Mastocytosis is a rare myeloid disorder characterized by expansion and accumulation of mast cells in 1 or more organ systems (1, 2). According to the WHO classification, mastocytosis can be divided into 2 main categories: cutaneous mastocytosis (CM) and systemic mastocytosis (SM) (3). Cutaneous lesions present with a high level of heterogeneity among patients. A generally accepted approach is to divide CM into maculopapular cutaneous mastocytosis (MPCM), also known as urticaria pigmentosa, diffuse cutaneous mastocytosis (DCM), and mastocytoma of the skin (4). According to a consensus statement, CM in adults should prompt a preliminary diagnosis of “mastocytosis in the skin” (MIS), with subsequent investigations being initiated to demonstrate or rule out SM (5). Adult-onset MIS almost always persists throughout life and is usually associated with SM. In contrast, childhood-onset MIS presents spontaneous resolution around puberty and the majority of paediatric patients present no evidence of histological involvement of other organs (4).
The aim of this study is to describe the differences in cutaneous involvement in children and adults with MIS. This is the first study to present data regarding the Greek population with MIS.
MATERIALS AND METHODS and RESULTS
This is a retrospective observational study, involving patients with MIS, between 2009 and 2022, from the Mastocytosis & Mast Cell Activation Disorders Clinic at Allergy Unit “D. Kalogeromitros” of “Attikon” University General Hospital of Athens, and the Allergy Department, 2nd Pediatric Clinic of the University of Athens at Panagiotis & Aglaia Kyriakou Children’s Hospital, Greece.
Patients were divided into 2 groups based on age at diagnosis of MIS, as follows: < 16 years (children) and ≥ 16 years (adults). Furthermore, adults were divided in 2 sub-groups based on the reported age of onset of disease, as follows: <16 years (childhood onset) and ≥16 years (adulthood onset).
All patients were diagnosed and classified according to WHO criteria (1). Children without evidence of extracutaneous involvement were classified as CM and received the diagnosis according to predefined clinical features. Only children with red flags (Fig. S1) received full Bone Marrow (BM) investigation. Adult patients with skin lesions who declined a BM investigation received a provisional diagnosis of MIS.
Results
A total of 108 patients with MIS were included in the study. Of these, 46 were children (mean age 2.2; age range 0–10 years) and 62 were adults (mean age 37.9; age range16–66 years) at the time of diagnosis. Although the prevalence of MIS was similar in females (53%) and males (47%), a slight predominance of males (62.2%) was found in the children, while in adults, females (62.9%) were more frequently diagnosed than males (p = 0.022) (Table SI).
Overall, 33 (30.6%) patients were diagnosed with SM, 52 (48.1%) with CM, and 23 adults (21.3%) with MIS. Among the 33 patients with SM, there was 1 (3%) child, and 32 (97%) adults.
Most adults presented with onset of skin involvement in adulthood (56/62, 90.3%) and only 6 (9.7%) with childhood-onset disease, with a mean age at onset of 5.3 years (age range 1–12 years). Among children, the vast majority presented with polymorphic MPMC (31/46, 83.8%) (Fig. 1A); 6 out of 46 (13%) with monomorphic MPMC (Fig. 1B) and 9 children (19.6%) with solitary mastocytoma (Fig. 1C). In all except 1 child (97.8%) the first lesions were presented during the first 2 years of life. In all children Darier sign was positive and 28/46 (60/9) reported symptoms of itching and 12/46 (26.1%) flushing after friction, crying or fever.
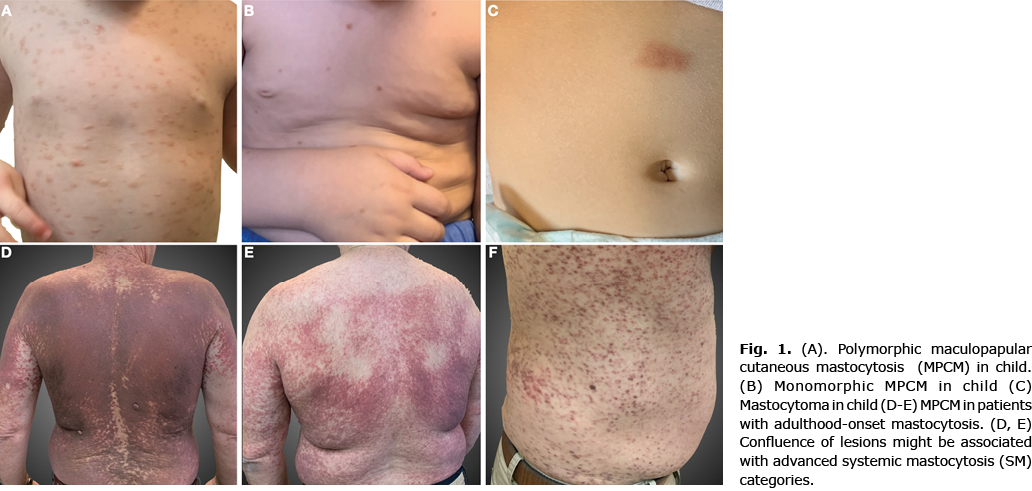
In adults, data regarding the form of cutaneous lesions was available for only 38 patients. Among them 37/38 (97.4%) presented monomorphic MPMC (Fig. 1D–F) and 1 (2.6%) presented atypical skin involvement (mast cell infiltrates in the skin biopsy) with no macroscopic skin lesions. Adult patients based on the time of onset of the lesions were classified into groups: childhood onset (6/62, 9.7%) with mean age of onset 5.3 years (age range 1–12 years) and adulthood onset (56/62, 90.3%) with mean age of onset 31.9 years (age range 16–61 years). Darier sign was positive in 66.1% (41/62) of patients; and cutaneous symptoms (itching, flushing) were reported in 25/62 (40.3%) patients.
No organomegaly, or pathological findings in blood count or general symptoms were noted in the paediatric population in the current study. Due to elevated serum tryptase (40 ng/ml) in 1 male child, full BM investigation was performed, which showed systemic involvement with c-KIT D816V mutation positive, CD2+, CD25+ on the BM sample, with a final diagnosis of indolent systemic mastocytosis (ISM). All other children (45/46) had normal serum tryptase levels (mean: 5.4 ng/mL). Peripheral blood test analysis for c-KIT D816V mutation was performed in 18 children and was negative in all of them.
Full BM investigation was performed in 39 adult patients; in 7/39 no BM infiltration of neoplastic mast cells was detected and a diagnosis of CM was made; 32/39 fulfilled the criteria of SM, with 30 of them receiving a diagnosis of ISM and 2 a diagnosis of aggressive systemic mastocytosis (ASM). Twenty-three adult patients declined or postponed BM investigation and received a provisional diagnosis of MIS. Adults with SM had a significantly higher mean value of serum tryptase than adults with CM (50.18 nm/mL vs 7.25 ng/ml; p = 0.0002).
DISCUSSION
This study found a reversal of the sex ratio of patients with MIS among children and adults; in patients with childhood-onset disease there was a higher proportion of males, compared with a higher proportion of females among patients with adult-onset disease. The age of onset in most childhood cases was before the age of 2 years, and in adult cases between the third and fourth decades of life.
Distribution of the cutaneous forms within the current study population is consistent with that reported previously (4). In the current study, lesions of childhood onset were more often large and widespread, whereas those of adulthood onset were more often small in diameter. It was also observed that confluence of lesions might be associated with advanced SM categories (Fig. 1D). In agreement with the literature (4), lesions in adult cases were frequently located on the trunk and extremities, while in paediatric cases the head/neck region was more frequently involved. There are only 9 paediatric cases with solitary mastocytoma; we assume that these deviations from published data are due to good prognosis of these lesions, which tend to be followed-up by paediatricians.
The true incidence of SM in children is unknown. A Spanish study of 111 children with MIS (6), recognized only 2 cases of SM; an Australian study (7) found SM in 2 of 173 children with MIS; whereas Lange et al. (8) reported that only 1 of 101 children with MIS in Poland progressed to SM.
In the current study population only 1 child with MIS underwent full BM investigation, due to a high tryptase level. Among adults the current study data confirmed the correlation of tryptase levels with SM. In addition, the current study analysed data for 6 adults with childhood-onset MIS. Among those with a final diagnosis of SM, in 2 out of 3 patients the tryptase levels were higher than normal (32 and 40 mg/dl, respectively), but in the other patient it was only 6 mg/dl. Tryptase levels were normal in the patients without BM involvement.
In contrast to the childhood-onset population, SM is a predominantly clinical manifestation of mastocytosis in adults (2, 9, 10). In the current study population of adults with adulthood-onset MIS, the vast majority (87%) had involvement of BM.
This study is limited by its retrospective nature. There were a limited number of skin biopsies and investigations for KIT mutations, especially in the paediatric population. Furthermore, for some of the adults, data regarding the classification of skin lesions were not available. Further studies are necessary, with extensive follow-up to adulthood of all children with MIS.
In conclusion, this study demonstrates the different types of CM lesions in children and adults; and different extracutaneous involvement, which suggest that MIS should be approached differently based on age.
ACKNOWLEDGEMENTS
Ιnstitutional review board (IRB) approval status: All procedures performed in studies involving human participants were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethics standards.
Informed consent was obtained from all individual participants included in the study.
The authors have no conflicts of interest to declare.
REFERENCES
- Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017; 129: 1420–1427.
- Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification and management. Am J Haematol 2015; 90: 250–262.
- Horny H-P, Akin C, Arber DA, Peterson LC, Tefferi A, Metcalfe DD, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edn. Lyon: International Agency for Research on Cancer; 2017, p. 62–69.
- Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137: 35–45.
- Valent P, Akin C, Escribano L et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest 2007; 37: 435–453.
- Alvarez-Twose I, Vano-Galvan S, Sanchez-Munoz L, Morgado JM, Matito A, Torrelo A, et al. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy 2012; 67: 813–821.
- Hannaford R, Rogers M. Presentation of cutaneous mastocytosis in 173 children. Aust J Dermatol 2001; 42: 15–21.
- Lange M, Niedoszytko M, Renke J, Gleń J, Nedoszytko B. Clinical aspects of paediatric mastocytosis: a review of 101 cases. J Eur Acad Dermatol Venereol 2013; 27: 97–102.
- Verstovsec S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Hematol 2012; 90: 89–98.
- Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood 2009; 113: 5727–5736.