ORIGINAL ARTICLE
Dasatinib Attenuates Fibrosis in Keloids by Decreasing Senescent Cell Burden
Claudia C. DARMAWAN1,2, Keunyoung HUR1–3, Novi KUSUMANINGRUM1,2, Jin Ho CHUNG1–3, Si-Hyung LEE1–3 and Je-Ho MUN1–3
1Department of Dermatology, Seoul National University College of Medicine, 2Institute of Human-Environment Interface Biology, Seoul National University and 3Department of Dermatology, Seoul National University Hospital, Seoul, Korea
Keloids are skin tumours caused by aberrant growth of dermal fibroblasts. Cellular senescence contributes to aging and various pathological conditions, including cancer, atherosclerosis, and fibrotic diseases. However, the effects of cellular senescence and senolytic drugs on keloids remain largely unknown. This study investigated senescent fibroblasts in keloids and assessed the effects of dasatinib on these cells. Tissues acquired from keloid removal surgery were analysed for senescence-associated β-galactosidase-positive cells, p16 expression, and the effects of dasatinib treatment on keloids. Keloid tissue was xenotransplanted into mice, and the effect of intralesional dasatinib injection on keloid growth was observed. The results showed that the numbers of β-galactosidase-positive and p16-expressing cells were higher in the keloids compared with in the controls. Dasatinib induced selective clearance of senescent cells and decreased procollagen expression in cultured keloid fibroblasts. In this xenotransplant keloid mouse model, intralesional injection of dasatinib reduced gross keloid tissue weight and the expression of both procollagen and p16. In addition, dasatinib-treated keloid fibroblasts conditioned medium reduced procollagen and p16 expression in cultured keloid fibroblasts. In conclusion, these results suggest that an increased number of senescent fibroblasts may play an important role in the pathogenesis of keloids. Therefore, dasatinib could be an alternative treatment for patients with keloids.
Key words: cellular senescence; fibrosis; keloid; senotherapeutics; therapy; therapeutics.
SIGNIFICANCE
Keloids are a fibroproliferative condition involving aberrant deposition of extracellular matrix including collagen because of dysregulated wound healing. Although surgical removal is possible, keloid recurrence is common, and other treatment modalities remain unsatisfactory. Senolysis is a therapeutic strategy to selectively eliminate senescent cells, consequently removing detrimental components of the senescence-associated secretory phenotype. This study showed that the anti-fibrotic effect of dasatinib in keloid fibroblast cultures and xenotransplant keloid tissue in mice involved the selective clearance of senescent cells and senescent-secreted secretome modulation. These results suggest a possible therapeutic application of senolytic treatment for keloids.
Citation: Acta Derm Venereol 2023; 103: adv4475. DOI https://doi.org/10.2340/actadv.v103.4475.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Dec 21, 2022; Published: Apr 6, 2023
Corr: Si-Hyung Lee and Je-Ho Mun, Department of Dermatology, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea. E-mails: always5515@gmail.com; jehomun@gmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
A keloid scar is a fibroproliferative disease following a dysregulated wound healing process that is characterized by overdeposition of extracellular matrix including collagen in the dermis. Clinically, keloids appear as firm nodules that extend beyond the borders of the original wound. In addition to cosmetic disfigurement, patients also report pruritus and pain (1). Several treatment modalities exist, including intralesional corticosteroid injections, revision surgery, cryotherapy, and laser therapy. However, the efficacy of these treatments remains unsatisfactory, and keloid recurrence is common (2).
Cellular senescence, a state in which cells undergo stress-induced cell cycle arrest, has recently been implicated in aging and age-related diseases, such as cancer, atherosclerosis, and osteoarthritis (3). Therefore, removal of senescent cells may be an effective treatment for these diseases. Senolysis is a therapeutic strategy for selectively eliminating senescent cells, consequently removing detrimental components of the senescence-associated secretory phenotype (SASP). Several drugs, including dasatinib, quercetin, navitoclax, and 17-dime-thylaminoethylamino-17-demethoxygeldanamycin, have shown selective effects on senescent cells (4). Dasatinib, a tyrosine kinase inhibitor, was originally developed to target oncogenic breakpoint cluster region-Abelson tyrosine-protein kinase 1 (BCR-ABL) and is used to treat chronic myeloid leukaemia (5, 6). During the past decade, dasatinib, in combination with quercetin, has shown senolytic activity by targeting crucial signalling pathways involved in the maintenance of senescent cells, such as tyrosine kinases and phosphoinositide 3-kinase (7–10). In patients with systemic sclerosis-associated interstitial lung disease, dasatinib showed clinical improvement, with decreased expression of SASP and other senescence-related genes, thereby suggesting that cellular senescence is an important pathological component of fibrotic diseases (7, 11). Moreover, dasatinib and quercetin alleviated physical dysfunction in idiopathic pulmonary fibrosis in a human open-label trial (12). However, the role of senescent cells in keloids remains largely unexplored.
The aim of this study was to investigate whether senescent cells are increased in keloids and to further explore the effect of dasatinib as a senolytic drug to treat keloids.
MATERIALS AND METHODS
Keloid biopsy from patients
After obtaining informed consent from 6 patients with keloids, tissues were acquired following keloid removal surgery. A dermal fibroblast cell line was isolated from tissues and cryosectioned. The biopsied specimens were divided into 2 groups: lesional and perilesional normal skin. This study was approved by the Seoul National University Institutional Review Board and conducted according to the principles of the Declaration of Helsinki.
Isolation of keloid dermal fibroblast
For the primary culture of dermal fibroblasts, human dermal fibroblasts were isolated by mechanical and enzymatic digestion, using a previously described protocol, and cultured in Dulbecco’s modified Eagle medium (DMEM, Welgene, Gyeongsan, South Korea) with 10,000 U/mL penicillin, 10,000 μg/mL streptomycin (Life Technologies, Waltham, MA, USA), and 10% foetal bovine serum (GE Healthcare, Little Chalfont, UK and Life Sciences, UT, USA). Cells were maintained in a humidified 5% CO2 atmosphere at 37°C. Only cells cultured between passages 1 and 4 were used for subsequent experiments.
Dasatinib treatment
The cells were cultured to 60% confluence, serum starved for 24 h, and treated with 1, 5, or 10 nM dasatinib or phosphate-buffered saline (PBS) for 24 h.
Senescence-associated β-galactosidase analysis
Cells were cultured to 40% confluence and senescence-associated β-galactosidase (SA-β-galactosidase) activity was measured using a senescence detection kit (Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer’s instructions. The number of positive cells was quantified after 24 h.
RNA and protein extraction
mRNA and protein were extracted from snap-frozen tissue samples comprising keloids or perilesional tissue. The crushed tissue was lysed using the TRIzol reagent according to the manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA, USA) for both RNA and protein analyses.
qRT-PCR
Total RNA (1 µg) was used in a 20 µL reaction mixture for first-strand cDNA synthesis using the First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). cDNA was amplified using a 7500 real-time PCR system (Thermo Fisher Scientific) and SYBR Premix Ex Taq (Perfect Real-time; Takara Bio, CA, USA) according to the manufacturer’s protocol, with the following primer pairs: 36B4 (forward, 5-TGGGCTCCAAGCAGATGC-3; reverse, 5-GGCTTCGCTGGCTCCCAC-3), p16 (forward, 5-CAACGCACCGAATAGTTACG-3; reverse, 5-CAGCTCCTCAGCCAGGTC-3), and procollagen (forward, 5-CTCGAGGTGGACACCACC CT-3; reverse, 5-CAGCTGGATGGCCACATCGG-3). The PCR thermocycling conditions were as follows: 50°C for 2 min and 95°C for 2 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The relative mRNA expression levels were normalized to those of 36B4, and the relative expression levels of the target genes were calculated using the 2−ΔΔCq method (13). Each experiment was repeated 3 times.
Immunoblotting
The cells were washed with ice-cold PBS and lysed with radio-immunoprecipitation assay lysis buffer (Millipore, Billerica, MA, USA) mixed with a protease inhibitor mixture (Roche, Basel, Switzerland) and phosphatase inhibitor mixture (Sigma-Aldrich, St Louis, MO, USA). The cell lysates were centrifuged at 13,500 × g at 4°C for 15 min, and the supernatants were collected. The total extracted protein concentration was quantified using a bicinchoninic acid assay reagent (Sigma-Aldrich). Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Roche). The membranes were blocked with 5% skimmed milk diluted in Tris-buffered saline containing 0.1% Tween-20, and then incubated with primary antibodies: mouse anti-β-actin (Santa Cruz Biotechnology, Dallas, TX, USA), mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Thermo Fisher Scientific, Waltham, MA, USA), mouse anti-procollagen (clone SP1.D8, Developmental Studies Hybridoma Bank, Iowa City, IA, USA), rabbit anti-p16 (Abcam, Cambridge, UK). β-actin and GAPDH were used as loading controls. The membranes were then washed and incubated with a mouse polyclonal antibody (Genetex, Irvine, CA, USA) against β-actin and procollagen and a rabbit polyclonal antibody (Genetex) against p16 and p21. Immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Thermo Fisher Scientific), and band density was measured using the ImageJ software (version 1.51; National Institutes of Health, Bethesda, MD, USA). The protein expression was normalized to that of β-actin.
Cell proliferation assay
The cells were seeded in 6-well plates, cultured to 60% confluence, starved for another 24 h in serum-free DMEM, and treated with dasatinib for 24 h. Cell viability was tested using the Ezy-Cytox Cell Viability Assay Kit (Daeil Bio, Seoul, South Korea) according to the manufacturer’s instructions. In summary, the Ezy-Cytox reagent was added to each well, incubated for 1 h, and transferred to a 96-well plate. The absorbance of the collected medium was determined spectrophotometrically using a microplate reader (Molecular Devices, San Jose, CA, USA) at 450/650 nm.
Xenotransplantation of human keloid tissue
Keloid tissues were obtained from patients during surgical excision. For xenotransplantation, donor tissues were prepared using a 5-mm punch (weight range 0.09–0.1 g) and preserved in normal saline (0.9% sodium chloride solution). Female athymic nude BALB/c mice (age 6 weeks) were anaesthetized with isoflurane, and human keloid tissues were transplanted at the recipient site, which was prepared using a 5-mm punch on each side of the back, within 2 h of keloid tissue acquisition. On the fifteenth day after transplantation, the tissue had completely healed and was intralesionally injected with vehicle (PBS) or 10 nM dasatinib for 2 weeks (3 times/week). Lesions were obtained for further analysis 7 days after the final treatment. Measurement of graft weight and western blot analysis of xenotransplant tissue was carried out according to a previously described protocol (14). The study was approved by the Institutional Care and Use Committee (IACUC) of Seoul National University, and the number of animals used was kept to a minimum to reduce suffering, according to the protocol.
Immunohistochemical and immunofluorescence staining
Tissues from both patients and xenotransplanted mice were fixed in a 4% paraformaldehyde solution overnight at 4 °C. The tissue blocks were embedded in paraffin, cut into approximately 5-µM sections using a microtome, and affixed on slides, which were de-paraffinized and rehydrated using a series of xylene and ethanol solutions. The antigens were retrieved with Tri-sodium citrate (0.1 nM sodium citrate, 0.05% Tween 20, pH 6.0) in a high-pressure cooker for 30 min. The slides were then incubated with rabbit anti-p16 antibody (ab54210; Abcam). The primary antibody was washed with PBS and incubated with a rabbit secondary antibody for 1 h. The colour was developed using a liquid AEC substrate kit (GBI Labs, Bothell, WA, USA). The slides were mounted and observed. The number of p16-positive cells from 3 fields of each sample was quantified manually by 3 researchers and is represented as the mean value±standard deviation (SD). To observe the thickness of collagen, tissue sections were stained with haematoxylin and eosin (H&E) and Masson’s trichrome.
For immunofluorescence staining, the tissue sections were incubated with rabbit anti-p16 antibody (ab5421, Abcam). The primary antibody was washed with PBS and incubated with the fluorescent-tagged anti-rabbit Alexa Fluor 488 secondary antibody (Invitrogen, Life Technologies, Carlsbad, CA, USA) for 1 h at room temperature. The nuclei were counterstained with DAPI. The sections were mounted on slides using an Immu-Mount (Thermo Fisher Scientific). Fluorescence images were acquired using a Leica (Wetzlar, Hesse, Germany) TCS SP8 microscope.
Conditioned medium and cytokine array
The cells were seeded in a 100-mm plate and treated with 10 nM dasatinib (conditioned medium (CM)) or vehicle for 48 h. The cells were reseeded in DMEM without growth factors for 96 h and incubated at 37°C. CM was used for downstream analysis of keloid fibroblasts. The remaining CM was harvested and stored at −80°C for protein cytokine array analysis using a RayBio Human Cytokine Antibody Array 5 (RayBiotech, Peachtree Corners, GA, USA). Membranes were treated and analysed according to the manufacturer’s protocol. Densitometric analyses were performed using ImageJ software. Keloid fibroblasts were treated with 10 ng/mL CXCL5, CCL5, CCL7, and CCL8 (R&D Systems, Minneapolis, MN, USA) for 24 h.
Statistical analysis
The results of at least 3 independent representative experiments with each of the data are represented as mean±standard deviation (SD) values. The Mann–Whitney U test was used to analyse differences between groups using SPSS (version 23.0; IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05.
RESULTS
Senescence-associated-β-galactosidase-positive cells and p16 expression are increased in keloids
First, this study compared the presence of senescent fibroblasts in keloid and perilesional normal tissues using SA-β-galactosidase activity analysis in primary cell cultures. Quantitative analysis of cultured fibroblasts demonstrated that the number of SA-β-galactosidase-positive cells was significantly higher in lesional keloid fibroblasts than in perilesional normal fibroblasts (p < 0.05, Fig. 1). To confirm these findings, p16 expression in the lesional and perilesional tissues was investigated. Compared with perilesional skin, keloid lesions showed significantly increased numbers of p16+ cells (Fig. 1C, D). In addition, p16 overexpression was revealed by real-time quantitative reverse transcription PCR (qRT-PCR) in keloid lesions (Fig. 1E).
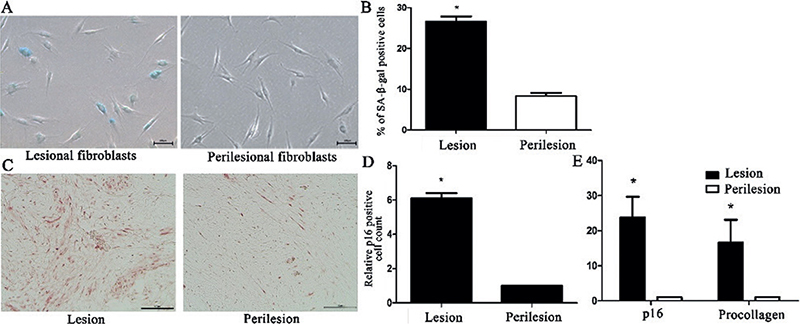
Fig. 1. Increased number of β-galactosidase+ cells and p16 expression in keloid lesion. (A) Senescence-associated-β-galactosidase (SA-β-galactosidase)-positive fibroblasts in keloid and perilesional skin (scale bars: 100 μm). (B) SA-β-galactosidase-positive fibroblast quantification in keloid and perilesional skin (p < 0.05). (C) Representative image of sections immunostained with anti-p16 antibody (scale bars: 100 μm) (D) Relative changes in the number of p16-expressing cells in lesional and perilesional tissues (p < 0.05). (E) p16 and procollagen mRNA expression in keloid tissue and perilesional skin (p < 0.05). The data are expressed as mean±SD. Representative data are shown from 6 independent experiments.
Dasatinib selectively eliminated p16-positive cells and suppressed procollagen expression
To evaluate the effect of dasatinib on cultured keloid cells, doses ranging from 1 nM to 1 µM were administered. Cell viability and senescence were evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide and SA-β-Gal assays, respectively. Although up to 10 nM dasatinib did not affect the viability of fibroblasts from perilesional skin, the number of fibroblasts from keloid lesions significantly decreased after treatment with the lowest dose of 1 nM dasatinib. The percentage of β-galactosidase-positive cells also decreased significantly in keloid fibroblasts after treatment with 1 nM dasatinib (Fig. 2A). These findings suggested that dasatinib selectively targets β-galactosidase-positive cells in keloid fibroblasts. Treatment with 1–10 nM dasatinib also significantly reduced p16 and procollagen protein expression in keloid fibroblasts in a concentration-dependent manner (Fig. 2B, C). The number of SA-β-galactosidase-positive cells exposed to dasatinib also decreased in a concentration-dependent manner (Fig. 2D).
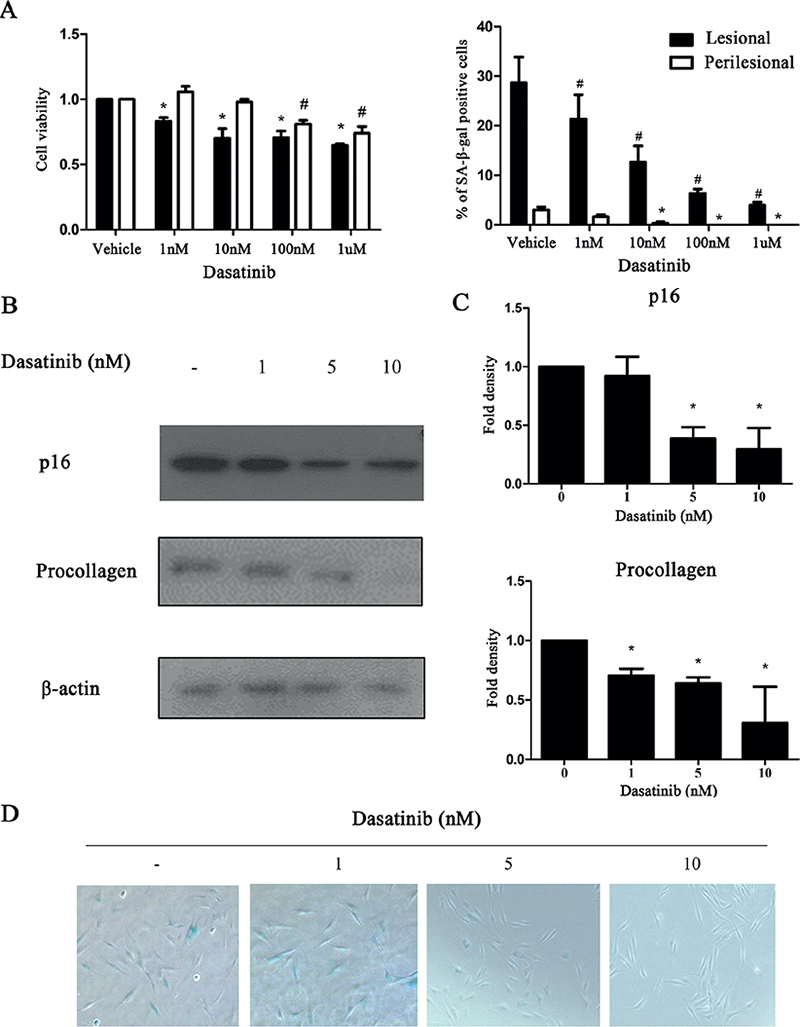
Fig. 2. Dasatinib reduced p16 and procollagen expression and the number of cells with β-galactosidase activity in keloid fibroblasts. (A) Cell viability and senescence-associated-β-galactosidase (SA-β-galactosidase)-positive cell percentage were measured in fibroblasts cultured from lesional or perilesional tissue after 1 nM–1 µM dasatinib treatment for 24 h. (B) Western blot showing decreased procollagen and p16 expression in dasatinib-treated lesional fibroblasts. (C) Quantification of western blot results showing reduced procollagen and p16 expression in a concentration-dependent manner (p < 0.05). (D) The SA-β-galactosidase staining shows a concentration-dependent reduction in SA-β-galactosidase-positive cells (1–10 nM). The data are expressed as mean±standard deviation (SD). Representative data are shown from 3 independent experiments. (*p < 0.05, lesional control vs dasatinib treatment; #p < 0.05, perilesional control vs dasatinib treatment).
Intralesional injection of dasatinib reduced xenotrans-planted keloid tissue size and suppressed procollagen and p16 expression
To validate the senolytic effect of dasatinib on keloids in vivo, human keloid tissues were implanted into the backs of athymic nude BALB/c mice. After 6 intralesional dasatinib injections over 2 weeks (three times/week), xenotransplant tissues were extirpated and evaluated (Fig. 3A). Tissue weight in the dasatinib-treated lesions was significantly lower compared with that in the vehicle-treated lesions (p < 0.05, Fig. 3B). Furthermore, both p16 and procollagen protein expression levels were lower in the dasatinib-treated lesions (p < 0.05, Fig. 3C, D). Histopathological analysis using both H&E and Masson’s trichrome staining showed reduced collagen bundle thickness in dasatinib-treated tissues (Fig. 4A). Immunofluorescence staining and quantification of p16-positive cells showed a significant reduction of p16-positive cells in dasatinib-treated lesions (p < 0.05, Fig. 4B).
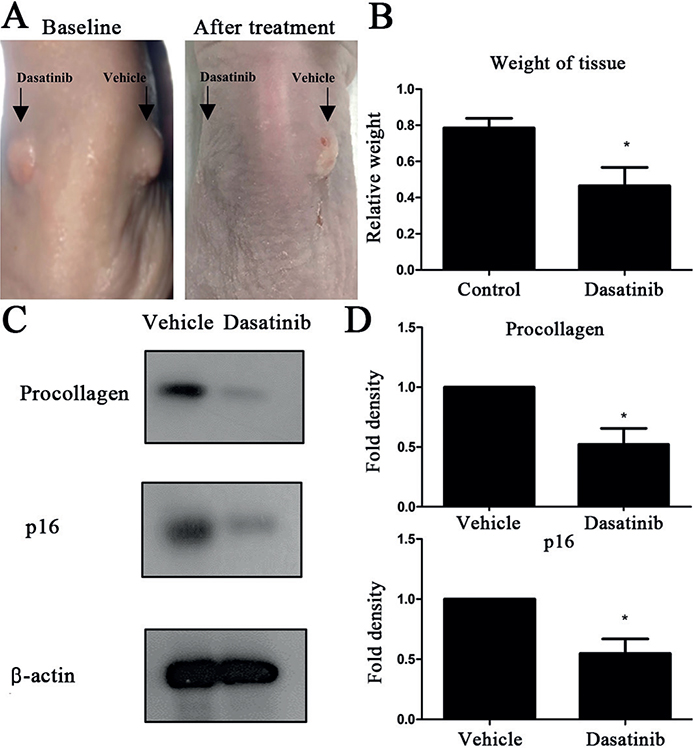
Fig. 3. Intralesional dasatinib reduced xenotransplanted lesion size and procollagen and p16 expression. (A) Macroscopic examination of the dasatinib-treated (right) and vehicle-treated (left) keloid tissues at baseline and after treatment. (B) Tissue weight after intralesional 10 nM dasatinib or phosphate-buffered saline (PBS) treatment for 2 weeks (thrice/week, p < 0.05). (C) Western blot analysis of procollagen and p16 expression from dasatinib-treated and vehicle-treated lesions (p < 0.05). (D) Quantification of western blot results (p < 0.05). The data are expressed as mean±standard deviation (SD). Representative data are shown from 3 independent experiments.
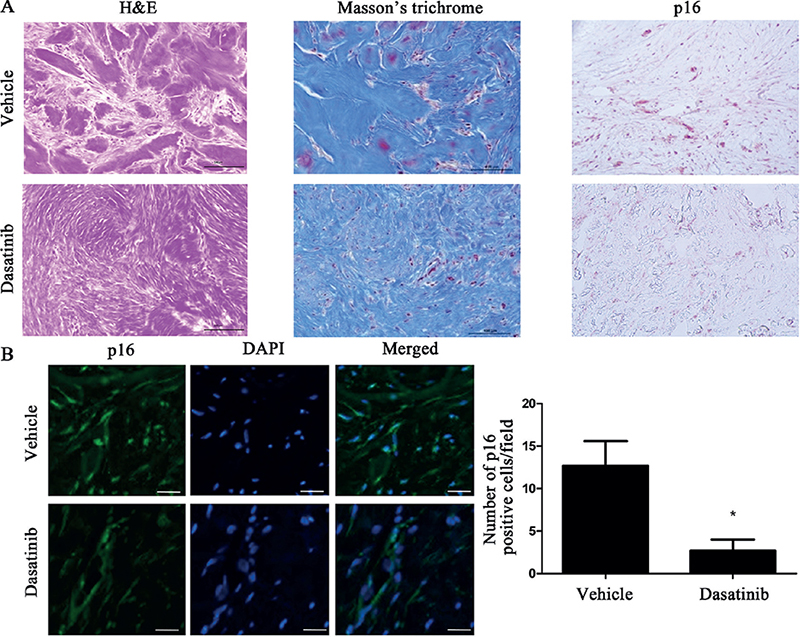
Fig. 4. Intralesional dasatinib reduced collagen bundle thickness and p16+ cells in the xenotransplanted keloid model. (A) Haematoxylin and eosin, Masson’s trichome, and p16 staining of vehicle- and dasatinib-treated lesions (scale bars: 100 µm). (B) Immunofluorescence staining of p16 and quantification of p16-positive cells on the vehicle-treated and dasatinib-treated lesion (scale bars: 100 µm; p < 0.05). Data expressed as mean±standard deviation (SD). Representative data are shown from 3 independent experiments.
Profibrotic effect of senescence-associated secretory phenotype was attenuated by dasatinib treatment
To determine whether the antifibrotic effect of dasatinib is mediated by SASP reduction in keloids, an in vitro assay was performed to test the effect of CM from keloid fibroblasts, with and without dasatinib treatment, on keloid fibroblasts. The cells cultured in dasatinib-treated CM showed significantly reduced expression of procollagen and p16 (Fig. 5A). In addition, CM was analysed using a cytokine array, with some notable differences between the vehicle- and dasatinib-treated CM groups. The 4 secretomes that were significantly reduced by dasatinib treatment were CCL5, CCL7, CCL8, and CXCL5 (p < 0.05). The effects of CXCL5, CCL8, CCL7, and CCL5 on keloid fibroblasts were investigated; CCL8, CCL7, and CCL5 induced procollagen overexpression (p < 0.05).
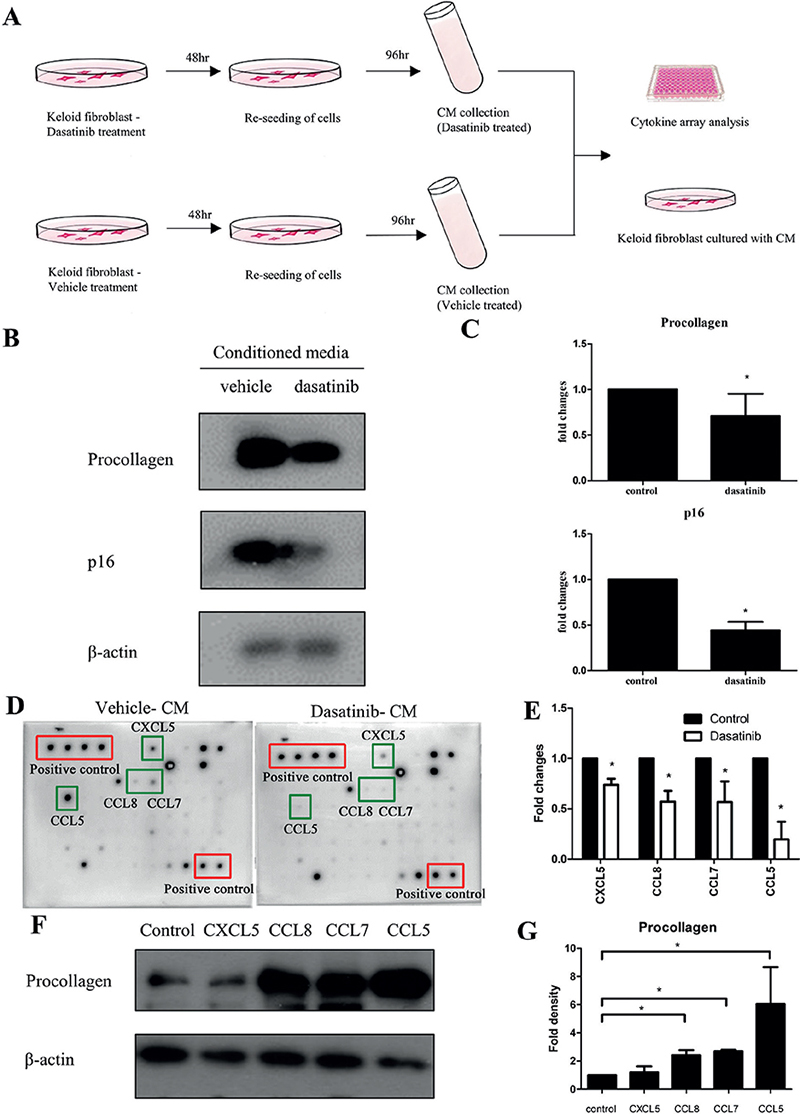
Fig. 5. Dasatinib-treated conditioned medium (CM) reduced procollagen and p16 expression in keloid fibroblasts and downregulated several cytokines (CXCL5, CCL8, CCL7, and CCL5). (A) Schematic representation of experimental methods. Keloid fibroblasts were treated with dasatinib or vehicle for 48 h. Cells were re-seeded with fresh medium and incubated for 96 h. The CM was collected, used for cytokine array analysis, and its effect on cultured keloid fibroblast was evaluated. (B) Western blot analysis of procollagen and p16 expression from keloid fibroblast cultured with dasatinib-treated and vehicle-treated CM (p < 0.05). (C) Quantification of western blot results (p < 0.05). (D) Cytokine antibody arrays with conditioned media from dasatinib- and vehicle-treated keloid fibroblasts (red: positive control; green: downregulated cytokines; top-right: CXCL5, CCL8, CCL7, CCL5). (E) Fold change in cytokine expression in CM from cultured keloid fibroblasts treated with dasatinib or vehicle. (F) Western blot analysis of procollagen expression from keloid fibroblasts cultured with 10 ng/mL CXCL5, CCL8, CCL7, and CCL5 (p < 0.05). (G) Quantification of western blot results (p < 0.05). Data expressed as mean±SD. Representative data are shown from 3 independent experiments.
DISCUSSION
Fibrosis is a major pathological condition characterized by distortion of normal tissue architecture by uncontrolled production of extracellular matrix, which eventually disrupts proper organ function. Recently, the accumulation of senescent cells has been suggested to be an important cause of internal organ fibrosis (12, 15). Therefore, senotherapeutics has emerged as a feasible approach for treating fibrotic disorders (15). Although the association between cellular senescence and abnormal skin wounds is largely unknown, a recent study showed that abnormal scars, including keloids and hypertrophic scars, showed increased senescence markers (p16) using immunochemical staining (16).
This study examined the presence of senescent cells in keloid fibroblasts and tissues and found an increased number of senescent cells in keloids. These findings are consistent with those of previous reports (16, 17). We further investigated the antifibrotic and senolytic effects of dasatinib in keloid fibroblasts. Dasatinib reduced procollagen expression and keloid fibroblast senescence in vitro. Moreover, a keloid xenotransplant model, in which human keloid tissues were implanted in athymic nude mice, showed that intralesional dasatinib injection reduced the macroscopic size and weight of the xenotransplant keloid tissue. In addition, p16 and procollagen expression levels were significantly decreased by dasatinib treatment. H&E and Masson’s trichrome staining revealed reduced collagen bundle thickness in the dasatinib-treated tissues. The expression of p16-positive cells indicated by immunofluorescence staining in keloid tissue was also significantly reduced by dasatinib treatment compared with vehicle-treated controls.
To elucidate the mechanism underlying the antifibrotic effect of dasatinib, we analysed the effect of CM from keloid fibroblasts treated with or without dasatinib on keloid fibroblasts. Keloid fibroblasts incubated with CM from dasatinib-treated keloid fibroblasts showed significantly reduced procollagen and p16 expression compared with cultured keloid fibroblasts incubated with CM from vehicle-treated keloid fibroblasts (p < 0.05). The expression of cytokines, such as CCL-5, CCL-7, CCL8, and CXCL-5, was downregulated in the dasatinib-treated CM compared with that in the vehicle-treated CM (p < 0.05). These cytokines are involved in fibrosis in several organs, such as the liver, kidneys, and lungs (18–22). CCL-5 contributes to early-stage liver fibrosis in non-alcoholic fatty liver disease; and, it has been considered as a potential therapeutic target for renal interstitial fibrosis (19, 22). CCL-7, previously known as monocyte chemoattractant protein 3, is overexpressed in systemic sclerosis and is associated with the extent of skin sclerosis and severity of pulmonary fibrosis (20, 21). CCL8 has been proposed as a candidate molecule for differential diagnosis and prediction of survival in idiopathic pulmonary fibrosis (18). Consistently, these cytokines also showed a pro-fibrogenic effect on cultured keloid fibroblasts in this study.
In addition to keloids, recent data demonstrated that senescent cell accumulation plays a direct role in fibrosis and contributes to fibrotic diseases in the liver, kidneys, and lungs (12, 15, 23). Therefore, senotherapeutics may be a viable strategy for treating multiple fibrotic conditions (15). Recent clinical evidence has shown a benefit of senolytic therapy for fibrotic diseases, such as pulmonary fibrosis and systemic sclerosis-related interstitial lung disease (10, 11).
In conclusion, the results of this study demonstrate that dasatinib has antifibrotic effects in keloid fibroblasts and xenotransplant keloid tissue in mice through the selective clearance of senescent cells and modulation of the senescence-secreted secretome. These findings suggest a possible therapeutic application of senolytic treatments for keloids.
ACKNOWLEDGEMENTS
The authors thank the patients who participated in this study.
This work was supported by a National Research Foundation of Korea (NRF) grant from the Korean Government (MSIT) (number 2021R1C1C1014154).
Data will be made available by the corresponding authors upon reasonable request.
This study was approved by the Seoul National University Institutional Review Board and was conducted according to the principles of the Declaration of Helsinki. This study was approved by The Institutional Animal Care and Use Committee (IACUC) of Seoul National University, and the number of animals used was kept to a minimum to reduce their suffering, according to the protocol.
REFERENCES
- Lee SS, Yosipovitch G, Chan YH, Goh CL. Pruritus, pain, and small nerve fiber function in keloids: a controlled study. J Am Acad Dermatol 2004; 51: 1002–1006.
- Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci 2018; 19: 711.
- Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 2017; 16: 718–735.
- Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc 2017; 65: 2297–2301.
- Shah NP, Kantarjian HM, Kim DW, Réa D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol 2008; 26: 3204–3212.
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270.
- Martyanov V, Kim GJ, Hayes W, Du S, Ganguly BJ, Sy O, et al. Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis-associated interstitial lung disease. PLoS One 2017; 12: e0187580.
- Montero JC, Seoane S, Ocaña A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res 2011; 17: 5546–5552.
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’s hill of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015; 14: 644–658.
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016; 15; 973–977.
- Martyanov V, Whitfield ML, Varga J. Senescence signature in skin biopsies from systemic sclerosis patients treated with senolytic therapy: potential predictor of clinical response? Arthritis Rheumatol 2019; 71: 1766–1767.
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 2019; 40: 554–563.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3: 1101–1108.
- Darmawan CC, Montenegro SE, Jo G, Kusumaningrum N, Lee SH, Chung JH, et al. Adiponectin-based peptide (ADP355) inhibits transforming growth factor-β1-induced fibrosis in keloids. Int J Mol Sci 2020; 21: 2833.
- Schafer MJ, Haak AJ, Tschumperlin DJ, LeBrasseur NK. Targeting senescent cells in fibrosis: pathology, paradox, and practical considerations. Curr Rheumatol Rep 2018; 20: 3.
- Limandjaja GC, Belien JM, Scheper RJ, Niessen FB, Gibbs S. Hypertrophic and keloid scars fail to progress from the CD34-/α-smooth muscle actin (α-SMA)+ immature scar phenotype and show gradient differences in α-SMA and p16 expression. Br J Dermatol 2020; 182: 974–986.
- Varmeh S, Egia A, McGrouther D, Tahan SR, Bayat A, Pandolfi PP. Cellular senescence as a possible mechanism for halting progression of keloid lesions. Genes Cancer 2011; 2: 1061–1066.
- Lee JU, Cheong HS, Shim EY, Bae DJ, Chang HS, Uh ST, et al. Gene profile of fibroblasts identify relation of CCL8 with idiopathic pulmonary fibrosis. Respir Res 2017; 18: 3.
- Li BH, He FP, Yang X, Chen YW, Fan JG. Steatosis induced CCL5 contributes to early-stage liver fibrosis in nonalcoholic fatty liver disease progress. Transl Res 2017; 180: 103–117.e4.
- Ong VH, Evans LA, Shiwen X, Fisher IB, Rajkumar V, Abraham DJ, et al. Monocyte chemoattractant protein 3 as a mediator of fibrosis: overexpression in systemic sclerosis and the type 1 tight-skin mouse. Arthritis Rheum 2003; 48: 1979–1991.
- Yanaba K, Komura K, Kodera M, Matsushita T, Hasegawa M, Takehara K, et al. Serum levels of monocyte chemotactic protein-3/CCL7 are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Ann Rheum Dis 2006; 65: 124–126.
- Zhang C, Hu X, Qi F, Luo J, Li X. Identification of CD2, CCL5 and CCR5 as potential therapeutic target genes for renal interstitial fibrosis. Ann Transl Med 2019; 7: 454.
- Xiao M, Chen W, Wang C, Wu Y, Zhu S, Zeng C, et al. Senescence and cell death in chronic liver injury: roles and mechanisms underlying hepatocarcinogenesis. Oncotarget 2017; 9: 8772–8784.