, Department of Maxillo-facial Surgery, 5University of Tours, University of Nantes, INSERM 1246-SPHERE, 6CHRU Tours, Clinical Investigation Center INSERM 1415, 7Department of Neuroradiology, CHRU Tours, Tours, 8Department of Dermatology and Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Necker), University Hospital Necker-Enfants Malades and 9Department of Pathology, University Hospital of Lariboisière, AP-HP, Paris, France
Intramuscular capillary-type haemangiomas (ICTH) are rare vascular anomalies that can easily be misdiagnosed as other entities. A systematic review was performed of all cases of ICTH in the literature since its first description in 1972. An adjudication committee reviewed cases to include only ICTHs. Among 1,143 reports screened, 43 were included, involving 75 patients. The most frequent differential diagnosis was intramuscular venous malformations. The mean age of patients at diagnosis was 21.2 years. ICTH was mainly described as a gradually increasing mass (81.8%), painless (73.9%), that could occur anywhere in the body but most frequently on the head and neck (44.0%). Magnetic resonance imaging (MRI) was mainly used for diagnosis (69.1%) and displayed specific features. The most frequent treatment was complete surgical removal (73.9%), which could be preceded by embolization, and led to complete remission without recurrence in all but 1 case.
Key words: intra-muscular haemangioma; intramuscular capillary-type haemangioma; fast-flow; intramuscular vascular malformation; extracranial arteriovenous malformation; intramuscular fast-flow vascular anomaly.
SIGNIFICANCE
Intramuscular capillary-type haemangiomas (ICTH) are rare and poorly understood vascular anomalies that can easily be misdiagnosed as other entities. This study reviewed cases of ICTH published in the literature to enable better understanding and management of this entity. The review found that ICTHs most frequently occur in young adults, anywhere in body but mainly on the head and neck, usually increase gradually, and are painless. Magnetic resonance imaging (MRI) (either with or without microscopy observation from a biopsy) is required for diagnosis. Surgery (either preceded or not by embolization) appear to be the best therapeutic option.
Citation: Acta Derm Venereol 2023; 103: adv00893. DOI https://doi.org/10.2340/actadv.v103.4432.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Nov 29, 2022; Published: Mar 20, 2023
*Corr: Jordan Orly, Department of Dermatology, CHRU Tours, Avenue de la République, FR-37044 Tours Cedex 9, France. E-mail: jordan.orly41@gmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Intramuscular haemangiomas (IMHs), also called haemangiomas of the skeletal muscle, are a rare and poorly known condition that includes heterogeneous entities. In 1972, IMHs were classified into 3 subgroups by Allen & Enzinger (1) by considering histopathological features of the lesions, especially the size of intratumoural vessels (vessels < 140 μm in diameter defined as the “small-vessel group”, vessels > 140 μm the “large-vessel group”, and both types of vessels in nearly equal proportions the “mixed group”). IMH has been an ill-defined condition with evolving nomenclature, leading to a better-defined entity called intramuscular capillary-type haemangiomas (ICTHs), with specific clinical, histology and imaging features (2, 3). Because the nomenclature has changed, ICTHs might be reported in the literature under various names, such as “intramuscular haemangioma small vessel type” (1) “intramuscular angioma-capillary type” (4), or “infiltrating angiolipoma” (5).
The features common to ICTHs are firm intramuscular lesions, which might occur at any age, but occur more frequently in young adults, appearing on imaging as intramuscular fast-flow lesions, without extension to the subcutaneous tissue or the underlying bone (2). They can easily be misdiagnosed as 4 main conditions: (i) intramuscular venous malformations (IVMs), characterized by slow flow on imaging, phleboliths, large venous vessels on microscopy, and the possibility of underlying bone changes (6); (ii) high-flow anomalies with extension to the skeletal muscle, such as extracranial arteriovenous malformations (AVMs) (7); extra-renal angiomyolipomas (now called angiomyolipomas), which are tumours of uncertain differentiation whose diagnosis is based on microscopy observation of smooth muscles, thick-walled blood vessels and mature fat in varying proportions: they usually are located in subcutaneous tissue, with potentially intramuscular infiltration (8, 9); (iii) phosphatase and TENsin homolog (PTEN)-related hamartomatous tumour syndrome, which involves clinically heterogeneous disorders that share a germline mutation in PTEN and damage to the derivatives of the 3 embryonic laminae, leading to hamartomas, overgrowth and neoplasia (10); and (iv) highly vascularized intramuscular tumours, such as rhabdomyosarcomas (11).
According to the International Society for the Study of Vascular Anomalies, vascular anomalies are classified as vascular tumours and vascular malformations (12). However, ICTH was included in the group of “provisionally unclassified vascular anomalies”, which highlights the lack of knowledge on this entity. In several cases of ICTH, recent molecular analyses identified somatic mutations in MAP2K1 and KRAS genes, similar to those detected in several cases of extracranial AVMs (13, 14), which suggests the hypothesis of overlap between those 2 groups of entities (15). A better understanding of ICTH is necessary in order to harmonize its diagnostic and therapeutic management.
The aim of this study was to investigate the clinical, radiological, pathological and molecular features of ICTH as well as treatments and outcome, through a systematic review of the literature.
METHODS
This systematic review followed the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered on the PROSPERO database (CRD42021237224).
Search strategy and information sources
The electronic databases MEDLINE via PubMed, CENTRAL and EMBASE were searched on 1 March 2021. The search equations are shown in Appendix S1. There were no restrictions on language, and the search included articles from 1972 (year of the first description by Allen & Enzinger (1)) to 1 March 2021.
Eligibility criteria
All original reports describing ICTH cases were included. Criteria for diagnosis were based on clinical, radiological and pathological features. Clinically, the mass would be strictly in the muscle without extension to the skin. If performed, ultrasonography had to show an intramuscular lesion and colour and spectral Doppler traces to show fast-flow waveforms or arterial supply. For diagnosis, at least 1 set of images was required from CT scan, MRI or angiogram, showing features of intramuscular well-delineated lesions without cutaneous involvement. MRI imaging had to show characteristic signals (hyperintense on T2-weighted images with hypointense flow voids, with homogeneous contrast enhancement) (2). The presence of fat might be observed. Angiography (not mandatory) could show enlarged but non-tortuous arterial afferences and inhomogeneous capillary type tissular blush without an early venous drainage. Pathological features (required in case of doubtful imaging) had to be consistent with the diagnosis of ICTH (1, 2, 15), showing an increased number of capillary-sized vessels, consisting of plump endothelial cells (positive for CD31 on immunohistochemistry when performed) and pericytes (16), separating skeletal muscle fibres. Immunohistochemistry for lesional endothelial cells for GLUT-1 and podoplanin (D2-40) should be negative if performed (17).
Exclusion criteria were cases with too much missing data, differential diagnoses and doubtful cases. Also excluded were reports describing slow-flow vascular malformations, especially IVMs that contained phleboliths; typical AVMs (flow voids without a tissular component on MRI); all intramuscular vascular anomalies that extended to the subcutaneous/cutaneous tissue; other tumours (infantile haemangiomas, congenital haemangiomas, malignant tumours etc.); and fibro-adipose vascular anomaly (FAVA) or PTEN-hamartoma of soft tissue (PHOST) (15, 18).
Articles that did not report original observations, such as editorials, general reviews, and expert opinions, were excluded.
Study selection strategy and data extraction
Two authors (JO and AM) independently selected studies on the basis of the title and abstract and then examined the full text. Then, 2 authors (JO and AE) independently extracted the following data: first author, publication year, characteristics of the study and study participants, triggering factors, location of the ICTH, histology and molecular features, imaging and follow-up data, including outcomes and treatment. In doubtful cases, especially for retaining a diagnosis of ICTH, the paper was reviewed by an adjudication committee of experts (AB, DH, MW and AM).
Statistical analyses
Descriptive data are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) for quantitative data, and number (%) for categorical data. Quantitative variables were compared by Wilcoxon test and categorical variables by χ2 test or Fisher exact test. p < 0.05 was considered statistically significant. R v3.6.2 was used for analysis. No methods were used to handle missing data.
RESULTS
Among 1,143 reports initially screened, 43 were included for analysis (Fig. 1), involving a total of 75 patients from 29 clinical cases and 14 case series. The most frequent differential diagnosis that explained the exclusion of reports was the description of typical IVMs under the name IMH (90 cases). Nine cases of AVM were excluded (description of a nidus/arteriovenous shunt in 7 cases, extension to skin in 2 cases).
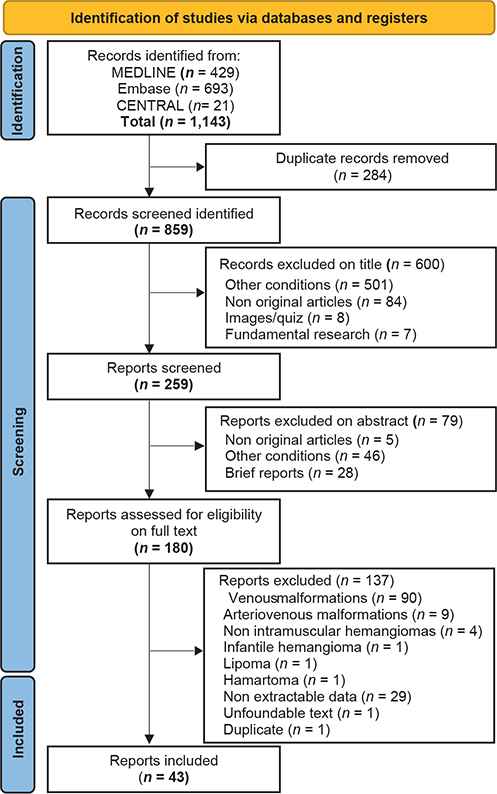
Fig. 1. Flow of reports included in the review.
Table I summarizes the characteristics of reports and patients. The mean age of patients at diagnosis was 21.2 ± 17.1 years and the median age was 17.0 years (IQR 7.0-31.0 years; range 1 day to 67 years). Among the 75 patients, 33 (44.0%) were < 16 years old. The ratio of males to females was 1/2. The median time to diagnosis was 12 months (IQR 4.9–36).
ICTH was most commonly a gradually increasing mass, not warm and not pulsatile, with a firm consistency and mostly painless (Table II). A triggering factor was suspected in 7 cases, especially local trauma (n = 5). ICTH could occur all over the body, but the most affected body region was the head and neck area (n = 33, 44.0%). Overall, 29 muscles or muscle groups with ICTH were reported among the 61 cases that included a description of the affected muscle(s) (Fig. 2A, B), the most commonly affected being the triceps surae (n = 7), sternocleidomastoid (n = 5), temporalis and quadriceps femoris (n = 4), gluteus maximus (n = 3), paraspinal (n = 3), extraocular muscles (n = 3) and lip muscles (n = 3), masseter (n = 2).
| Variable | Description | Missing data |
| Clinical data | ||
| Size (cm2), mean (IQR) | 19.5 (9.0-48.8) | 21 |
| Pain, n (%) | 12 (26.1) | 29 |
| Location | 0 | |
| Head and neck | 33 (44.0) | |
| Trunk | 15 (20.0) | |
| Lower limb | 15 (20.0) | |
| Upper limb | 12 (16.0) | |
| Progressive increase | 36 (90.0) | 35 |
| Rapid increase | 4 (10.0) | |
| Warmth, n (%) | 0 | 29 |
| Pulsatility (on palpation), n (%) | 3 (6.5) | 29 |
| Firm consistency, n (%) | 23 (59.0) | 36 |
| Soft consistency, n (%) | 13 (33.3) | |
| Identified triggering factor, n (%) | 7 (14.3) | 26 |
| Trauma | 5 | |
| Pregnancy | 1 | |
| Air travel | 1 | |
| Imaging data | ||
| MRI, described in n cases (%) | 47 (62.7) | 28 |
| Well-delineated mass* | 10 (76.3) | |
| Heterogeneity/homogeneity* | ||
| Heterogeneous mass | 23 (88.5) | |
| Homogeneous mass | 3 (11.5) | |
| T1-weighted sequence description* | ||
| Hyperintense signals | 3 (9.4) | |
| Hypointense signals | 2 (6.2) | |
| Isointense signal | 27 (84.4) | |
| T2-weighted sequence description* | 31 (100) | |
| Hyperintense signals | ||
| Contrast enhancement* | 34 (100) | |
| Flow voids* | 21 (100) | |
| Signal modifications of the underlying bone* | 2 (4.1) | |
| CT scan, described in n cases (%) | 22 (29.3) | 53 |
| Well-delineated mass* | 4 (66.7) | |
| Heterogeneous mass* | 4 (100) | |
| Contrast enhancement* | 9 (100) | |
| Angiogram, n (%) | 27 (36.0) | 48 |
| Pathology and molecular data | ||
| Biopsy/excision performed, n (%) | 68 (90.7) | 7 |
| Presence of adipose tissue* | 31 (93.9) | |
| Description of the vessel size* | ||
| Small vessels only | 42 (67.7) | |
| Small and other vessels | 20 (32.3) | |
| Search of somatic mutations* | 6 | |
| MAP2K1 gene | 4 | |
| KRAS gene | 2 | |
| *For each of these variables, percentages were calculated as number of patients with the positive variable to the number patients for whom this variable was described. Thus, the denominator was not the same for each because all variables were not reported in all patients (i.e. percentage calculations take into account missing data for each variable). | ||
| IQR: interquartile range; CT: computed tomography; MRI: magnetic resonance imaging. | ||
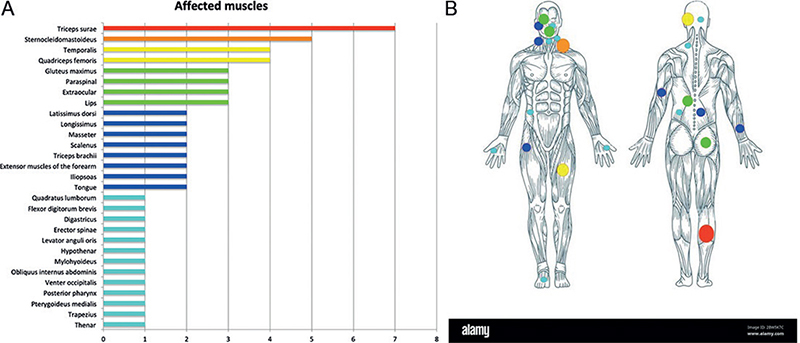
Fig. 2. Muscles affected by the intramuscular capillary-type haemangioma (ICTH). (A) Muscles affected by ICTH according to the number of patients (n = 61 of 75 for whom the muscle was precisely described); (B) overview of the body and the location where ICTH occurred (the size of the spots are proportional to the number of muscles affected).
Diagnosis required MRI in 47 (62.7%) cases; the most common features were a well-delineated mass with heterogeneous signals, isointense on T1-weighted images, and always displaying hypersignals on T2-weighted images and after contrast enhancement (Table II). Flow voids were reported in 21 cases. Angiography was performed in 27 (36.0%) cases, mostly describing highly vascularized lesions with no nidus, inhomogeneous parenchymal staining and emerged arterial feeders.
Pathology data on biopsy or surgical excision were provided for 68 (90.7%) (Table II) cases. Descriptions mainly included endothelial cell proliferation, with small-sized vessels alone in 67.7% of cases or concomitant with other-sized vessels, and in most, the presence of adipose tissue. When described, all cases were negative for GLUT-1 and the lymphatic marker D2-40. One article reported mutations in the genes KRAS (n = 4) and MAP2K1 (n = 2) in a series of 8 cases (15).
Therapeutic management was reported in 46 cases (Table III). The most frequent treatment consisted of complete surgical removal (n = 34, 73.9%), often described as a bleeding surgery, which led to complete remission without recurrence in all cases except 1 (n = 1/22), with a median post-treatment follow-up of 14.0 months (IQR 6.0-24.0). Most ICTH cases that underwent complete surgery were located on the head and neck (n = 22 of 34, 64.7%). No treatment with embolization alone was described, but 5 cases of embolization were followed by surgery, with complete remission in 2 (3 missing data). The “wait and see” attitude was reported in 3 cases and showed stabilization of the lesion.
ICTH occurred earlier for trunk than head and neck lesions (median 19.7 vs 31.0 years, p = 0.002), were larger (median 36.0 vs 13.0 cm2, p = 0.014), and were the most frequent (Appendix S2). Pain did not differ by topography, and patients ≤ 16 years and > 16 years old did not differ in characteristics of ICTH.
DISCUSSION
Since its first description by Allen & Enzinger, in 1972 (1), ICTH (i.e. IMH) remains poorly defined and understood. This review of the literature allows for a better characterization: ICTH can occur at every age, but mostly in young adults, and is often a firm, painless, progressively increasing mass located in a muscle on the head and neck (temporalis or masseter), the trunk (sternocleidomastoideus) or limb (triceps surae, quadriceps femoris, gluteus maximus). MRI is the most widely used imaging technique for diagnosis, showing features of a well delineated hypervascular heterogeneous lesion, isointense on T1-weighted images, hyperintense on T2-weighted images, enhanced after injection of contrast medium and showing flow voids. Pathology examination of samples is also useful for diagnosis and shows a proliferation of capillaries, with GLUT-1 negative endothelial cells, sometimes associated with larger arteries and veins, and with adipose tissue.
The most common differential diagnosis of ICTH is another type of vascular anomaly, IVMs. Among the 1,143 articles screened, 90 cases were excluded after reinterpretation by an adjudication committee because they were considered a misdiagnosis of venous malformations instead of ICTH. Indeed, venous malformations might also be located in a muscle, commonly the lower limbs and head and neck, but are often painful due to localized intravascular coagulopathy within the malformation and lead to thrombosis and phleboliths (19). In venous malformations, vessels appear much more dilated on microscopy (cavernous vessels) (20) and are not associated with a capillary component. ICTH must be distinguished from IVMs because the management differs (21–23).
The pathogenesis of ICTH remains unknown. Local triggering factors were suspected in very few cases, which raised the hypothesis of post-traumatic-induced vascular lesions (24, 25). However, no evidence supports this hypothesis.
Regarding treatment, surgical removal, when possible, seems the best therapy, and might be preceded by embolization to control bleeding. Contrary to extracranial AVMs, no exacerbation of lesions was described after embolization alone or partial excision (26). Except for corticosteroids, which were not found efficient for reducing the mass, no drugs were reported as a treatment for ICTH. In a recent study of ICTHs (named intramuscular fast-flow vascular anomalies) somatic mutations in KRAS and MA2PK1 genes, similar to extracranial AVMs, were identified by Goss et al. (15). This suggests a common pathogenesis or a spectrum of lesions for ICTHs and AVMs. However, ICTHs seem clinically different from usual extracranial AVM, with a very limited local aggressive potential and lower recurrence rate (27). Beyond this, ICTH and its analogy to extracranial AVMs questions the dichotomy between tumoural and malformative vascular anomalies. However, future molecular analyses of ICTH are needed to confirm the findings by Gross et al., as very few molecular findings are currently published. Future targeted therapies could be useful for non-operable huge lesions if post-zygotic mutations are confirmed, as is increasingly described for AVM treatment (28).
Study limitations
The main limitations of the review were, first, that numerous articles reporting IMHs involved other conditions, mostly IVMs; hence a large number of reports were excluded. It cannot definitely be ruled out that some included cases did not correspond to the definition of ICTH because of limited imaging or pathological descriptions. Secondly, the included reports had a lot of missing data regarding treatments and follow-up, with very little long-term follow-up information.
Conclusion
This review highlights that ICTH is still often misdiagnosed, and allows for better understanding of ICTH and its management.
ACKNOWLEDGEMENT
Data will be made available, in addition to study protocols, the statistical analysis plan, and the informed consent form upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. A written agreement must be signed with the University Hospital of Tours and the coordinator of the study. Proposals should be submitted to dpo@chu-tours.fr.
REFERENCES
- Allen PW, Enzinger FM. Hemangioma of skeletal muscle. An analysis of 89 cases. Cancer 1972; 29: 8–22.
- Yilmaz S, Kozakewich HP, Alomari AI, Fishman SJ, Mulliken JB, Chaudry G. Intramuscular capillary-type hemangioma: radiologic-pathologic correlation. Pediatr Radiol 2014; 44: 558–565.
- Griffin N, Khan N, Thomas JM, Fisher C, Moskovic EC. The radiological manifestations of intramuscular haemangiomas in adults: magnetic resonance imaging, computed tomography and ultrasound appearances. Skeletal Radiol 2007; 36: 1051–1059.
- Beham A, Fletcher CD. Intramuscular angioma: a clinicopathological analysis of 74 cases. Histopathology 1991; 18: 53–59.
- DeGiovanni JC, Simmonds J, Lang-Orsini M, Lee A. Recurrent intramuscular hemangioma (infiltrating angiolipoma) of the lower lip: a case report and review of the literature. Ear Nose Throat J 2022; 101: 306–311.
- Hein KD, Mulliken JB, Kozakewich HPW, Upton J, Burrows PE. Venous malformations of skeletal muscle. Plast Reconstr Surg 2002; 110: 1625–1635.
- Ros de San Pedro J, Cuartero Pérez B, Ferri Ñíguez B, Villanueva San Vicente V. Arteriovenous malformations of the temporalis muscle: a comprehensive review. Oper Neurosurg 2018; 14: 325–340.
- Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol 2021; 28: 44–58.
- Hoeft S, Luettges J, Werner JA. Infiltrating angiolipoma of the M. temporalis. Auris Nasus Larynx 2000; 27: 265–269.
- Kurek KC, Howard E, Tennant LB, Upton J, Alomari AI, Burrows PE, et al. PTEN hamartoma of soft tissue: a distinctive lesion in PTEN syndromes Am J Surg Pathol 2012; 36: 671–687.
- Stein-Wexler R. Pediatric soft tissue sarcomas. Semin Ultrasound CT MR 2011; 32: 470–488.
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 2015; 136: e203–204.
- Couto JA, Huang AY, Konczyk DJ, Goss JA, Fishman SJ, Mulliken JB, et al. Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am J Hum Genet 2017; 100: 546–554.
- Al-Olabi L, Polubothu S, Dowsett K, Andrews KA, Stadnik P, Joseph AP, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest 2018; 128: 1496–1508.
- Goss JA, Konczyk DJ, Smits PJ, Kozakewich HPW, Alomari AI, Al-Ibraheemi A, et al. Intramuscular fast-flow vascular anomaly contains somatic MAP2K1 and KRAS mutations. Angiogenesis 2019; 22: 547–552.
- Chen H, Xu B, Wang G, Guo Y, Hou K, Yu J, et al. Posterior occipital intramuscular hemangioma mimicking arteriovenous malformation: Case report. Medicine (Baltimore) 2019; 98: e14678.
- Russo D, Fraitag S, Bruneau B, Stock N, Aillet S, Dupuy A, et al. Non-congenital dorsal tumefaction with rapid growth in a young child identified as an intramuscular hemangioma. JAAD Case Rep 2020; 6: 616–618.
- Lipede C, Nikkhah D, Ashton R, Murphy G, Barnacle AM, Patel PA, et al. Management of Fibro-adipose Vascular Anomalies (FAVA) in paediatric practice. JPRAS Open 2021; 29: 71–81.
- Dompmartin A, Acher A, Thibon P, Tourbach S, Hermans C, Deneys V, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol 2008; 144: 873–877.
- Colletti G, Biglioli F, Poli T, Dessy M, Cucurullo M, Petrillo M, et al. Vascular malformations of the orbit (lymphatic, venous, arteriovenous): diagnosis, management and results. J Craniomaxillofac Surg 2019; 47: 726–740.
- Maruani A, Tavernier E, Boccara O, Mazereeuw-Hautier J, Leducq S, Bessis D, et al. Sirolimus (rapamycin) for slow-flow malformations in children: The observational-phase randomized clinical PERFORMUS trial. JAMA Dermatol 2021; 157: 1289–1298.
- Chen RJ, Vrazas JI, Penington AJ. Surgical management of intramuscular venous malformations. J Pediatr Orthop 2021; 41: e67–e73.
- Rabe E, Pannier F. Sclerotherapy in venous malformation. Phlebology 2013; 28: 188–191.
- Yonehara Y, Nakatsuka T, Ichioka I, Takato T, Matsumoto S, Yamada A. Intramuscular haemangioma of the anterior chest wall. Br J Plast Surg 2000; 53: 257–259.
- Lescura CM, de Andrade BAB, Bezerra KT, Agostini M, Ankha MVA, de Castro F, et al. Oral intramuscular hemangioma: report of three cases. J Cutan Pathol 2019; 46: 603–608.
- Lyness RW, Williams R. Intramuscular haemangioma arising in the lateral rectus extraocular muscle: a case report and review of the published work. Orbit 1986; 5: 159–167.
- Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK. Extracranial arteriovenous malformations: natural progression and recurrence after treatment Plast Reconstr Surg 2010; 125: 1185–1194.
- Nicholson CL, Flanagan S, Murati M, Boull C, McGough E, Ameduri R, et al. Successful management of an arteriovenous malformation with trametinib in a patient with capillary-malformation arteriovenous malformation syndrome and cardiac compromise. Pediatr Dermatol 2022; 39: 316–319.