SHORT COMMUNICATION
Aggressive Squamous Cell Carcinoma in a Case of Epidermodysplasia Verruciformis Carrying a TMC6 Splice-site Mutation
Kazunori YOKOI1, Noriko ARASE1, Takashi SHIMBO2, Manabu FUJIMOTO1 and Atsushi TANEMURA1
1Department of Dermatology, Graduate School of Medicine, Osaka University, 2-2 Yamadaoka, Suita-shi, Osaka 565-0871 and 2Department of Stem Cell Therapy Science, Graduate School of Medicine, Osaka University, Suita, Japan.
Citation: Acta Derm Venereol 2023; 103: adv00858. DOI https://doi.org/10.2340/actadv.v103.4550.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Dec 6, 2023; Published: Jan 27, 2023
E-mail: kyokoi@derma.med.osaka-u.ac.jp
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Epidermodysplasia verruciformis (EV; MIM #226400) is a rare inherited skin disease, first described by Lewandowsky & Lutz in 1922 (1). The disease is caused by abnormal susceptibility of the skin to β-human papillomavirus (HPV) (2). In particular, human papilloma virus (HPV)-5 and HPV-8 were classified as “possibly carcinogenic” in patients with epidermodysplasia verruciformis (EV)” by the WHO (3). Approximately half of patients with EV develop non-melanoma skin cancers (NMSC), mainly on sun-exposed areas by around their 40s (4). EV is classified into a genetic type and an acquired type as a result of immunosuppression caused by HIV or solid-organ transplantation (5). Genetic EV is classified into classical EV associated with TMC6 or TMC8 gene mutations and non-classical EV associated with non-TMC gene mutations, such as RHOH, MST-1 and CORO1A (5). Classical EV and most non-classical EV are inherited in an autosomal recessive manner. On the other hand, cases of X-chromosome recessive or autosomal dominant inheritance have been reported with unspecified loci (5). Approximately half of all patients with EV have been classified as classical EV, with mutations in TMC6 or TMC8 (5).
We describe here a case of EV with an aggressive squamous cell carcinoma. The c.892-2A > T mutation of the TMC6 gene was detected in the patient. We report, for the first time, that the c.892-2A > T mutation is estimated to induce an amino acid mutation of p.Gly298*.
CASE REPORT
The patient was a 50-year-old man, who presented with small pale-red spots, which had been present on his limbs and trunk in childhood. Keratotic lesions of various sizes, including seborrhoeic keratosis, actinic keratosis and verruca vulgaris, were found on his neck, trunk, and limbs. A large erythema with keratosis and partial ulceration was found on the left side of his chest (Fig. 1a). The lesion on his chest was diagnosed as squamous cell carcinoma (SCC) from a biopsy (Fig. 1b). Extended resection and left lymph node dissection were performed for the SCC. He received postoperative adjuvant therapy with peplomycin sulphate. Six months later, a large metastatic right lymph node was found on positron emission tomography–computed tomography (PET-CT) (Fig. 1c). Right lymph node dissection was performed (Fig. 1d). Since then, Bowen’s disease, 3 SCCs and a sebaceous carcinoma were found within 15 years, none of which showed evidence of metastasis. Histopathological examination of the SCC on his back revealed koilocytosis, which suggested viral infection (Fig. 1e–h). His older sister, who was deceased, had experienced similar skin symptoms. His parents, who were also deceased, and his 2 surviving daughters showed no symptoms. His maternal grandfather and paternal grandmother were related to each other, as shown in the family tree (Fig. 1i). The patient gave his informed consent to provide a blood sample and tissue biopsy specimens of the skin and lymph node. This research was approved by Osaka University ethics committee (number 683).
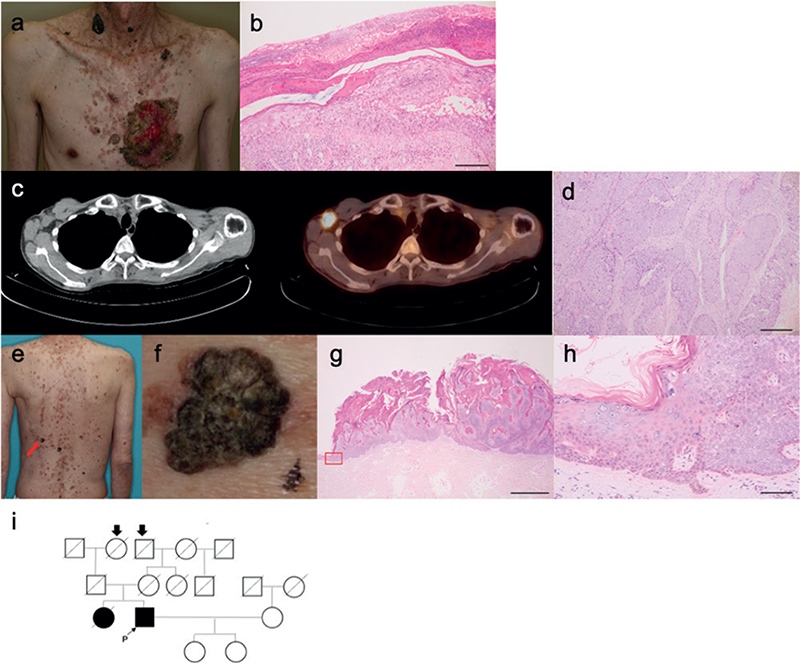
Fig. 1. Clinical features of the patient with epidermodysplasia verruciformis (EV) in this report. (a) Clinical symptoms of the case. A large squamous cell carcinoma (SCC) was present on the patient’s chest. Various sized keratotic lesions, including seborrhoeic keratosis, actinic keratosis, and verruca vulgaris, were observed on his neck and trunk. Bar = 100 μm. (b) Biopsy specimen from the SCC. Atypical keratinocytes proliferated in the epidermis. (c) Positron emission tomography – computed tomography (PET-CT) images. A metastatic right axillary lymph node was found. (d) Biopsy specimen from the lymph node. Lymphoid follicles were destroyed and replaced by atypical tumour cells. Bar = 100 μm. (e) Clinical photograph of the SCC on his back (red arrow). (f) Enlarged image of the SCC. (g) Biopsy specimen of SCC on the patient’s back. Bar = 2 mm. (h) Magnified image of the base of the tumour (framed by red lines) shows koilocytosis, suggesting viral infection of the keratinocytes. Bar = 100 μm. (i) Family tree of the current case. His maternal grandfathers and paternal grandmothers (arrows) were related.
Genomic DNA and total RNA were extracted from the blood of the patient and a healthy control. Sequencing analyses of TMC6 were performed. Details were shown in Appendix S1.
Genotyping of β-HPV was performed with the previously reported method using the single tube nested “hanging droplet” PCR (6).
RESULTS
Genomic DNA sequencing found a c.892-2A > T mutation on the intron 8 of TMC6 (Fig. S1a). Sequence analysis of cDNA reverse transcribed from the mRNA found that c.892_932 of TMC6 (the first 41 base pairs of exon 9) was deleted. This splicing abnormality was estimated to induce the 298th amino acid to become a stop codon (p.Gly298*), suggesting that the amino acids after exon 9 were not translated (Fig. S1b). This splice site mutation may be caused by the sequence of c.930_931AG, which was estimated to function as a novel end of intron 8 instead of c.892-2_892-1AG (Fig. S1c). HPV-5 was detected in the SCC on the patient’s back (Fig. S2).
DISCUSSION
To date, various types of TMC6 mutations have been reported in classical EV (7–15) (Table I). In the current study, a c.892-2A > T mutation was detected. This is not a novel mutation of TMC6 in EV; 2 cases with this mutation have been reported from Japan. One showed compound heterozygous mutation of c.744C > T and c.892-2A > T (9) in TMC6. The other showed homozygous mutation of c.892-2A > T (10). Although c.892-2A > T was estimated to be a splice site mutation, and c.892-2A > T (VCV000662928.2) has already been reported as probably pathogenic in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) (accessed 3 June 2022), its actual effect is unknown. We report here, for the first time, that c.892-2A > T induced an amino acid mutation of p.Gly298*. Nonsense mutations, such as p.Gln393* (VCV001437451.1) and p.Ser678* (VCV001456110.1), which are located downstream of the 298th amino acid, have already been reported as pathogenic mutations in ClinVar. Therefore, c.892-2A > T, which was found in the current patient, was considered to be a pathogenic mutation. This splice site mutation was caused by the sequence of c.930_931AG, which was estimated to function as a novel splice site instead of c.892-2_892-1AG. In comparison with the previously reported patients with EV with c.892-2A > T mutation, the current case presented very severe clinical symptoms with rapidly progressing SCC with lymph node metastasis. The case reported by Tate et al. (9) was a 65-year-old woman who developed Bowen’s disease on her hands at 51, 52 and 64 years of age. An SCC and Bowen’s disease were found on her face at 65 years of age. In this case, neither HPV-5 nor HPV-8 was detected. The case reported by Sunohara et al. (10) was a 53-year-old man who developed Bowen’s disease on his face several times. HPV-3, HPV-14 and HPV-38 were detected in this case. Neither case was associated with advanced cutaneous malignancies that metastasized to other sites. HPV-5, which has been classified as “possibly carcinogenic” in patients with EV, might contribute to the aggravation of SCC in the current case.
| TMC6 mutation | Type | Proteins | Origin | Reference |
| c.[220C >T];[220C >T] | Nonsense | p.[(Gln74*)];[(Gln74*)] | Japan | (7) |
| c.[280C >T];[280C >T] | Nonsense | p.[(Arg94*));[(Arg94*)] | Algeria | (8) |
| c.[280C >T];[280C >T] | Nonsense | p.[(Arg94*)];[(Arg94*)] | Algeria | (8) |
| c.[744C >A];[892-2A >T] | Nonsense & splice site | p.[(Tyr248*)];[?] | Japan | (9) |
| c.[892-2A >T];[892-2A >T] | Splice site | p.[?];[?] | Japan | (10) |
| c.[892-2A >T];[892-2A >T] | Splice site | p.[(Gly298*)];[(Gly298*)] | Japan | Present study |
| c.[916_917insCATGT];[916_917insCATGT] | Frameshift | p.[(Tyr306fs)];[(Tyr306fs)] | China | (11) |
| c.[968delT];[968delT] | Frameshift | p.[(Leu323fs)];[(Leu323fs)] | Pakistan | (12) |
| c.[1110C >G];[1110C >G] | Nonsense | p.[(Tyr370*)];[(Tyr370*)] | France | (13) |
| c.[1110C >G];[1110C >G] | Nonsense | p.[(Tyr370*)];[(Tyr370*)] | Mexico | (14) |
| c.[1726G >T];[1726G >T] | Nonsense | p.[(Glu576*)];[(Glu576*)] | Colombia | (8) |
| c.[2278-2A >G];[2278-2A >G] | Frameshift & nonsense | p.[(Glu760Glyfs*17)];[(Glu760Glyfs*17)] | China | (15) |
| Mutations of c.892-2A > T have only been reported from Japan. It was found, for the first time, that c.892-2A > T mutation caused amino acid change of p.Gly298*. | ||||
ACKNOWLEDGEMENTS
The authors thank Dr Megumi Fujimoto (Department of Dermatology, Osaka University Hospital) for clinical support and Mr Hiroaki Tojo (Department of Dermatology, Graduate School of Medicine, Osaka University) for sequence analysis.
REFERENCES
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol 1922; 14: 193–203.
- Cardoso JC, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat 2011; 20: 145–154.
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi El F, et al. A review of human carcinogens – part B: biological agents. Lancet Oncol 2009; 10: 321–322.
- Przybyszewska J, Zlotogorski A, Ramot Y. Re-evaluation of epidermodysplasia verruciformis: reconciling more than 90 years of debate. J Am Acad Dermatol 2017; 76: 1161–1175.
- Huang S, Wu JH, Lewis DJ, Rady PL, Tyring SK. A novel approach to the classification of epidermodysplasia verruciformis. Int J Dermatol 2018; 57: 1344–1350.
- Forslund O, Ly H, Higgins G. Improved detection of cutaneous human papillomavirus DNA by single tube nested ‘hanging droplet’ PCR. J Virol Methods 2003; 110: 129–136.
- Aochi S, Nakanishi G, Suzuki N, Setsu N, Suzuki D, Aya K, et al. A novel homozygous mutation of the EVER1/TMC6 gene in a Japanese patient with epidermodysplasia verruciformis. Br J Dermatol 2007; 157: 1265–1266.
- Ramoz N, Rueda LA, Bouadjar B, Montoya LS, Orth G, Favre M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet 2002; 32: 579–581.
- Tate G, Suzuki T, Kishimoto K, Mitsuya T. Novel mutations of EVER1/TMC6 gene in a Japanese patient with epidermodysplasia verruciformis. J Hum Genet 2004; 49: 223–225.
- Sunohara M, Ozawa T, Morimoto K, Harada T, Ishii M, Fukai K. Dye laser photodynamic therapy for Bowen’s disease in a patient with epidermodysplasia verruciformis. Osaka City Med J 2012; 58: 77–82.
- Zuo YG, Ma D, Zhang Y, Qiao J, Wang B. Identification of a novel mutation and a genetic polymorphism of EVER1 gene in two families with epidermodysplasia verruciformis. J Dermatol Sci 2006; 44: 153–159.
- Gober MD, Rady PL, He Q, Tucker SB, Tyring SK, Gaspari AA. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol 2007; 127: 817–820.
- Youssefian L, Vahidnezhad H, Mahmoudi H, Saeidian AH, Daneshpazhooh M, Kamyab HK, et al. Epidermodysplasia verruciformis: genetic heterogeneity and EVER1 and EVER2 mutations revealed by genome-wide analysis. J Invest Dermatol 2019; 139: 241–244.
- López-Ramírez S, Santillán-Hernández Y, Carrasco-Gerard E, Rodas-Serrano A, Zenteno JC. Next-generation sequencing identifies a homozygous nonsense p.Tyr370* mutation of the TMC6 gene in a Mexican pedigree with epidermodysplasia verruciformis. Rev Invest Clin 2021; 73: 129–131.
- Wang R, Liu J, Yang X, Habulieti X, Yu X, Sun L, et al. Identification and splicing characterization of novel TMC6 and TMC8 variants associated with epidermodysplasia verruciformis in three Chinese families. Front Genet 2021; 12: 712275.