ORIGINAL REPORT
Effect of Disease Severity on Comorbid Conditions in Atopic Dermatitis: Nationwide Registry-Based Investigation in Finnish Adults
Ville KIISKI1, Liisa UKKOLA-VUOTI2#, Johanna VIKKULA2#, Martta RANTA3, Mariann I. LASSENIUS2 and Jaakko KOPRA3
1Independent Researcher, Helsinki, 2Medaffcon Oy, Espoo, and 3AbbVie Oy, Helsinki, Finland
#These authors contributed equally.
The majority of registry studies on atopic dermatitis include only patients and diagnoses from specialized healthcare. The aim of this retrospective, real-world cohort study was to evaluate the effect of atopic dermatitis severity on comorbidities and total morbidity, with comprehensive data from both primary and specialty healthcare registries covering the entire Finnish adult population. In total, 124,038 patients were identified (median age 46 years; 68% female) and stratified by disease severity. All regression analyses (median follow-up 7.0 years) were adjusted at a minimum for age, sex, obesity, and educational level. Compared with mild atopic dermatitis, severe atopic dermatitis was significantly associated with multiple morbidities, including neurotic, stress-related and somatoform disorders, abscesses, erysipelas/cellulitis, impetigo, herpes zoster, extragenital herpes, bacterial conjunctivitis, septicaemia, lymphomas, alopecia areata, urticaria, other dermatitis, contact allergy, osteoporosis, and intervertebral disc disorders (p < 0.001). In addition, there were significant associations with alcohol dependence, depression, condylomas, rosacea, migraine, sleep apnoea, hypertension, enthesopathies, atherosclerosis, and drug-induced cataract (p < 0.05). Odds ratios were modest and mostly were between 1.10 and 2.75. Furthermore, patients with severe atopic dermatitis had lower incidences of prostate cancer, cystitis, and anogenital herpes than patients with mild atopic dermatitis (p < 0.05). These results suggest that severe atopic dermatitis results in significant overall morbidity.
Key words: atopic dermatitis; cohort study; comorbidities; epidemiology; registries; severity.
SIGNIFICANCE
Atopic dermatitis is an itchy, persistent skin disorder that significantly reduces quality of life. In addition, patients may concurrently experience other adverse health conditions that increase the burden of this disorder. This first national study of adverse health conditions related to the severity of atopic dermatitis in Finnish adults shows that the risk of coexisting conditions, such as infectious, psychiatric, dermatological, and musculoskeletal disorders, is linked to the severity of atopic dermatitis. Therefore, this comorbidity load should be considered when treating patients with severe atopic dermatitis, aiming for better control of symptoms and a comprehensive approach.
Citation: Acta Derm Venereol 2023; 103: adv00882. DOI https://doi.org/10.2340/actadv.v103.4447.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Dec 7, 2022; Published: Mar 8, 2023
Corr: Jaakko Kopra, AbbVie Oy, Veturitie 11 T 132, FIN-00520 Helsinki, Finland. E-mail: Jaakko.kopra@abbvie.com
Competing interests and funding: MR and JK are employees of AbbVie and own AbbVie stock. LUV, JV, and MIL are employed by Medaffcon Oy. VK is or has been a consultant or advisory board member for AbbVie, LEO Pharma, and Merck.
INTRODUCTION
Atopic dermatitis (AD) is a prevalent, itchy, inflammatory skin disorder with a chronic or recurrent course (1). It affects people of all ages and ethnic groups worldwide, although the prevalence varies (2). AD often considerably impairs the quality of life of those affected (3). The pathogenesis of AD is complex, and is characterized by skin barrier dysfunction and immune dysregulation (4). Patients with AD have an increased risk of other atopic conditions, such as food allergies, allergic rhinoconjunctivitis, asthma, and nasal polyposis. In addition, many cutaneous bacterial and viral infections are more prevalent and more severe in patients with AD (5–7). Furthermore, a higher risk of extracutaneous infections has been reported in patients with AD, especially among those with severe disease, including upper and lower respiratory tract infections, endocarditis, septicaemia, and encephalitis (5, 8, 9).
Over the past decade, many studies have focused on non-infectious comorbid conditions associated with AD. Among psychiatric conditions, depression and anxiety seem to be linked to AD, and the risk appears to increase with disease severity (8, 10). Suicidal ideation, bipolar disorder, and schizophrenia have also been associated with AD (11–15). Several studies have indicated a positive association between attention deficit hyperactivity disorder (ADHD) and AD, mostly in paediatric populations (16). Sleep disturbances are frequent in AD (17). Many autoimmune diseases seem to be more common in patients with AD; of these, alopecia areata and vitiligo have the strongest association (18).
Atopic dermatitis is a highly prevalent disease in Finland (19). Previous studies in the Finnish population have shown an association between AD and many psychiatric conditions, such as depression, anxiety, bipolar disorder, schizophrenia, personality disorders, and eating disorders (14, 21). However, there are no prior studies on AD and comorbid conditions in relation to disease severity. Furthermore, previous studies have only included patients treated in a specialty care setting, typical of most registry studies (11, 20).
This real-world registry cohort study aimed to identify and describe Finnish patients with AD and to evaluate the effect of AD severity on total morbidity and disease burden by combining nationwide data from both primary and specialty healthcare, private and public settings, covering the entire adult Finnish population.
MATERIALS AND METHODS
This was a retrospective registry study, using existing data generated during routine clinical practice in primary and specialty care and available in the Finnish nationwide electronic healthcare registries (Appendix S1). All methods were carried out in accordance with relevant guidelines and regulations.
Real-world data (RWD) were retrieved for the study cohorts from 6 nationwide databases: the National Institute of Health and Welfare; the Social Insurance Institution of Finland; the Finnish Centre for Pensions; Statistics Finland; and 2 nationwide private healthcare providers, Terveystalo and Mehiläinen (Fig. 1).

Fig. 1. Flow chart of the data formation (excluded data, blue boxes) and analyses (grey boxes). AD: atopic dermatitis; ICD-10: International Classification of Diseases, 10th Revision; ICPC2: International Classification of Primary Care, 2nd edition.
Inclusion criteria
Inclusion criteria for patient identification were the diagnosis codes for AD given by specialty or primary healthcare physicians (International Classification of Diseases, 10th Revision (ICD-10), code L20* (* indicates any number) and the International Classification of Primary Care, 2nd edition (ICPC2), code S87) in the Care Register for Healthcare of the Finnish Institute for Health and Welfare, drug reimbursement codes, and reimbursed drug purchases for AD in the Social Insurance Institution registries in 2005–2019.
Inclusion criteria for the analysis cohort were the AD diagnosis (ICD-10 code L20; ICPC-2 code S87) recorded by a physician or a granted reimbursement code for AD medication between 2005 and 2019. Patients were included from both public and private healthcare providers based on AD diagnoses.
Because the primary care diagnoses are available from 2012 onwards, to maximize the cohort size and the observational period, both prevalent and incident patients with AD were included. Prevalent patients were identified during the baseline period (1 January 2005 to 31 December 2012) and incident patients from 1 January 2013 to 31 December 2019. For prevalent patients, the index date was 1 January 2013 (cross-sectional cohort setting); for incident patients, the index was set to the first fulfilment of the inclusion criteria (incident cohort setting). The identified AD cohort was followed from index until death or the end of follow-up (i.e. 31 December 2019). Therefore, the maximum observational period for a patient was 7 years.
Patient stratification by atopic dermatitis severity
Adult patients were stratified to subgroups by disease severity (mild, moderate, or severe) using disease-specific treatment and care history obtained from the health and drug reimbursement-related registries (Tables SI and SII). The severity criteria were developed together with AD clinical experts. For patient characteristics and analyses of co-diagnoses, the highest severity grade during the follow-up per patient was used.
Patient characteristics
Demographic characteristics were assessed at the end of the study (31 December 2019). Co-diagnoses were analysed from the follow-up period (the time between the index and the end of follow-up) using diagnosis data (with ICD-10 diagnosis codes) from public and private healthcare settings, considering both the primary diagnosis and comorbidities.
Statistical analyses
Descriptive statistics were used to summarize the patient cohort, including mean with standard deviation (SD), median with interquartile range (IQR), minimum and maximum values for continuous variables, and number and percentage of patients for categorical variables. The difference between strata was tested using analysis of variance for normally distributed continuous variables, Kruskal–Wallis test for skewed continuous variables and χ2 test for categorical variables. All analyses assumed a significance level of 0.05, and all tests were 2-sided.
The relationship between occurrence of various co-diagnoses (71 diagnoses of interest; Table SIII) and the severity of AD was assessed using logistic regression analysis with various confounding factors (Appendix S1). Only prevalent patients were included in the regression analyses to unify the length of the follow-up; incident patients were excluded owing to shorter follow-up. The p-values were corrected using the Benjamini-Hochberg method owing to multiple testing. Both original and corrected p-values are reported along with the adjusted odds ratios (ORs) and 95% confidence intervals (95% CI).
Statistical analyses were performed using R version 4.0.2. Only existing data were used, and no imputation of missing values was performed. The proportion of missing values are reported where applicable.
RESULTS
Patient characteristics
A total of 128,428 adult patients with AD during the years 2005 through 2019 were identified (Fig. 1). The number of patients at the end of study was 124,038, comprising a total of more than 600,000 patient-years (Table I); 46,805 patients were included in the regression analyses (Fig. 1). The median age at the end of study was 46 years, and 68% of patients were female. Among the prevalent patients at the end of study, 30.3% had severe AD, 46.9% had moderate AD, and 22.8% had mild AD (Table SIV). A total of 46,805 patients were included in the regression analyses (Fig. 1).
Association of infections and autoimmune diseases with atopic dermatitis severity
The risk of certain cutaneous and subcutaneous infections and conditions (allergic contact dermatitis and other dermatitis, extragenital HSV, urticaria, impetigo, cellulitis and erysipelas, varicella zoster virus, abscess (p < 0.001), condylomas, and rosacea (p < 0.05)), infections (sepsis, and conjunctivitis (p < 0.001)), autoimmune disease alopecia areata (p < 0.001), and other atopic diseases (asthma, and allergic conjunctivitis and rhinitis (p < 0.001)) increased with increased AD severity (Fig. 2). In contrast, patients with severe AD had significantly fewer anogenital HSV symptoms and less cystitis compared with patients with mild AD (p < 0.05). The risk of rheumatoid arthritis was higher in patients with moderate AD compared with those with mild AD. The severity of AD was not significantly associated (corrected p > 0.05) with the risk of viral warts, candidiasis, dermatophytosis, acne, otitis media, gastroenteritis, Crohn’s disease, hypothyroidism, ulcerative colitis, nasal polyposis, influenza, pneumonia, bronchitis, or upper respiratory infections (Fig. S1A–D).
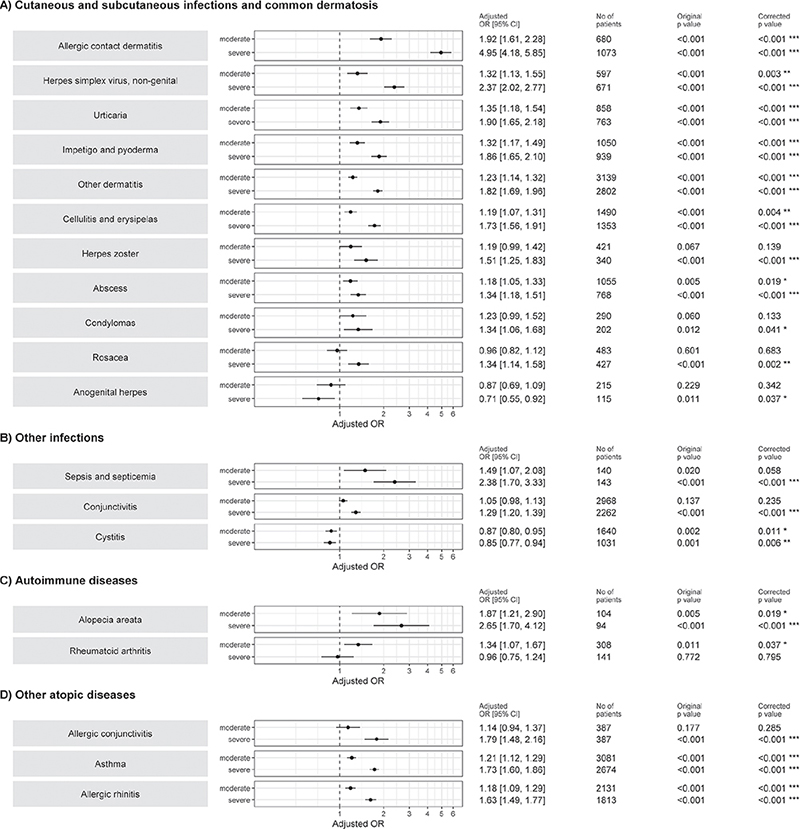
Fig. 2. Logistic regression model for association of (A) cutaneous and subcutaneous infections, (B) other infections, and (C) autoimmune disease, and (D) other atopic disease comorbidities with atopic dermatitis severity. Independent variable atopic dermatitis severity on left side of the figure (mild as reference). ICD-10: International Classification of Diseases, 10th Revision; OR: odds ratio. Confounding variables for adjusting analyses were age, sex, educational level, and obesity (ICD-10 code E66). p-value has been corrected for multiple testing using Benjamini-Hochberg method. *p < 0.05; **p < 0.001; ***p < 0.001.
Association of mental health disorders, neurological, cardiovascular and metabolic, and muscular diseases with atopic dermatitis severity
Severe AD increased the risk of certain psychiatric disorders (alcohol dependence, ADHD, depression (p < 0.05) and other neurotic, stress-related, and somatoform diseases (p < 0.001)), neurological diseases (migraine, sleep apnoea and other sleep disorders (p < 0.05)), cardiovascular and metabolic diseases (hypertension and atherosclerosis (p < 0.05)), and musculoskeletal diseases (intervertebral disc disorders (p < 0.001) and enthesopathies (p < 0.05)) compared with mild AD (Fig. 3A–D). There was no correlation (p > 0.05) between AD severity and the risk of other studied mental health disorders, neurological diseases, cardiovascular and metabolic, or musculoskeletal diseases (Fig. S2A–D).
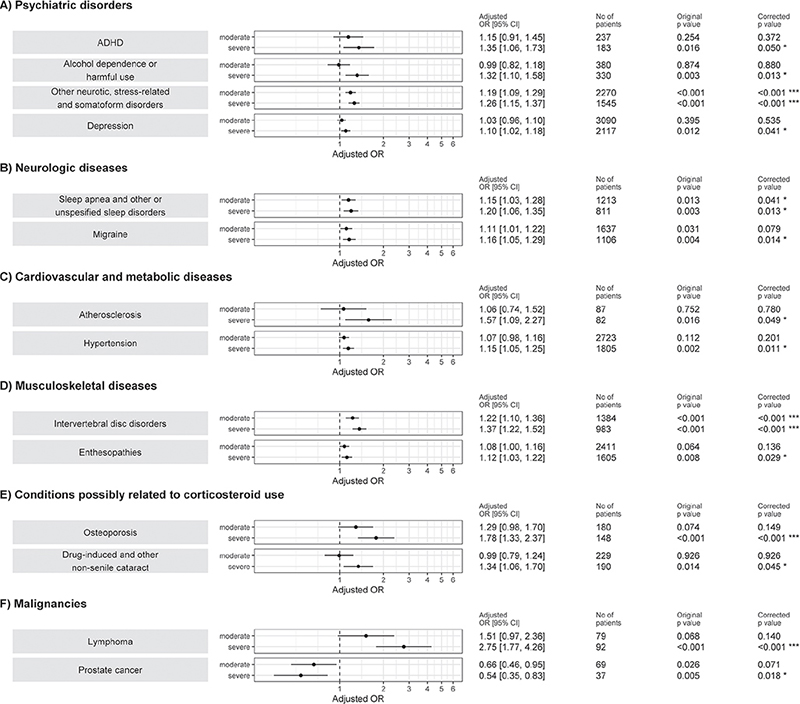
Fig. 3. Logistic regression model for association of (A) mental health disorders, (B) neurological diseases, (C) cardiovascular and metabolic diseases, (D) muscular diseases, (E) conditions possibly related to corticosteroid use, and (F) malignancies with atopic dermatitis severity. Independent variable atopic dermatitis severity on left side of the figure (mild as reference). ADHD: attention-deficit/hyperactivity disorder; ICD-10: International Classification of Diseases, 10th Revision; OR: odds ratio. Confounding variables for adjusting analyses were age, sex, educational level, and obesity (ICD-10 code E66). p-value has been corrected for multiple testing using Benjamini-Hochberg method. *p<0.05; ***p<0.001.
Association of conditions possibly related to corticosteroid use and malignancies with atopic dermatitis severity
The presence of severe AD was significantly associated with osteoporosis (p < 0.001), drug-induced cataract (p < 0.05), and lymphoma (p < 0.001; Fig. 3E–F). Patients with moderate or severe AD had a lower risk of prostate cancer compared with patients with mild AD (p < 0.05), but not with breast cancer (Fig. S2F). Furthermore, there were no associations with AD severity and glaucoma, senile cataract, common fractures, or non-melanoma skin cancer (Fig. S2E–F).
The prevalence of other diagnoses among patients with AD overall and by severity of AD during the follow-up period is shown in Table SV.
DISCUSSION
In this extensive registry study of more than 124,000 patients with AD and a median follow-up of 7.0 years, we report that severe AD compared with mild AD, bears an increased risk of morbidity, including mental health disorders, bacterial and viral infections, lymphomas, alopecia, urticaria, rosacea, migraine, sleep apnoea, hypertension, atherosclerosis, enthesopathies, and intervertebral disc disorders. Furthermore, AD severity was associated with an increased risk of osteoporosis and drug-induced cataract. It is important to note that the reference cohort comprised patients with mild AD, who might already have an increased risk of some conditions compared with healthy individuals. To our knowledge, the current study is the first one to combine nationwide patient data from primary care, specialty care, and private healthcare registries covering the entire Finnish adult population with comprehensive data on prescription medications, healthcare resource utilization, and diagnoses. Data on medications and phototherapy made it possible to stratify patients by AD severity estimates.
Skin barrier dysfunction, immune dysregulation, and cutaneous microbiome dysbiosis with prevalent Staphylococcus aureus colonization (4, 5, 7, 21) explain the increased risk of cutaneous infections in patients with AD. In the current study, severe AD was associated with an increased risk of cutaneous and subcutaneous bacterial infections, varicella zoster virus, condylomas, extragenital herpes infections, and septicaemia compared with patients with mild AD. This concurs with earlier studies (5–9, 22–24). Somewhat surprisingly, the situation with genital herpes was reversed: patients with severe AD had significantly fewer genital HSV symptoms. We hypothesize that this could be explained by sexual dysfunction associated with severe AD (25), leading to a smaller number of sexual partners. This hypothesis is, however, challenged by the opposite finding regarding the risk of condylomas.
Urticaria and contact dermatitis were more frequent in patients with severe AD. The observed association between severe AD and contact allergy is unlikely to represent a true association, but instead, to be due to the more frequent patch-testing of patients with severe AD. It has been previously well reported that AD is not truly associated with an increased risk of contact allergies (26, 27). The increased risk of other dermatitis is explained by nummular dermatitis, for which AD is a known risk factor (22). To our knowledge, the association of AD severity with rosacea has not been reported previously. However, rosacea is a known adverse effect of topical corticosteroids frequently used in the treatment of AD (28). The higher rate of healthcare visits in patients with more severe AD might partially explain the increased detection of some diseases, particularly other dermatological conditions.
Considering the more frequent use of systemic corticosteroids in severe AD in the past, it is not surprising that, in the current study, severe AD increased the risk of osteoporosis and drug-induced cataract. Both conditions are previously reported comorbidities of AD and known adverse effects of systemic corticosteroids (29, 30). However, an earlier study by Haeck et al. (31) found that lower bone density in patients with AD was independent of corticosteroid use within the previous 5 years; hence other explanations are possible. Nonetheless, this observed association should further warrant a critical view on systemic corticosteroids in the treatment of AD.
In the current study, severe AD increased the risk of ADHD, alcohol dependence, depression, and other neurotic, stress-related, and somatoform diseases, which have all been linked to AD (10, 13, 23, 32), including an earlier study in the Finnish population by Kauppi et al. (11). However, the prevalence of anxiety did not increase together with AD severity. Seeking help for a mental health disorder can be burdensome, even more so for patients already struggling with severe skin disease, which may impact observed associations. Of the autoimmune diseases, severe AD was only associated with a higher risk of alopecia, in line with previous studies (18, 32). There is a known connection between migraine and sleep disorders (33), which can partly explain the increased risk of migraine in patients with severe AD, aligning with earlier research (23, 34).
AD has been associated with a slightly elevated cardiovascular risk, even though research on this area is inconclusive (15, 23, 32, 34). In the current study, the risk of hypertension followed AD severity, which concurs with a recent meta-analysis by Yousaf et al. (35). Furthermore, vascular inflammation seems to correlate with the severity of AD (36). In line with this, the risk of peripheral atherosclerosis in the current study was significantly higher in patients with severe AD, and there was a trend toward a higher risk of coronary and cerebral arterial diseases. The current study is among the first extensive studies assessing cardiovascular risk and the effect of AD severity. However, even if we could adjust for educational level, obesity, hyperlipidaemias, diabetes, and hypertension, we lacked data on some important cardiovascular risk factors, such as smoking.
Studies on the effect of AD severity on the risk of musculoskeletal diseases other than osteoporosis are sparse. AD has been previously shown to be associated with other musculoskeletal injuries and fractures (37). The current study showed associations between enthesopathies and intervertebral disc disorders and the severity of AD. The risk of lymphoma increased with AD severity, as expected from previous studies (38). Unexpectedly, patients with severe AD had a significantly lower occurrence of prostate cancer. To our knowledge, this has not been reported previously, although a decreased risk of lung and central nervous system cancer in patients with AD has been proposed (39).
No association was found between AD severity and educational level, aligned with prior studies in the Finnish population (19). Only one-third of patients with AD in the current study were men. This considerably skewed sex ratio probably represents the missingness of male subjects from the data. One-fifth of the patients were classified as having severe AD. However, the current data did not include over-the-counter medications, such as mild topical hydrocortisone preparations. Many patients with mild AD may have no or very infrequent need for healthcare services or prescription drugs. Consequently, the number of patients with mild AD may be significantly underestimated, and the analysed cohort skewed toward moderate and severe disease. This may have diluted the observed associations. However, we find that this is mainly reflected in the skewed AD severity grouping already, and the effect on the analyses is probably small.
There are differences between countries in terms of treatment patterns, healthcare systems, and healthcare utilization, and this may affect the generalizability of the results. However, because of the nature of the study objectives (comorbidities) and the extensive real-life data from primary and specialty care and the public and private sectors, the results are generally transferable to other adult populations of European descent.
Study limitations
The real-world evidence (RWE) registry study setting has its limitations. The retrospective data used were initially recorded for purposes other than research. Thus, some information may have been inconsistently recorded and the diagnoses or the indirect disease severity stratification cannot be validated by a specialist, potentially affecting the outcomes. As stated above, many male patients and patients with mild AD are probably missing from the data, and the differences in healthcare systems affect generalizability to some extent. In addition, the lack of a non-AD control group is a limitation.
Conclusion
Most previous studies on comorbidities and the burden of AD have only included data from tertiary care, representing mostly patients with moderate to severe AD. Therefore, RWE studies stratified by disease severity with comprehensive patient data are necessary. The current RWD analysis of Finnish registry data demonstrated the increased total morbidity of patients with severe AD, with regard to numerous infectious, dermatological, mental health, metabolic, and musculoskeletal diseases and lymphomas. This suggests that the effect of severe AD results in significant overall morbidity, which is not limited to the skin.
ACKNOWLEDGEMENTS
The authors wish to thank Anne Lehtonen, Jaakko Aaltonen, Mari Renlund, and Mikko Kosunen for their significant input in the design and initiation of this study as well as for their support in its execution. We also thank Anita Remitz and Lari Kurkisuo for their input in the study design.
The study was conducted with permission from the Finnish Social and Health Data Permit Authority Findata (Findata, permit no. THL/3438/14.02.00/2020) and Statistics Finland (data permit no. TK/451/07.03.00/2020) by the provision of the Act on the Secondary Use of Health and Social Data (Finland’s Ministry of Justice 552/2019), therefore no informed consent from the patients was required.
AbbVie sponsored the study; contributed to the design; and participated in the collection, analysis, and interpretation of data and in the writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship.
REFERENCES
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–60.
- Bylund S, Kobyletzki LB, Svalstedt M, Svensson Å. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol 2020; 100: adv00160.
- Koszorú K, Borza J, Gulácsi L, Sárdy M. Quality of life in patients with atopic dermatitis. Cutis 2019; 104: 174–177.
- Brunner PM, Leung DYM, Guttman-Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol 2018; 120: 34–41.
- Wan J, Shin DB, Syed MN, Abuabara K, Lemeshow AR, Gelfand JM. Risk of herpesvirus, serious and opportunistic infections in atopic dermatitis: a population-based cohort study. Br J Dermatol 2022; 184: 664–672.
- Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis 2020; 31: 157–164.
- Wang V, Boguniewicz J, Boguniewicz M, Ong PY. The infectious complications of atopic dermatitis. Ann Allergy Asthma Immunol 2021; 126: 3–12.
- Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Ann Allergy Asthma Immunol 2018; 120: 66–72.e11.
- Serrano L, Patel KR, Silverberg JI. Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: a systematic review and meta-analysis. J Am Acad Dermatol 2019; 80: 904–912.
- Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 2013; 131: 428–433.
- Kauppi S, Jokelainen J, Timonen M, Tasanen K, Huilaja L. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol 2019; 99: 647–651.
- Sandhu JK, Wu KK, Bui TL, Armstrong AW. Association between atopic dermatitis and suicidality: a systematic review and meta-analysis. JAMA Dermatol 2019; 155: 178–187.
- Thyssen JP, Hamann CR, Linneberg A, Dantoft TM, Skov L, Gislason GH, et al. Atopic dermatitis is associated with anxiety, depression, and suicidal ideation, but not with psychiatric hospitalization or suicide. Allergy 2018; 73: 214–220.
- Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. Br J Dermatol 2019; 181: 554–565.
- Cho YT, Hsieh WT, Chan TC, Tang CH, Chu CY. Prevalence of baseline comorbidities in patients with atopic dermatitis: a population-based cohort study in Taiwan. JAAD Int 2020; 1: 50–58.
- Schmitt J, Apfelbacher C, Heinrich J, Weidinger S, Romanos M. [Assoziation von Neurodermitis und Aufmerksamkeits-Defizit/Hyperaktivitäts-Syndrom]. Z Kinder Jugendpsychiatr Psychother 2013; 41: 35–42; quiz 42–44.
- Bawany F, Northcott CA, Beck LA, Pigeon WR. Sleep disturbances and atopic dermatitis: relationships, methods for assessment, and therapies. J Allergy Clin Immunol Pract 2021; 9: 1488–1500.
- Lu Z, Zeng N, Cheng Y, Chen Y, Li Y, Lu Q, et al. Atopic dermatitis and risk of autoimmune diseases: a systematic review and meta-analysis. Allergy Asthma Clin Immunol 2021; 17: 96.
- Kiiski V, Salava A, Susitaival P, Barnhill S, Remitz A, Heliovaara M. Atopic dermatitis in adults: a population-based study in Finland. Int J Dermatol 2022; 61: 324–330.
- Kauppi S, Jokelainen J, Timonen M, Tasanen K, Huilaja L. Atopic dermatitis and the risk of eating disorders: a population-based cohort study. J Am Acad Dermatol 2022; 87: 474–476.
- Kuo IH, Yoshida T, de Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol 2013; 131: 266–278.
- Leshem YA, Sugerman PB, Weil C, Chodick G, Liang H, Wang H, et al. Cutaneous comorbidities associated with atopic dermatitis in Israel: a retrospective real-world data analysis. Dermatitis 2022; 33: S61–S68.
- Roh YS, Huang AH, Sutaria N, Choi U, Wongvibulsin S, Choi J, et al. Real-world comorbidities of atopic dermatitis in the US adult ambulatory population. J Am Acad Dermatol 2022; 86: 835–845.
- Droitcourt C, Vittrup I, Kerbrat S, Egeberg A, Thyssen JP. Risk of systemic infections in adults with atopic dermatitis: a nationwide cohort study. J Am Acad Dermatol 2021; 84: 290–299.
- Linares-Gonzalez L, Lozano-Lozano I, Gutierrez-Rojas L, Lozano-Lozano M, Rodenas-Herranz T, Ruiz-Villaverde R. Sexual dysfunction and atopic dermatitis: a systematic review. Life (Basel) 2021; 11: 1314.
- Hamann CR, Hamann D, Egeberg A, Johansen JD, Silverberg J, Thyssen JP. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 70–78.
- Diepgen TL, Ofenloch RF, Bruze M, Bertuccio P, Cazzaniga S, Coenraads PJ, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol 2016; 174: 319–329.
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006; 54: 1–15; quiz 16–18.
- Wu D, Wu XD, Zhou X, Huang W, Luo C, Liu Y. Bone mineral density, osteopenia, osteoporosis, and fracture risk in patients with atopic dermatitis: a systematic review and meta-analysis. Ann Transl Med 2021; 9: 40.
- Beck KM, Seitzman GD, Yang EJ, Sanchez IM, Liao W. Ocular co-morbidities of atopic dermatitis. Part i: associated ocular diseases. Am J Clin Dermatol 2019; 20: 797–805.
- Haeck IM, Hamdy NAT, Timmer-de Mik L, Lentjes EGWM, Verhaar HJJ, Knol MJ, et al. Low bone mineral density in adult patients with moderate to severe atopic dermatitis. Br J Dermatol 2009; 161: 1248–1254.
- Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol 2017; 137: 18–25.
- Tiseo C, Vacca A, Felbush A, Filimonova T, Gai A, Glazyrina T, et al. Migraine and sleep disorders: a systematic review. J Headache Pain 2020; 21: 126.
- Smirnova J, Montgomery S, Lindberg M, Svensson Å, von Kobyletzki L. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol 2020; 20: 23.
- Yousaf M, Ayasse M, Ahmed A, Gwillim EC, Janmohamed SR, Yousaf A, et al. Association between atopic dermatitis and hypertension: a systematic review and meta-analysis. Br J Dermatol 2022; 186: 227–235.
- Villani AP, Pavel AB, Wu J, Fernandes M, Maari C, Saint-Cyr Proulx E, et al. Vascular inflammation in moderate-to-severe atopic dermatitis is associated with enhanced Th2 response. Allergy 2021; 76: 3107–3121.
- Garg N, Silverberg JI. Association between eczema and increased fracture and bone or joint injury in adults: a US population-based study. JAMA Dermatol 2015; 151: 33–41.
- Mansfield KE, Schmidt SAJ, Darvalics B, Mulick A, Abuabara K, Wong AYS, et al. Association between atopic eczema and cancer in England and Denmark. JAMA Dermatol 2020; 156: 1086–1097.
- Wang L, Bierbrier R, Drucker AM, Chan AW. Noncutaneous and cutaneous cancer risk in patients with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol 2020; 156: 158–171.