QUIZ SECTION
Progressive Necrotic Black Eschar and Reticular Purpura: A Quiz
Zhen-Ting LIN, Hong-Hao HU, Hao GUO* and Jiu-Hong LI*
Department of Dermatology, The First Hospital of China Medical University, 155N. Nanjing Street, Shenyang 110001, PR China. E-mails: guohao27@126.com; pfkl2011@126.com
Citation: Acta Derm Venereol 2023; 103: adv4540. DOI https://doi.org/10.2340/actadv.v103.4540.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Published: May 10, 2023
A 55-year-old woman presented to our department with progressive necrotic black eschar on the lower extremities that had persisted for 2 months. The lesions initially appeared as painful, but invisible, skin changes and subsequently progressed to reticular purpura throughout the lower extremities. Subsequently, the abdomen was also covered with scattered lesions. She had been diagnosed with vasculitis and treated with intravenous glucocorticoids (40 mg/day) for 2 weeks, without improvement. The lesions worsened gradually, presenting as multiple ulcers covered with massive black eschars (Fig. 1).
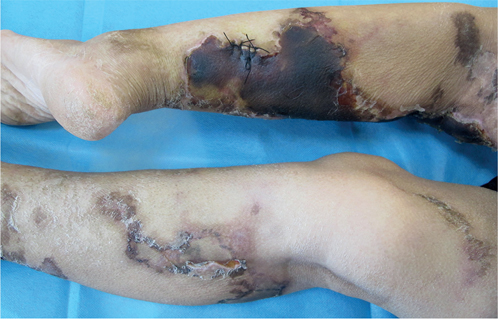
Fig. 1. Skin lesions. Necrotic black eschar overlying ulcers on the lower extremities, surrounded by diffuse reticular purpura. Note sutured site of biopsy on left leg.
The patient had nephritis for 10 years with elevated blood creatinine levels (291 µmol/L; normal 44–133 µmol/L). Two months previously, she had undergone a parathyroidectomy for the management of secondary hyperparathyroidism with elevated blood calcium (5.40 mmol/L; normal 2.10–2.55 mmol/L) and parathyroid hormone (1,292 pg/mL; normal 15–65 pg/mL) levels. Pedal pulses were palpated on both sides; however, the physical examinations and family history were unremarkable. Laboratory tests revealed a C-reactive protein (CRP) level of 203.4 mg/L; normal 0–10 mg/L). Relevant laboratory tests, such as cancer biomarkers, lupus anticoagulant, anticardiolipin antibodies and antineutrophil cytoplasmic antibodies were identified with no abnormalities. A biopsy specimen was obtained from the edge of the black eschar on the left lower limb (Fig. 1).
What is your diagnosis? See next page for answer.
ANSWERS TO QUIZ
Progressive Necrotic Black Eschar and Reticular Purpura: A Commentary
Diagnosis: Calciphylaxis
Histopathological examination revealed epidermal necrolysis and thrombosis in the small vessels of the dermis (Fig. 2A), with calcium deposition in the subcutaneous adipose tissue (Fig. 2B). Furthermore, Von-Kossa staining was positive and highlighted as dark granules in small vessels (Fig. 2C). Computed tomography (CT) of the chest, abdomen, and pelvis revealed multiple calcium depositions in the aortic wall and abdominal vessels.
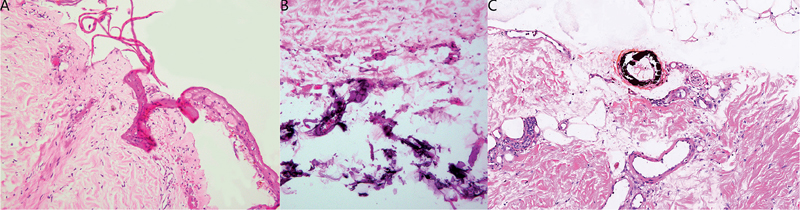
Fig. 2. Histopathological examination findings. (A) A biopsy specimen taken from the edge of the black eschar showing epidermal necrolysis and thrombosis in small vessels of the dermis (haematoxylin-eosin, original magnification ×200). (B) Another micro field of the same specimen showing calcium deposition in the subcutaneous adipose tissue (haematoxylin-eosin, original magnification ×200). (C) Von-Kossa stains showing calcium deposition in the small vessels (original magnification ×100).
Based on the clinical manifestations, laboratory tests, imaging and histological examination, the diagnosis of calciphylaxis was confirmed, which was consistent with the Mayo Clinic diagnostic criteria (1). After 2 weeks of treatment with intravenous sodium thiosulphate, the patient had no new necrotic skin lesions, and was transferred to the nephrology department of the local hospital for further treatment.
Calciphylaxis is a rare disorder characterized by micro-vascular calcium deposition and subsequent thrombosis, resulting in intensely painful ischaemic lesions. These lesions most commonly develop in fat-rich areas, such as the extremities, abdomen, and buttocks. Patients may not initially display any visible skin changes but have intense pain. Subsequent varied visible lesions include firm subcutaneous nodules and reticular purpura, which may progress to ulcers covered by black eschar (2). Calciphylaxis predominantly affects patients with end-stage renal disease. Both uraemic and non-uraemic patients present with skin lesions. The 1-year mortality of this disease is estimated to range from 45% to approximately 80%, with lower mortality rates in non-nephrogenic patients. Sepsis caused by infection of wounds is the most common cause of death (1).
The exact aetiology of calciphylaxis remains unknown. However, calcium deposition in the small vessels may be the first event, subsequently followed by thrombosis and skin ischaemia. Vascular calcifications are always circumferential and located in the intima of otherwise normal-appearing vessels, which suggests a rapid development (3). Associated risk factors include longer dialysis vintage, hyperphosphataemia, female sex, obesity (body mass index (BMI) > 30 kg/m2), hypercoagulable states, and medications, including warfarin and systemic glucocorticoids (2).
Clinical suspicion is important for early diagnosis. Calciphylaxis should be considered in patients with both risk factors and typical clinical manifestations. If not, we should rely on the results of histopathological examination and computed tomography. Non-invasive imaging tools have been reported to aid in the diagnosis of calciphylaxis, which may avoid possible infection risks from biopsy (4). Typical histological features include a combination of small vessel calcification and thrombosis. If the histological findings are not apparent on haematoxylin and eosin staining, Von-Kossa staining shows calcium deposits as dark granules in the small vessels (5).
Given the diversity of calciphylaxis lesions, other diseases must be excluded in order to make a definitive diagnosis as quickly as possible. Cellulitis has been reported as one of the most common misdiagnosed diseases in cases of calciphylaxis. The lesions present with painful erythema, oedema, and warmth. Clinical manifestations and positive blood culture results can help distinguish between these 2 entities (6). Although painful ulcers can also present in pyoderma gangrenosum (PG) and peripheral artery disease (PAD), PG cases are usually associated with systemic diseases, such as inflammatory bowel diseases and rheumatoid arthritis (7). Furthermore, pedal pulses are difficult to palpate in patients with PAD (8). Histological findings are thickened vessel walls with media hypertrophy, suggesting a slower process than calciphylaxis.
Currently, there is no standard treatment for calciphylaxis. Topical therapies include debridement, irrigation with normal saline, and topical growth factors (9, 10). Hyperbaric oxygen support therapy can improve the prognosis. Systemic therapies include treatment of the primary disease and intravenous sodium thiosulphate to neutralize calcium deposition (2).
ACKNOWLEDGEMENTS
The authors thank the patient for granting permission to publish this information.
This work is supported by grants from the National Natural Science Foundation of China (82273547 HG) for data collection and patient management.
REFERENCES
- McCarthy JT, El-Azhary RA, Patzelt MT, Weaver AL, Albright RC, Bridges AD, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc 2016; 91: 1384–1394.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med 2018; 378: 1704–1714.
- Colboc H, Moguelet P, Bazin D, Carvalho P, Dillies AS, Chaby G, et al. Localization, morphologic features, and chemical composition of calciphylaxis-related skin deposits in patients with calcific uremic arteriolopathy. JAMA Dermatol 2019; 155: 789–796.
- Shmidt E, Murthy NS, Knudsen JM, Weenig RH, Jacobs MA, Starnes AM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol 2012; 67: 1296–1301.
- Ellis CL, O’Neill WC. Questionable specificity of histologic findings in calcific uremic arteriolopathy. Kidney Int 2018; 94: 390–395.
- Gabel CK, Blum AE, François J, Chakrala T, Dobry AS, Garza-Mayers AC, et al. Clinical mimickers of calciphylaxis: a retrospective study. J Am Acad Dermatol 2021; 85: 1520–1527.
- Peterson DM, Damsky WE, Vesely MD. Fever, Hypotension, and a worsening necrotic wound. JAMA 2022; 327: 1496–1497.
- Layden J, Michaels J, Bermingham S, Higgins B. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ 2012; 345: e4947.
- Han CM, Cheng B, Wu P. Clinical guideline on topical growth factors for skin wounds. Burns Trauma 2020; 8: tkaa035.
- Twu O, Mednik S, Scumpia P, Doaty S, Worswick S. Use of Becaplermin for nondiabetic ulcers: pyoderma gangrenosum and calciphylaxis. Dermatol Ther 2016; 29: 104–108.