ORIGINAL ARTICLE
Antibiotic Prophylaxis of Surgical Site Infections in Cutaneous Surgery: A Prospective Observational Study
Benjamin KENDZIORA1, Kathrin PATZER1, Lars E. FRENCH1,2, Justin G. SCHLAGER1# and Daniela HARTMANN1#
1Department of Dermatology and Allergy, University Hospital, Ludwig-Maximilian University Munich, Munich, Germany and 2Dr Phillip Frost Department of Dermatology & Cutaneous Surgery, Miller School of Medicine, University of Miami, Miami, FL, USA
#These authors contributed equally to this article.
The use of perioperative antibiotic prophylaxis in cutaneous surgery is controversial due to unclear efficacy and, thus, potentially unnecessary side-effects. This prospective observational study analysed the efficacy of oral perioperative antibiotic prophylaxis in preventing surgical site infections. Adult patients undergoing cutaneous surgery between August 2020 and May 2021 at Ludwig-Maximilian University Hospital Munich, Germany, without prior signs of infection were eligible. Propensity score weighting was used for covariate adjustment to account for non-randomized treatment assignment. Of 758 included patients, 23 received perioperative antibiotic prophylaxis (3.0%). In this group, a surgical site infection occurred in 1 of 45 lesions (2.2%) compared with 76 of 1,189 lesions (6.5%) in the group without perioperative antibiotic prophylaxis (735 patients, 97.0%). With covariate adjustment, the odds ratio for the occurrence of a surgical site infection in patients receiving perioperative antibiotic prophylaxis was 0.114 (95% confidence interval 0.073–0.182; p <0.001) on a per lesion level. The number of lesions needed to treat to prevent 1 surgical site infection was 17.6 (95% confidence interval 16.8–19.2). This prospective observational study shows a reduction in the incidence of surgical site infection in cutaneous surgery performed with perioperative antibiotic prophylaxis. The large size difference between the 2 study groups limits the study.
Key words: surgical wound infection; antibiotic prophylaxis; observational study; propensity score.
SIGNIFICANCE
Wound infection after skin surgery can lead to serious complications, such as sepsis and the need for further surgery. Whether prophylactic use of an antibiotic immediately before or during the initial surgery reduces the risk of wound infection is unclear. This study, which included 758 patients, compared 2 groups of patients; a group given perioperative antibiotics and a group without perioperative antibiotics. Because assignment to groups was not random, which can lead to bias, propensity score weighting was used as a statistical technique. The study showed a reduction in the risk of wound infection after skin surgery with the use of perioperative antibiotics.
Citation: Acta Derm Venereol 2023; 103: adv4469. DOI: https://doi.org/10.2340/actadv.v103.4469.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jan 13, 2023; Published: May 10, 2023
Corr: Benjamin Kendziora, Department of Dermatology and Allergy, University Hospital, Ludwig-Maximilian University (LMU), Frauenlobstr. 9-11, DE-80337 Munich, Germany. E-mail: benjamin.kendziora@med.uni-muenchen.de
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
The aims of perioperative antibiotic prophylaxis in cutaneous surgery include the prevention of infective endocarditis, haematogenous joint infection, and surgical site infection. The efficacy of perioperative antibiotic prophylaxis in reducing the incidence of such complications is unclear, since large-scale prospective clinical trials are missing. In addition, the overuse of perioperative antibiotics may lead to an increased incidence of side-effects and the development of multidrug-resistant bacteria. The use of perioperative antibiotic prophylaxis in cutaneous surgery is thus controversial; there is no broad consensus regarding indications, and recommendations are extrapolated from guidelines issued by organizations outside of dermatology (1). The guidelines for the prevention of infective endocarditis of the European Society of Cardiology published in 2015 and the American Heart Association published in 2017 recommend antibiotic prophylaxis for procedures that breach the oral mucosa or infected skin in patients at high risk for infective endocarditis; for example, those with a prosthetic cardiac valve (2, 3). The 2012 guidelines for the prevention of prosthetic joint infections of the American Dental Association and the American Academy of Orthopaedic Surgeons suggest that antibiotic prophylaxis for patients with prosthetic joints undergoing dental procedures may not be necessary, except for immunocompromised patients (4, 5). This can, at least, be extrapolated to dermatological procedures affecting the oral mucosa. Regarding the prevention of surgical site infections, an advisory statement for antibiotic prophylaxis in dermatological surgery, published by Wright et al. in 2008, recommends the use of perioperative antibiotic prophylaxis for groin and lower extremity procedures, wedge excisions on the lip or ear, skin flaps on the nose, skin grafts, and procedures on highly inflammatory skin (4). In contrast, recent literature tends to advise against the use of oral prophylactic antibiosis for the prevention of surgical site infections in cutaneous surgery, and favours the use of oral antibiotics after diagnosis of infection, mainly because of unclear efficacy and the risk of multidrug resistance in bacteria (6).
The aim of this prospective observational study was to analyse the efficacy of oral perioperative antibiotic prophylaxis in the prevention of surgical site infections in patients undergoing cutaneous surgery.
MATERIALS AND METHODS
Ethics review and informed consent
This prospective observational single-centre study was approved by the ethics committee of the Faculty of Medicine at Ludwig-Maximilian University, Munich, Germany (process number 20-141). It was performed in accordance with the ethics standards of the responsible committee on human experimentation (institutional and regional) and with the Declaration of Helsinki 1975, as revised in 1983. Prior to initiation, a study-protocol was published (available at https://www.researchregistry.com; identifying number: researchregistry5879). Both oral and written informed consent were obtained from all study participants. Beforehand, all participants received written information validated by the ethics committee.
Patients
All adult patients admitted for cutaneous surgery to the Department of Dermatology and Allergy at the Ludwig-Maximilian University Hospital between 10 August 2020 and 31 May 2021 were eligible for inclusion. After obtaining informed consent, patient and lesion characteristics were collected by study personnel. Cancellation of surgery, transfer to an external department, loss to follow-up, and incomplete patient or lesion characteristics resulted in exclusion before analysis.
Cutaneous surgery
Surgery was performed in an in- or out-patient setting. Due to local COVID-19 restrictions during the COVID-19 pandemic, most cutaneous surgeries were performed in an inpatient setting after obtaining a negative PCR test for COVID-19. Only minor surgeries, such as punch biopsies and shave excisions, were performed in an outpatient setting. In both settings, surgeons and assistants wore sterile surgical gowns, surgical caps, masks, and sterile gloves. In the inpatient setting, patients wore clean surgical dresses. Surgeries were performed with local or general anaesthesia. According to the site of surgery and possible contraindications, antiseptic skin preparation was performed using an alcohol-based or aqueous solution of povidone-iodine, octenidine dihydrochloride, or chlorhexidine. Immediate or delayed wound closure was conducted by simple suture, flaps, grafts, or secondary intention. Non-absorbable and monofilament suture made of polyamide, non-absorbable and braided suture made of polyester fibres, absorbable and monofilament suture composed of polymer polydiaxone, absorbable and braided suture made of polyglactin, surgical staples, and/or adhesive closure strips were used for wound closure, except in secondary wound healing. Wound care was performed using sterile wound dressing.
Perioperative antibiotic prophylaxis
Administration of oral or intravenous, systemic antibiotics within 60 min before surgery or during the surgical procedure was defined as perioperative antibiotic prophylaxis. Perioperative antibiotics for the prevention of infective endocarditis were given according to the guidelines for the management of infective endocarditis published in 2015 by the European Society of Cardiology (7) using aminopenicillins. For the prevention of surgical site infections, perioperative antibiotics were given at the discretion of the treating surgeons who identified patients at risk for surgical site infection based on risk factors previously described in the literature and clinical experience. Aminopenicillins, cephalosporins, clindamycin, or doxycycline were used for the latter indication.
Follow-up
Depending on the surgical site and wound condition, suture material was removed between 5 and 14 days after wound closure. Suture removal and regular wound dressing changes were performed in the outpatient clinic whenever possible. Irrespective of where suture removal and regular wound dressing changes were performed, standardized follow-up by phone was conducted at day 14 after wound closure. Patients were interviewed regarding any adverse events and antibiotic intake.
Outcome measure
The outcome of interest was surgical site infection within the first 14 days after skin surgery. The definition of surgical site infection differs in clinical practice. In this study, surgical site infection was defined and diagnosed according to the European Center for Disease Prevention and Control (8).
Statistical analysis
In observational studies, non-random treatment assignment usually leads to an imbalance of covariates across compared patient groups, which may cause confounding bias and thus prevent the estimation of causal effects. Propensity score weighting is a widely used statistical method that accounts for an imbalance of covariates and thus allows the estimation of causal effects (9). An imbalance of patient and lesion characteristics was expected between patients who received perioperative antibiotic prophylaxis and patients who did not, due to non-random assignment. Propensity score weighting was used to account for this expected imbalance.
Sex, age, diabetes, hypertension, body mass index (BMI), smoking status, alcohol abuse, coronary artery disease, hypercholesterolaemia, history of haematological cancer, human immunodeficiency virus (HIV) infection, glucocorticoid intake, intake of a biological, intake of another immunosuppressive medication, anticoagulation, antiplatelet medication, malignancy of operated skin lesion, planned wound closure, ulceration, defect size, and localization were included as covariates in a logistical regression model to calculate the probability of treatment assignment given the covariate values, which is the definition of the propensity score. In selecting the variables to be included, care was taken to include variables that might affect the decision to administer perioperative antibiotic prophylaxis. The setting (inpatient vs outpatient) was not included, since it depended on defect size, localization, and planned wound closure, which were included as covariates.
The hypothesis that the use of perioperative antibiotics reduced the incidence of surgical site infection was tested using a logistical regression model. In this model, perioperative antibiotic prophylaxis was included as covariate. Observations were weighted according to the corresponding propensity score values. More specifically, the inverse probability of treatment was used to weight observations. The hypothesis was tested 2-sided with an alpha level of 0.05.
Sensitivity analysis
Classic propensity score weighting does not account for a multilevel data structure. In this study, a multilevel data structure was present, since observed units were organized within clusters. The observed units were wound lesions. One patient could have several wound lesions and, thus, wound lesions were nested within patients. Most of the covariates included in the models of the main analysis were patient characteristics. The study thus assumed no relevant additional variance in the intercept between patients in the models of the main analysis. Against this background, the study considered classic propensity score weighting as adequate for the analysis of this data.
However, as a sensitivity analysis, multilevel matching was used as a new approach to account for an imbalance of covariates in observational data with a multilevel structure (10). Multilevel matching aims at mimicking a cluster randomized trial, in which observed units are organized within clusters that were randomized to a treatment or control group. In the current case, multilevel matching mimics a cluster randomized trial, in which wound lesions are organized within patients who were randomized to the use or non-use of antibiotic prophylaxis. Multilevel matching was performed using a network flow algorithm, as presented by Pimentel et al. (10). Sex, age, diabetes, hypertension, BMI, smoking status, alcohol abuse, coronary artery disease, hypercholesterolaemia, history of haematological cancer, HIV infection, glucocorticoid intake, intake of a biological, intake of another immunosuppressive medication, anticoagulation, and antiplatelet medication were defined as covariates on the patient level. Malignancy of operated skin lesion, planned wound closure, ulceration, defect size, and localization were defined as covariates on a per lesion level. Patients were matched without matching lesions within pairs of matched patients. All patients receiving perioperative prophylaxis were kept in the matched dataset.
The hypothesis that the use of perioperative antibiotics reduced the incidence of surgical site infection was tested using a mixed effects logistical regression model. Perioperative antibiotic prophylaxis was included as fixed effect. A random intercept was used to cluster both within matched pairs of patients and within patients (10). The hypothesis was tested 2-sided with an alpha level of 0.05.
All statistics were performed in R, version 4.0.3 (Vienna, Vienna, Austria). Functions of the Stats package, version 4.0.3 (Vienna, Vienna, Austria), were used for propensity score estimation. Functions of the lme4 package, version 1.1-26 (Vienna, Vienna, Austria), were applied for outcome analysis. Functions of the matchMulti package, version 1.1.7 (Vienna, Vienna, Austria), were used for multilevel matching.
RESULTS
Patients
Between 24 August 2020 and 25 May 2021, 804 patients were eligible and consented to participate in this observational study. Of these 804 patients, 758 (94.3%) could be included in the complete case analysis. Most patients among the 758 complete cases did not receive perioperative antibiotic prophylaxis (n = 735, 97.0%). Perioperative prophylaxis was given to 23 patients (3.0%), primarily for preventing infectious endocarditis in 10 patients (43.5%), primarily for the prevention of surgical site infection in 13 patients (56.5%). Antibiotics used according to the European Society of Cardiology (7), the surgeon’s preference, and drug tolerability are listed in Table SI. A patient flow chart is shown in Fig. 1.
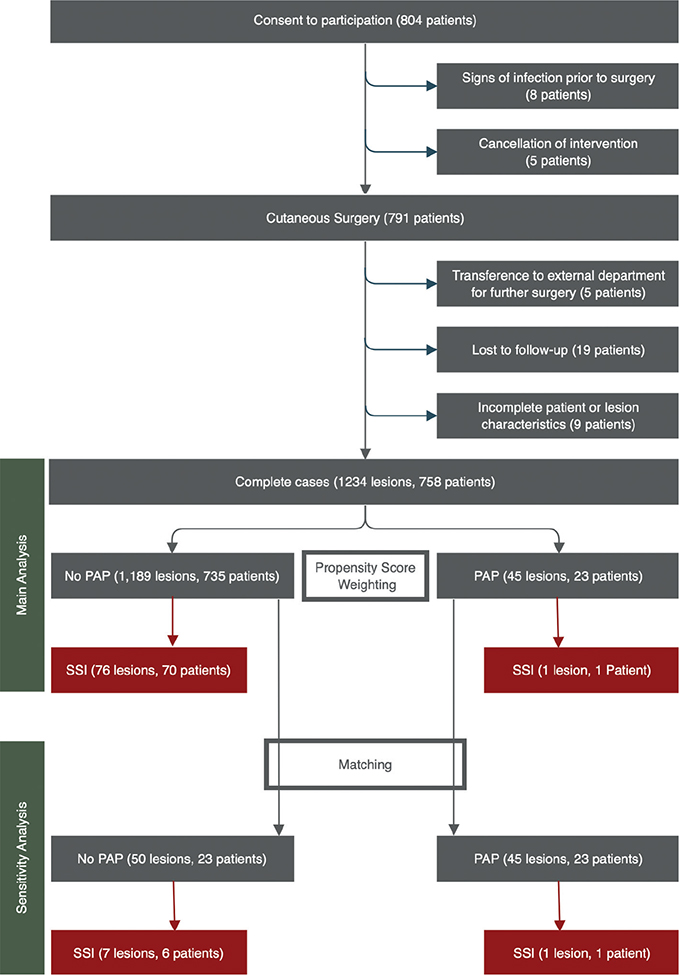
Fig. 1. Patient flow chart. PAP: perioperative antibiotic prophylaxis; SSI: surgical site infection.
Tables I–II show a considerable imbalance of several patient and lesion characteristics between patients who received perioperative antibiotic prophylaxis and patients who did not.
Outcome analysis
In the group of 23 patients who received perioperative antibiotic prophylaxis, 1 of 45 lesions (2.2%) was diagnosed with surgical site infection within the 14-day post-operative follow-up period. In the group of 735 patients who did not receive perioperative antibiotic prophylaxis, 76 of 1,189 lesions (6.4%) were diagnosed with surgical site infection within 14 days. With adjustment for covariate imbalances through propensity score weighting, the odds ratio for the occurrence of a surgical site infection with the prescription of perioperative antibiotic prophylaxis was 0.114 (95% confidence interval (95% CI) 0.073–0.182; p <0.001) on the per lesion level. The number of lesions needed to treat in order to prevent 1 surgical site infection was 17.6 (95% CI 16.8–19.2).
Sensitivity analysis
Of the 735 patients who did not receive perioperative antibiotic prophylaxis, 23 could be matched to the 23 who received antibiotic prophylaxis. Patient and lesions characteristics in the groups can be compared in Tables SII–SIII. In the group of 23 matched patients who did not receive perioperative antibiotic prophylaxis, 7 of 50 lesions (14.0%) were diagnosed with surgical site infection. Compared with the main analysis, the odds ratio for the occurrence of a surgical site infection with the prescription of perioperative antibiotic prophylaxis remained stable (odds ratio = 0.136; 95% CI 0.016–1.192; p = 0.072). The p-value showed a trend towards significance (< 0.1) without falling below the significance level of 0.05, which is explained by a loss of power resulting from a reduction in sample size of 94% compared with the main analysis.
DISCUSSION
This prospective observational study revealed a reduction in the risk of surgical site infection in cutaneous surgery performed with perioperative antibiotic prophylaxis when adjustments were made for differences in patient and lesion characteristics between treated and untreated patients that arose due to non-random assignment.
The shown effect of perioperative antibiotic prophylaxis on the incidence of surgical site infection should not be used to conclude that all patients undergoing cutaneous surgery should be treated with perioperative antibiotic prophylaxis. Antibiotics may lead to side-effects at the individual patient level or the emergence of multidrug resistant bacteria at the epidemiological level (11). Given these risks, the number needed to treat to prevent 1 surgical site infection should be reasonably low. This can be accomplished by a reliable selection of patients with increased risk for surgical site infection as candidates for the administration of perioperative antibiotic prophylaxis. In this study, patients who did not receive perioperative antibiotic prophylaxis for the prevention of infective endocarditis, but for the prevention of surgical site infection, were selected primarily based on clinical intuition because of heterogeneity in previously described risk factors (12–14). Future work should thus focus on defining accepted risk factors.
While the efficacy of perioperative prophylaxis in the reduction of surgical site infection has been shown with high evidence in general surgery (15–19) and other surgical disciplines (20–24), data available for cutaneous surgery are scarce. In a randomized controlled trial conducted in Australia and published in 2018, 154 patients undergoing flap or graft closure following skin cancer excision on the ear or nose were randomized to perioperative antibiotic prophylaxis or placebo (25). The authors showed a significant reduction in surgical site infection by perioperative antibiotic prophylaxis and a number needed to treat of 9.8 on the patient level. The number needed to treat is lower than the number needed to treat revealed from the current data; however, the current study calculated the number needed to treat on the per lesion level, which may, at least partially, explain this difference. The Australian study is limited by a restrict-ed spectrum of dermatological surgeries and moderate sample size, but its randomized nature gives the study a relatively high level of evidence. The same applies to a study conducted at the same institution, in which 54 patients undergoing lower limb skin lesion excision were randomized to perioperative antibiotic prophylaxis. The authors showed a trend towards a lower incidence of surgical site infection in the group of patients who received perioperative antibiotic prophylaxis. The number needed to treat was 4.3 (26). In a retrospective chart review of 271 patients undergoing Mohs micrographic surgery and wide local excisions below the knee, published in 2018 (27), and a registry study with data of 816 Mohs cases collected by the American College of Mohs Surgery, published in 2019 (27), no significant difference in infection rate between patients receiving perioperative antibiotic prophylaxis and patients who did not was found, which is contrary to the current study result. The latter 2 studies are limited primarily by their retrospective nature.
Study strengths and limitations
The current study gains strength of evidence by virtue of its prospective nature, its relatively high sample size, broad spectrum of dermatological surgical procedures included, and covariate adjustment by both classic propensity score weighting and multilevel matching to account for non-randomized treatment assignment.
Regarding limitations, the sample size was relatively high in total, but only a small minority of patients received perioperative antibiotic prophylaxis, resulting in inequality in size between the intervention and control group. This did not prevent the inclusion of all patients in the main analysis using propensity score weighting, but the majority of patients could not be included in the sensitivity analysis after matching patients. While the odds ratio for the occurrence of a surgical site infection when perioperative antibiotic prophylaxis was prescribed remained stable in the sensitivity analysis, the loss of power due to the exclusion of the majority of patients resulted in a trend toward significance in the sensitivity analysis without overcoming the hurdle of the significance level. Both propensity score weighting and matching aim at reducing covariate imbalance and allow the estimation of causal effects. However, both propensity score weighting and matching cannot rule out residual imbalance in covariates, which may have larger effects when groups are unequally sized. Randomization ensures covariate balance with a higher degree of confidence, especially of unobserved covariates.
Conclusions
This prospective observational analysis of 758 patients undergoing cutaneous surgery shows a reduction in the risk of surgical site infection by perioperative antibiotic prophylaxis. To decrease the number needed to treat to prevent a surgical site infection and, through this, avoid unnecessary side-effects, including the spread of multidrug resistant bacteria, targeted use of perioperative antibiotic prophylaxis should be preferred to widespread use. Future work should focus on defining accepted risk factors for surgical site infection in cutaneous surgery and establishing guidelines for the use of perioperative antibiotic prophylaxis based on risk factors and the efficacy of perioperative antibiotic prophylaxis.
ACKNOWLEDGEMENTS
The patients in this manuscript have provided written informed consent to publication of their case details. The authors would like to thank all involved physicians and nurses in the Department of Dermatology and Allergy at Ludwig-Maximilian University, Munich, Germany.
This study was founded with a grant of the Faculty of Medicine at Ludwig-Maximilian University, Munich, Germany (“Verein zur Förderung von Wissenschaft und Forschung an der Medizinischen Fakultät der Ludwig-Maximilians-Universität München e.V.”).
Approval by the ethics committee of the Faculty of Medicine at Ludwig-Maximilian University, Munich, Germany.
REFERENCES
- Harlan CA, Nguyen NB, Hirshburg JM, Hirshburg JM. Updates on recommendations for prophylactic antibiotics in dermatologic surgery. Dermatol Surg 2021; 47: 298–300.
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e1159–e1195.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075–3128.
- Sollecito TP, Abt E, Lockhart PB, Truelove E, Paumier TM, Tracy SL, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: Evidence-based clinical practice guideline for dental practitioners – a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 2015; 146: 11–16.e18.
- Watters W, 3rd, Rethman MP, Hanson NB, Abt E, Anderson PA, Carroll KC, et al. Prevention of orthopaedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg 2013; 21: 180–189.
- Johnson-Jahangir H, Agrawal N. Perioperative antibiotic use in cutaneous surgery. Dermatol Clin 2019; 37: 329–340.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075–3128.
- European Centre for Disease Prevention and Control (ECDC). Surveillance of surgical site infections and prevention indicators in European hospitals – HAI-Net SSI protocol, version 2.2. Stockholm: ECDC; 2017.
- Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int 2016; 113: 597–603.
- Samuel DP, Lindsay CP, Matthew L, Luke K. Optimal multilevel matching using network flows: an application to a summer reading intervention. Ann Appl Statist 2018; 12: 1479–1505.
- van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: an update. Infect Dis Clin North Am 2020; 34: 709–722.
- Schlager JG, Hartmann D, Ruiz San Jose V, Patzer K, French LE, Kendziora B. Procedure-related risk factors for surgical site infection in dermatologic surgery. Dermatol Surg 2022; 48: 1046–1050.
- Schlager JG, Hartmann D, Wallmichrath J, Ruiz San Jose V, Patzer K, French LE, et al. Patient-dependent risk factors for wound infection after skin surgery: a systematic review and meta-analysis. Int Wound J 2022; 19: 1748–1757.
- Schlager JG, Ruiz San Jose V, Patzer K, French LE, Kendziora B, Hartmann D. Are specific body sites prone for wound infection after skin surgery? A systematic review and meta-analysis. Dermatol Surg 2022; 48: 406–410.
- Boonchan T, Wilasrusmee C, McEvoy M, Attia J, Thakkinstian A. Network meta-analysis of antibiotic prophylaxis for prevention of surgical-site infection after groin hernia surgery. Br J Surg 2017; 104: e106–e117.
- Kasatpibal N, Nørgaard M, Sørensen HT, Schønheyder HC, Jamulitrat S, Chongsuvivatwong V. Risk of surgical site infection and efficacy of antibiotic prophylaxis: a cohort study of appendectomy patients in Thailand. BMC Infect Dis 2006; 6: 111.
- Liang B, Dai M, Zou Z. Safety and efficacy of antibiotic prophylaxis in patients undergoing elective laparoscopic cholecystectomy: a systematic review and meta-analysis. J Gastroenterol Hepatol 2016; 31: 921–928.
- Mazaki T, Mado K, Masuda H, Shiono M. Antibiotic prophylaxis for the prevention of surgical site infection after tension-free hernia repair: a Bayesian and frequentist meta-analysis. J Am Coll Surg 2013; 217: 788–801.e781–784.
- Uchino M, Ikeuchi H, Bando T, Chohno T, Sasaki H, Horio Y, et al. Efficacy of preoperative oral antibiotic prophylaxis for the prevention of surgical site infections in patients with Crohn disease: a randomized controlled trial. Ann Surg 2019; 269: 420–426.
- Li K, Sambare TD, Jiang SY, Shearer EJ, Douglass NP, Kamal RN. Effectiveness of preoperative antibiotics in preventing surgical site infection after common soft tissue procedures of the hand. Clin Orthop Relat Res 2018; 476: 664–673.
- Zhang Y, Dong J, Qiao Y, He J, Wang T, Ma S. Efficacy and safety profile of antibiotic prophylaxis usage in clean and clean-contaminated plastic and reconstructive surgery: a meta-analysis of randomized controlled trials. Ann Plast Surg 2014; 72: 121–130.
- Warnock M, Ogonda L, Yew P, McIlvenny G. Antibiotic prophylaxis protocols and surgical site infection rates in trauma surgery: a prospective regional study of 26,849 procedures. Ulster Med J 2019; 88: 111–114.
- Farmer N, Hodgetts-Morton V, Morris RK. Are prophylactic adjunctive macrolides efficacious against caesarean section surgical site infection: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2020; 244: 163–171.
- Platt R, Zucker JR, Zaleznik DF, Hopkins CC, Dellinger EP, Karchmer AW, et al. Perioperative antibiotic prophylaxis and wound infection following breast surgery. J Antimicrob Chemother 1993; 31 Suppl B: 43–48.
- Rosengren H, Heal CF, Buttner PG. Effect of a single prophylactic preoperative oral antibiotic dose on surgical site infection following complex dermatological procedures on the nose and ear: a prospective, randomised, controlled, double-blinded trial. BMJ Open 2018; 8: e020213.
- Smith SC, Heal CF, Buttner PG. Prevention of surgical site infection in lower limb skin lesion excisions with single dose oral antibiotic prophylaxis: a prospective randomised placebo-controlled double-blind trial. BMJ Open 2014; 4: e005270.
- Bari O, Eilers RE, Jr, Rubin AG, Jiang SIB. Clinical characteristics of lower extremity surgical site infections in dermatologic surgery based upon 24-month retrospective review. J Drugs Dermatol 2018; 17: 766–771.