Evidence of the association between a personal history of basal cell carcinoma and the risk of non-cutaneous malignancies is conflicting. The aim of this study was to retrospectively clarify the risk of non-cutaneous cancers in individuals with basal cell carcinoma using nationwide Finnish registry data for 96,304 patients and 394,503 randomly selected population controls. In this study, individuals with basal cell carcinoma have an increased risk of other cancers (odds ratio (OR) 1.38; 95% confidence interval (95% CI) 1.36–1.40). The risk was most prominent for lip cancer (OR 5.29; 95% CI 4.50–6.21), mycosis fungoides (OR 3.13; 95% CI 2.31–4.23) and soft tissue cancers (OR 2.77; 95% CI 2.43–3.16). In age-adjusted model, men had higher risk of cancers overall compared with women (p < 0.05). In conclusion, the study found increased overall cancer risk among patients with basal cell carcinoma compared with randomly selected population controls.
Key words: basal cell carcinoma; non-melanoma skin cancer.
Accepted Nov 17, 2022; Published Nov 30, 2022
Acta Derm Venereol 2022; 102: adv00826.
DOI: 10.2340/actadv.v102.4451
Corr: Laura Huilaja, Department of Dermatology, Oulu University Hospital, P.B.20, FIN-90029 Oulu, Finland. E-mail: laura.huilaja@oulu.fi
SIGNIFICANCE
Basal cell carcinoma (BCC) is the most common skin cancer in fair-skinned individuals, and its incidence is increasing worldwide. There are conflicting results regarding the risk of non-cutaneous cancers in individuals with basal cell carcinoma. This study analysed this risk in patients with basal cell carcinoma in Finland by using nationwide health-registry data. The study found that individuals with BCC have an increased risk of other cancers overall. The risk was especially increased for lip cancer, mycosis fungoides and for soft tissue cancers. Further studies are needed to clarify the reasons behind this association.
INTRODUCTION
Basal cell carcinoma (BCC), belonging to the group of non-melanoma skin cancers (NMSC), is the most common skin cancer in fair-skinned individuals and its incidence is increasing worldwide (1–4).
There is evidence that individuals with a personal history of BCC are at altered risk of developing non-cutaneous cancers. It is well established that exposure to ultraviolet (UV) radiation is the most important risk factor for BCC (5, 6). On the contrary, it has been postulated that exposure to UV radiation, through production of vitamin D and its possible anti-carcinogenic effects, might be inversely associated with several internal cancers (7, 8). This has raised the hypothesis that skin cancer, including BCC, could be an indicator of high vitamin D levels due to excess sun exposure and could thus be associated with decreased risk of internal malignancies, such as cancers of the colon, breast and prostate (9–11).
Alternatively, a growing body of evidence suggests that individuals with BCC are at increased risk of non-cutaneous cancers, both prior to (12, 13) and after the diagnosis of BCC (14–21). A higher risk of comorbid malignancies has been observed, especially in younger individuals with BCC (16, 21). Among the non-cutaneous cancers that have repeatedly been reported at increased incidence in individuals with BCC are cancers of the lip, salivary glands, lung, breast, prostate and non-Hodgkin’s lymphoma (12–17, 21, 22).
The objective of this study was to clarify the association between BCC and the risk of non-cutaneous cancers and possible differences in the risk between sexes. In addition, this study aimed to investigate whether the location of the first reported BCC affects the risk of non-cutaneous malignancies.
MATERIALS AND METHODS
Populations and databases
This was a retrospective nationwide registry study of individuals aged 18 years or older, born at the earliest in 1910, with a first-time diagnosis of BCC between 1 January 1987 and 31 December 2018. Data were obtained from the Finnish Care Register for Health Care (CRHC) maintained by the Finnish Institute of Health and Welfare. The CRHC contains data on all diagnoses set in inpatient care in specialized healthcare from 1969 onward and in specialized outpatient care from 1998 onward. The unique social security number given to every Finnish citizen ensures that each individual is included only once in the CRHC.
This study was exempted from review by the IRB (ethics committee of Northern Ostrobothnia Hospital District) since it was a registry-based study.
BCC was defined using codes 1730A to 1739A in the ninth revision of the International Classification of Diseases (ICD) and codes C44.01, C44.11, C44.21, C44.31, C44.41, C44.51, C44.61, C44.71, C44.81 and C44.91 in the tenth revision of the ICD. Only individuals with at least 1 registered BCC no later than 1 year before the end of data collection were included in the BCC population to ensure follow-up time. A group of controls was selected randomly from data from the Digital and Population Data Services Agency. Corresponding diagnosis data from CRHC was gathered for controls. Individuals with a record of BCC were excluded from the control population. Individuals with a record of albinism, Gorlin syndrome or xeroderma pigmentosum (XP) were excluded from both study and control populations because of their increased risk of developing BCC (23, 24). Diagnoses of comorbid cancers (Table SI) based on ICD-9 and ICD-10 codes were gathered for cases and controls from the CRHC.
Statistical analyses
The characteristics of the study population are presented as proportions and means. The associations between BCC and non-cutaneous cancer were evaluated using a conditional logistic regression model and presented with odds ratio (OR) and 95% confidence interval (95% CI). Estimates were adjusted for age (by birth year) and sex. The potential interaction between sex and a diagnosis of cancer was assessed by including an interaction term in the regression models and through stratified analyses. However, because the matching factors are associated with exposure, they were controlled in conditional logistic regression analysis. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary NC, USA). Two-sided p-values < 0.05 were considered statistically significant.
RESULTS
Characteristics of the study and control populations
Characteristics of the study and control populations are shown in Table I. Among 96,304 BCC patients, 31,667 (32.9%) and among 394,503 controls, 79,420 (20.1%) had been diagnosed with at least 1 non-cutaneous cancer. The majority (71.9%) of those with BCC had been diagnosed with only one non-cutaneous cancer (Table I). However, compared with controls, BCC cases were more likely to be diagnosed with more than 1 non-cutaneous cancer at different sites (p < 0.05).
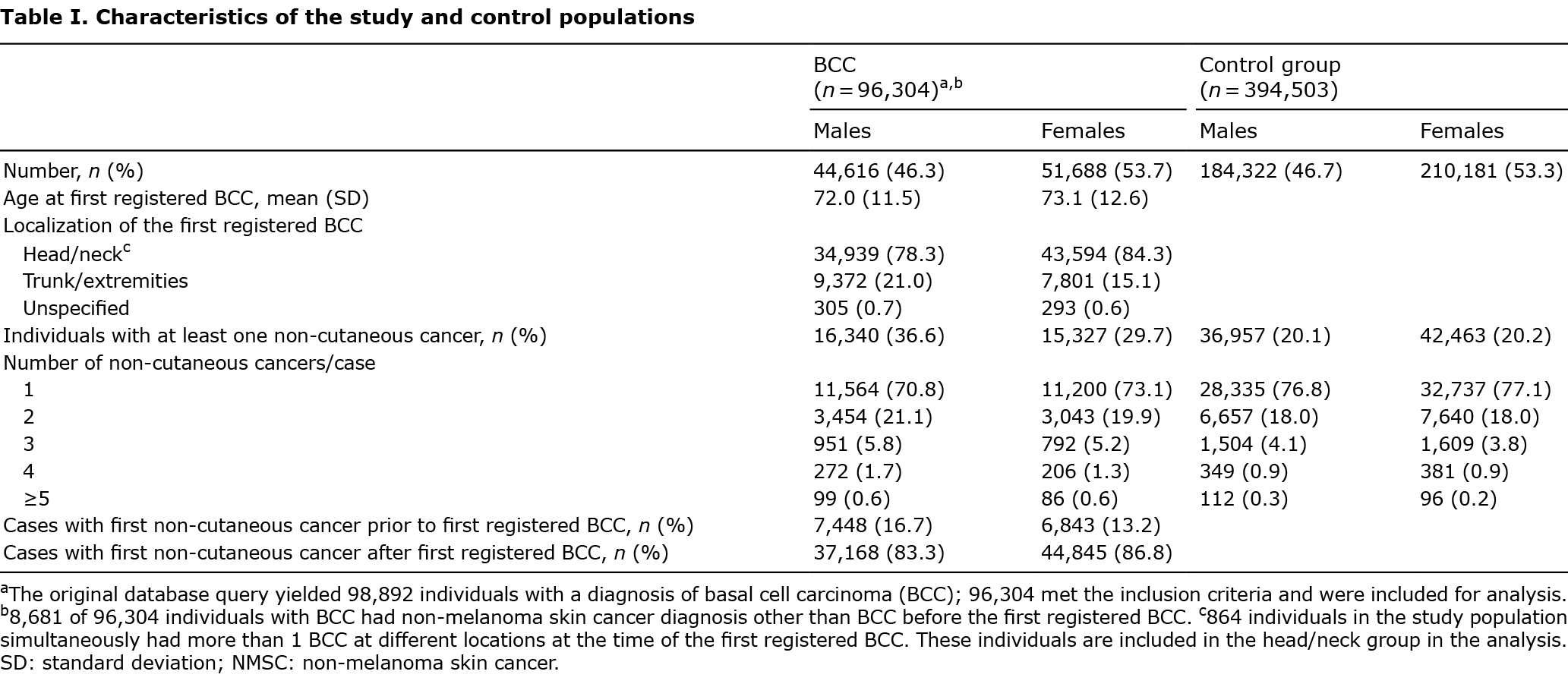
Risk of cancers other than skin in patients with a history of basal cell carcinoma
Those with BCC had higher overall cancer morbidity (odds ratio (OR) 1.38, 95% CI 1.36–1.40) compared with controls when adjusted for age and sex (Table SII). Several cancers at different sites were found at increased incidence in patients with BCC (Fig. 1, Tables SII and SIII). Among the selected cancers, lip cancer demonstrated the highest risk (OR 5.29; 95% CI 4.50–6.21), followed by mycosis fungoides (OR 3.13; 95% CI 2.31–4.23) and malignancies of connective tissue (OR 2.77; 95% CI 2.43–3.16), salivary glands (OR 2.35; 95% CI 1.95–2.84), nose and nasal cavity (OR 2.34; 95% CI 1.87–2.92), eye (OR 2.21; 95% CI 1.88–2.59) and Hodgkin’s lymphoma (OR 2.16; 95% CI 1.86–2.52). After exclusion of ocular melanoma, the risk of eye cancer was (OR 3.08; 95% CI 2.50–3.79).
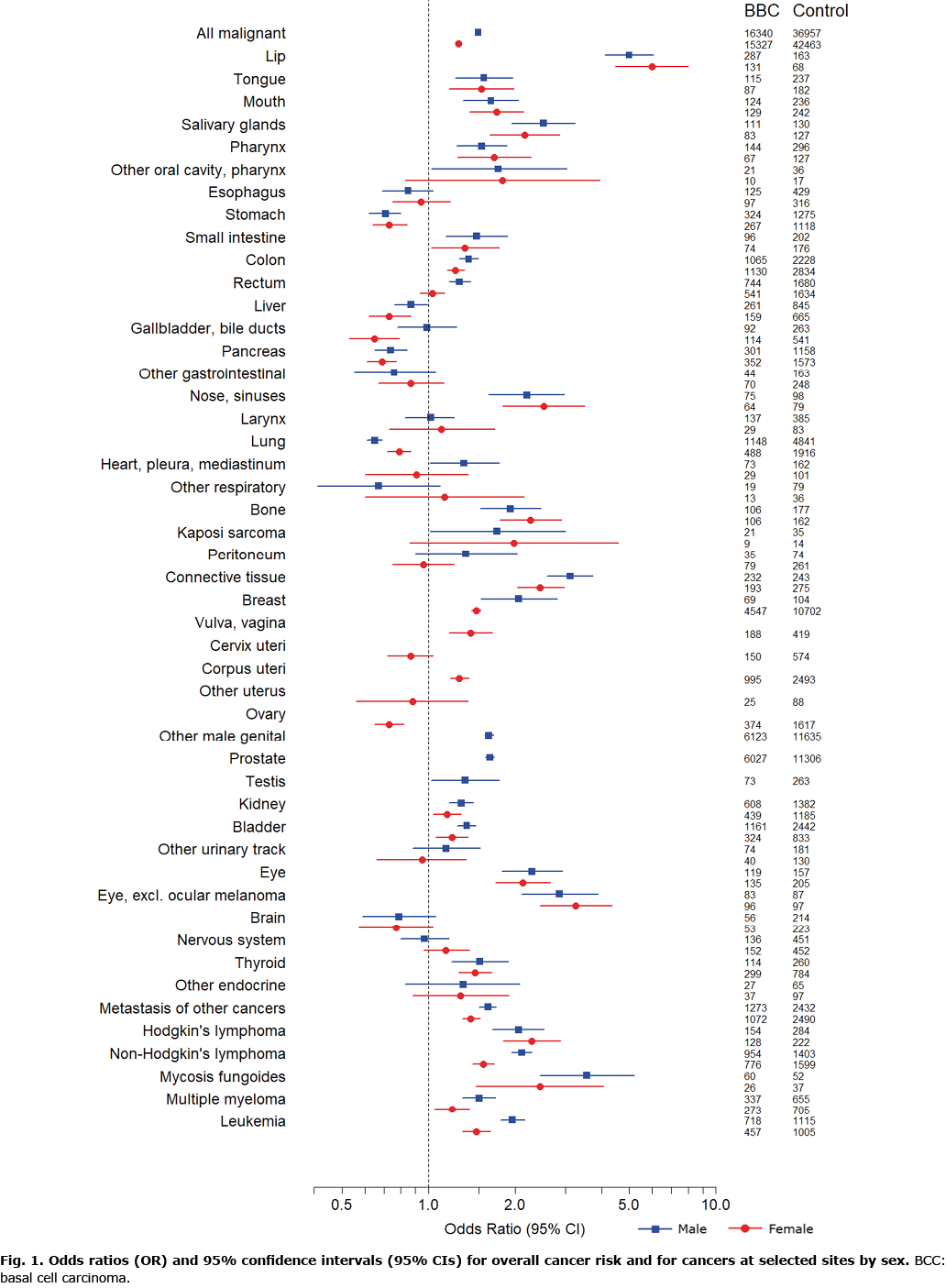
In contrast, subjects with BCC demonstrated decreased risks of malignancies of the lung (OR 0.69; 95% CI 0.65–0.73), pancreas (OR 0.71; 95% CI 0.65–0.77), stomach (OR 0.72; 95% CI 0.66–0.79), ovary (OR 0.73; 95% CI 0.65–0.82), gallbladder and bile ducts (OR 0.76; 95% CI 0.65–0.89), brain (OR 0.78; 95% CI 0.63–0.96) and liver (OR 0.81; 95% CI 0.73–0.91) compared with controls (Table SII).
Differences in cancer risk between sexes and effect of the location of the first basal cell carcinoma
The overall risk of non-cutaneous cancer was significantly (p < 0.05) higher among men with BCC (OR 1.49; 95% CI 1.45–1.52) compared with women with BCC (OR 1.27; 95% CI 1.24–1.30) (Fig. 1, Table SIII). A significant difference between sexes (p < 0.05) was observed for several selected cancers (Fig. 1). Women with the first-reported BCC on the trunk and extremities were more likely to have a diagnosis of non-cutaneous cancer compared with women whose first BCC was located in the head and neck area (OR 1.12; 95% CI 1.06–1.18). Among men, this risk was not observed (OR 1.04; 95% CI 0.99–1.09).
DISCUSSION
This study provides additional evidence of the association between BCC and risk of non-cutaneous cancers in a large study population and randomly selected population controls. The study observed higher overall cancer morbidity in both men and women with BCC compared with controls. In addition, various types of cancer were found significantly in excess on different body sites in individuals with BCC. Compared with controls, BCC cases were more likely to have a record of more than 1 non-cutaneous cancer. Furthermore, the study found evidence that women may carry different risk of non-cutaneous malignancies depending on the localization of the first diagnosed BCC, initial lesion on the trunk and extremities indicating a higher risk.
Previous reports on the association between a personal history of BCC and the risk of non-cutaneous malignancies have yielded conflicting findings. A cancer registry study of 13,961 BCC cases from the UK reported decreased risk of non-cutaneous cancers combined, as well as cancer of the breast in women, cancer of the bladder in men, and cancers of the cervix and prostate (11). A Swiss cancer registry study (22) and a cancer registry study from Northern Ireland (25)) did not find an association between BCC and risk of other cancers combined after exclusion of skin cancer. An international multicentre study incorporating 13 large cancer registries (26), including a total of 148,885 individuals with BCC, observed decreased overall risk of solid malignancies after the first BCC in sunny countries (Australia, Singapore and Spain), but, in contrast, increased risk in less sunny countries (Canada, Denmark, Finland, Iceland, Norway, Scotland, Slovenia and Sweden) (26). Several other studies have also reported increased risk of non-cutaneous malignancies in individuals with BCC both prior to (12, 13) and after the diagnosis of BCC (14–19, 21). A systematic review revising 18 registry-based studies and 3 cohort studies concluded in the direction of increased risk (20).
In the current study, lip cancer demonstrated the most prominent risk in individuals with BCC. The increase in the risk of lip cancer, well documented in a previous Finnish cancer registry study of 71,924 subjects with BCC (16) as well as in other previous studies (13–15, 21), is most likely due to a shared risk factor, since UV radiation is a known risk factor for both BCC (6) and lip cancer (27).
In addition to lip cancer, this study found an excess of several other selected cancers among individuals with BCC, including cancer of the breast in women and cancers of the prostate and colon. A growing number of studies have observed increased incidences for these 3 cancers in patients with BCC (13–17), but also decreased incidences have been reported (9–11). Two cancer registry studies from the Netherlands reported decreased risks in NMSC patients for prostate cancer (10) and for colorectal and breast cancer (9). For patients with BCC separately, a decrease in the risk of colorectal and breast cancer was significant only for certain stages of these cancers and for head and neck BCCs in men concerning colorectal cancer. Separate analyses of colon cancer and rectal cancer found no significant association (9). For prostate cancer, a separate analysis of individuals with BCC did not show a significant decrease in risk, apart from a significant risk reduction within the first year after the diagnosis of BCC (10).
The finding of increased risk of eye cancer in the current study is of interest. Krilaviciute et al. (15) reported a 2-fold risk of eye cancer in patients with BCC, although their result was not significant due to a small number of cases. Additional checking on morphology revealed that 5 of the 6 observed eye cancers were ocular melanoma and therefore, the authors postulated that the potential increased risk of eye cancer could be explained by UV exposure. Increased risk of eye cancer has also been reported previously in women by Milan et al. (16). To our surprise, only 75 of 254 individuals with eye cancer had ocular melanoma in the current study population and, thus, after exclusion of ocular melanomas, patients with BCC demonstrated even higher risk of eye cancer, suggesting that there may be an unknown shared risk factor other than UV exposure or true aetiological association between BCC and cancer of the eye.
The current study results indicate that mycosis fungoides (MF), a subtype of cutaneous T-cell lymphoma, is more common in individuals with BCC compared with controls. Increase in the risk of lymphomas, especially non-Hodgkin’s lymphoma, has previously been reported both prior to (13) and after BCC (14, 16), but in these studies, MF was not analysed separately, but was included under the diagnosis of non-Hodgkin’s lymphoma. Previous studies investigating the risk of secondary malignancies in patients with MF have not found an association between MF and BCC (28) or did not include BCCs (29–32).
In the current study, cancers of the lung, pancreas, stomach, liver, gallbladder and bile ducts, ovary and brain were less common in BCC compared with controls. Similar observations of decreased risks for cancers of the stomach, pancreas and gallbladder have been reported in a few previous studies (14, 22), although increased risk of stomach cancer, but also liver cancer (16) and cancer of the ovaries (13) has been observed in patients with BCC. In contrast to the results of the current study, lung cancer has been reported at increased incidence among patients with BCC, both in Finland (16) and elsewhere (14, 26). This discrepancy may reflect changes in the individual level risk factors of these cancers or lifestyle habits over time; it was not possible to control for potential confounders. In addition, inability to control for socioeconomic disparities may affect the observed decreased risks in the current study, since cancers of the lung, liver, pancreas, and stomach have been reported to be less common in individuals with higher socioeconomic status (SES) compared with lower SES (33, 34), whereas BCCs are more common in higher SES (35).
A limited number of previous studies have investigated the risk of internal cancers according to the location of the first BCC. The current study results suggest that women with their first BCC on the trunk and extremities are at moderately higher risk of non-cutaneous malignancies compared with women with first BCC in the head and neck area. Recent epidemiological studies of BCC have reported a rapid increase in incidence especially among younger women (2–4). This has been observed to be due to a proportional increase in truncal and leg lesions of BCC in women (2, 3). In addition, a higher risk of subsequent malignancies has been observed especially in younger individuals with BCC (16, 21). To the best of our knowledge, such an incidence trend has not yet been assessed in Finland, but could offer an explanation as to why especially women appear to demonstrate differences in risk according to the location of the first BCC.
A major strength of the current study is a large study population as well as a random, representative sample of the Finnish population as a control group. Our nationwide data is comprehensive, since public healthcare is equally available to every citizen and all cancers diagnosed and treated in public specialized healthcare are included in CRHC.
Since this was a registry-based study, misclassification of diagnoses cannot be excluded, and we have no control over the BCC patients’ and control subjects’ previous history of BCCs and cancers before 1987. The data used in the study is limited to the hospital setting. In addition, as a general limitation to registry-based studies, the current study was unable to control for UV exposure and other potential risk factors on an individual level, apart from age and sex. In addition, without information regarding SES, BCC cases as a study population may represent a selection bias, since BCCs are more common in individuals with higher SES (35). However, previous studies that have been able to adjust for potential confounding variables have not found any substantial differences between the age-adjusted and multivariable-adjusted risks (12, 17, 36), suggesting that the observed association between BCC and the risk of other cancers is unlikely to be explained by individual-level confounders.
Another limitation should be addressed. After cancer diagnosis, patients are more likely to be under intensified medical surveillance, which could represent a potential source of surveillance bias. Because this study included internal cancers both prior to and after BCC, treatment effects may affect the number of BCCs and second primary cancers and the observed cancer risks.
Even though reduced all-cause mortality has been reported among individuals with BCC (37, 38), there is evidence that cancer mortality might be higher in those with a personal history of BCC (38). Unfortunately, this study did not include data concerning deaths, and therefore the study was not able to include mortality analyses; neither could the study ensure completeness of follow-up. Secondly, the divergent prognoses of subsequent cancers may thus represent a potential source of bias, since the prognosis and mortality rates of non-cutaneous cancers differ between cancer types (33). However, considering the increasing incidence of BCC, any potential increase in cancer morbidity and mortality would be significant in terms of the number of patients at risk.
In conclusion, this study strengthens the evidence that several non-cutaneous cancers are found in modest excess in individuals with BCC. Especially women with the first reported BCC on trunk or extremities may be at altered and higher risk. Further studies are needed to clarify the underlying mechanisms of these associations.
The authors have no conflicts of interest to declare.
REFERENCES
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080.
- Adalsteinsson JA, Ratner D, Olafsdottir E, Grant-Kels J, Ungar J, Silverberg JI, et al. Basal cell carcinoma: an emerging epidemic in women in Iceland. Br J Dermatol 2020; 183: 847–856.
- Flohil SC, Seubring I, van Rossum MM, Coebergh JW, de Vries E, Nijsten T. Trends in Basal cell carcinoma incidence rates: a 37-year Dutch observational study J Invest Dermatol 2013; 133: 913–918.
- Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjaer SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978–2007: Rapid incidence increase among young Danish women. Int J Cancer 2010; 127: 2190–2198.
- Kricker A, Armstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? a case-control study in Western Australia. Int J Cancer 1995; 60: 489–494.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B 2001; 63: 8–18.
- Grant WB. Review of recent advances in understanding the role of vitamin D in reducing cancer risk: breast, colorectal, prostate, and overall cancer. Anticancer Res 2020; 40: 491–499.
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health 2006; 96: 252–261.
- Soerjomataram I, Louwman WJ, Lemmens VE, Coebergh JW, de Vries E. Are patients with skin cancer at lower risk of developing colorectal or breast cancer? Am J Epidemiol 2008; 167: 1421–1429.
- de Vries E, Soerjomataram I, Houterman S, Louwman MW, Coebergh JW. Decreased risk of prostate cancer after skin cancer diagnosis: a protective role of ultraviolet radiation? Am J Epidemiol 2007; 165: 966–972.
- Bower CP, Lear JT, Bygrave S, Etherington D, Harvey I, Archer CB. Basal cell carcinoma and risk of subsequent malignancies: a cancer registry-based study in southwest England. J Am Acad Dermatol 2000; 42: 988–991.
- Friedman GD, Tekawa IS. Association of basal cell skin cancers with other cancers (United States). Cancer Causes Control 2000; 11: 891–987.
- Lindelof B, Krynitz B, Ayoubi S, Martschin C, Wiegleb- Edström D, Wiklund K. Previous extensive sun exposure and subsequent vitamin D production in patients with basal cell carcinoma of the skin, has no protective effect on internal cancers. Eur J Cancer 2012; 48: 1154–1158.
- Nugent Z, Demers AA, Wiseman MC, Mihalcioiu C, Kliewer E v. Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 2584–2590.
- Krilaviciute A, Vincerzevskiene I, Smailyte G. Basal cell skin cancer and the risk of second primary cancers: a cancer registry-based study in Lithuania. Ann Epidemiol 2016; 26:511–514.
- Milan T, Pukkala E, Verkasalo PK, Kaprio J, Jansen CT, Koskenvuo M, et al. Subsequent primary cancers after basal-cell carcinoma: a nationwide study in Finland from 1953 to 1995. Int J Cancer 2000; 87: 283–288.
- Song F, Qureshi AA, Giovannucci EL, Fuchs CS, Chen WY, Stampfer MJ, et al. Risk of a second primary cancer after non-melanoma skin cancer in white men and women: a prospective cohort study. PLoS Med 2013; 10: e1001433.
- Chen J, Ruczinski I, Jorgensen TJ, Yenokyan G, Yao Y, Alani R, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst 2008; 100: 1215–1222.
- Lindelöf B, Sigurgeirsson B, Wallberg P, Eklund G. Occurrence of other malignancies in 1973 patients with basal cell carcinoma. J Am Acad Dermatol 1991; 25: 245–248.
- Wheless L, Black J, Alberg AJ. Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiol Biomarkers Prev 2010; 19: 1686–1695.
- Frisch M, Hjalgrim H, Olsen JH, Melbye M. Risk for subsequent cancer after diagnosis of basal-cell carcinoma: a population-based, epidemiologic study. Ann Intern Med 1996; 125: 815–821.
- Levi F, la Vecchia C, Te VC, Randimbison L, Erler G. Incidence of invasive cancers following basal cell skin cancer. Am J Epidemiol 1998; 147: 722–726.
- Nikolaou V, Stratigos AJ, Tsao H. Hereditary nonmelanoma skin cancer. Semin Cutan Med Surg 2012; 31: 204–210.
- Jaju PD, Ransohoff KJ, Tang JY, Sarin KY. Familial skin cancer syndromes: increased risk of nonmelanotic skin cancers and extracutaneous tumors. J Am Acad Dermatol 2016; 74: 434–437.
- Cantwell MM, Murray LJ, Catney D, Donnelly D, Autier P, Boniol M, et al. Second primary cancers in patients with skin cancer: a population-based study in Northern Ireland. Br J Cancer 2009; 100: 174–177.
- Tuohimaa P, Pukkala E, Scelo G, Olsen JH, Brewster DH, Hemminki K, et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer 2007; 43: 1701–1712.
- Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of lip cancer: a review of global incidence and aetiology. Oral Dis 1999; 5: 185–195.
- Väkevä L, Pukkala E, Ranki A. Increased risk of secondary cancers in patients with primary cutaneous t cell lymphoma. J Invest Dermatol 2000; 115: 62–65.
- Goyal A, O’Leary D, Goyal K, Rubin N, Bohjanen K, Hordinsky M, et al. Increased risk of second primary malignancies in patients with mycosis fungoides: a single-center cohort study. J Am Acad Dermatol 2020; 82: 736–738.
- Kantor AF, Curtis RE, Vonderheid EC, van Scott EJ, Fraumeni Jr JF. Risk of second malignancy after cutaneous T-cell lymphoma cancer 1989; 63: 1612–1615.
- Huang KP, Weinstock MA, Clarke CA, Mcmillan A, Hoppe RT, Kim YH. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sézary syndrome evidence from population-based and clinical cohorts. Arch Dermatol 2007; 143: 45–50.
- Hodak E, Lessin S, Friedland R, Freud T, David M, Pavlovsky L, et al. New insights into associated co-morbidities in patients with cutaneous T-cell lymphoma (mycosis fungoides). Acta Derm Venereol 2013; 93: 451–455.
- Pitkäniemi J, Malila N, Virtanen A, Degerlund H, Heikkinen S, Seppä K. Cancer in Finland 2018. Cancer Society of Finland Publication No. 94, Helsinki 2020.
- Larsen IK, Myklebust TÅ, Babigumira R, Vinberg E, Møller B, Ursin G. Education, income and risk of cancer: results from a Norwegian registry-based study. Acta Oncol 2020; 59: 1300–1307.
- Steding-Jessen M, Birch-Johansen F, Jensen A, Schüz J, Kjær SK, Dalton SO. Socioeconomic status and non-melanoma skin cancer: a nationwide cohort study of incidence and survival in Denmark. Cancer Epidemiol 2010; 34: 689–695.
- Karagas MR, Greenberg ER, Mott LA, Baron JA, Ernster VL. Occurrence of other cancers among patients with prior basal cell and squamous cell skin cancer. Cancer Epidemiol Biomarkers Prev 1998; 7: 157–161.
- Jensen AO, Lamberg AL, Jacobsen JB, Braae Olesen A, Sorensen HT. Non-melanoma skin cancer and ten-year all-cause mortality: a population-based cohort study. Acta Derm Venereol 2010; 90: 362–367.
- Jensen AO, Bautz A, Olesen AB, Karagas MR, Sorensen HT, Friis S. Mortality in Danish patients with nonmelanoma skin cancer, 1978–2001. Br J Dermatol 2008; 159: 419–425.