ORIGINAL REPORT
Economic Burden of Atopic Dermatitis in Taiwan
Ellen M. LEE1, Yung-Tsu CHO2, Tom C. CHAN2, Dereck SHEN3, Chia-Yu CHU2 and Chao-Hsiun TANG3
1Chang gung memorial hospital, Taoyuan, 2Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei and 3School of Health Care Administration, College of Management, Taipei Medical University, Taipei, Taiwan
Atopic dermatitis is a prevalent inflammatory skin disease that manifests clinically as pruritus and eczema. Severe forms of atopic dermatitis can be chronic and relapsing or associated with other dermatological complications and comorbidities, resulting in lifelong impacts across multiple aspects for patients. This study was conducted to calculate the atopic dermatitis-related economic burden in Taiwan. First, the out-of-pocket costs incurred by 200 patients with atopic dermatitis were estimated using a specifically designed questionnaire. Secondly, work impairment was converted into quantifiable costs. The costs reimbursed by the Taiwan National Health Insurance (NHI), which were estimated in our previous work, were included in the final calculation. The atopic dermatitis-related economic burden for patients in Taiwan in 2018 was estimated as (2018 New Taiwan dollars; NT$) 37.90 billion, which is 0.207% of Taiwan’s gross domestic product. This substantial economic burden suggests an existing need for more effective and equitable treatment for atopic dermatitis.
Key words: atopic dermatitis; cost of illness; burden of disease.
SIGNIFICANCE
Atopic dermatitis is common skin disease that can result in discomfort and inconvenience to patients and their family members from a range of different aspects, including physically, mentally, and financially. This study aims to calculate the total atopic dermatitis-related economic burden by adding up the estimated out-of-pocket costs, costs of work productivity loss from a meticulously designed questionnaire from 200 patients in Taiwan, and costs reimburs-ed by the Taiwan National Health Insurance estimated in our previous work. The results show that the out-of-pocket costs and costs of work productivity are major contributors to the total burdens and should not be overlooked.
Citation: Acta Derm Venereol 2023; 103: adv00866. DOI: https://doi.org/10.2340/actadv.v103.4556.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Dec 7, 2022; Published: Feb 14, 2023
Corr: Chao-Hsiun Tang, School of Health Care Administration, College of Management, Taipei Medical University, 172-1 Keelung Road, Section 2, Taipei City 106, Taiwan, and Chia-Yu Chu, Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine; 15F, No.7, Chung-Shan South Road, Taipei, Taiwan. E-mails: chtang@tmu.edu.tw; chiayu@ntu.edu.tw
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
A topic dermatitis (AD) is a prevalent inflammatory skin disease with reported prevalence rates ranging from 15% to 25% in children and from 1% to 10% in adults (1). Classical AD manifestations are pruritic eczematous skin lesions, often accompanied by other clinical findings (2). In severe cases, patients may experience chronic and relapsing eczema, intense pruritus, dermatological complications, and additional comorbidities, such as autoimmune disorders, atopic disorders, ocular disorders, chronic urticaria, ischaemic heart disorders, and cerebrovascular disorders (3, 4). Consequently, AD has adverse impacts on multiple aspects, including quality of life, social aspects, academic aspects, work productivity, and financial impacts (5–7). Therefore, due to the increasing prevalence rate and lifelong impacts associated with AD, the societal costs of AD have become an important issue (8, 9).
To fully assess the economic burdens associated with AD, considerations should include direct costs paid by the government and individuals and the costs of lost work productivity due to physical and mental anguish. Although the economic burden associated with AD has been examined in both Western and Eastern countries, only a few studies have included all related costs (10–12). Most studies calculating AD-related costs consider only some economic aspects. Several studies have emphasized the costs of reimbursements from either public or private health insurance programmes (13–16). A few studies have focused on out-of-pocket (OOP) payments paid by patients (17–19), and some studies have focused on the costs associated with lost work productivity (5, 20). Comprehensive estimates of the AD-related economic burden are relatively rare, especially for Asian countries, requiring further investigation.
Two separate studies have examined the healthcare costs associated with AD-related comorbidities in Taiwan, both of which utilized the National Health Insurance Research Database (NHIRD) (4, 16). In addition, Chan et al. (7) investigated AD-related work productivity and activity impartment using a questionnaire. However, estimates of AD-related OOP costs and the costs associated with work productivity impairment due to absenteeism or presenteeism have not yet been examined.
The aim of this study was to estimate the OOP costs paid by patients with AD and their family members based on a specifically designed questionnaire (Appendix S1). To obtain a more comprehensive view of the overall economic burden associated with AD, we also quantified the costs of work productivity impairment. In addition to individual expenses and loss, AD is associated with societal costs in the form of National Health Insurance (NHI) reimbursements, which were estimated in our previous work (16). These costs should also be taken into account when considering the total AD-related economic burden. The resulting comprehensive estimation of AD-related economic burden should both inform patients with AD of the potential economic burden associated with the disease and encourage the optimization of AD treatments.
MATERIALS AND METHODS
Study design and patient recruitment
From October 2018 to April 2019, a total of 200 patients aged 20 years or older who were diagnosed with AD by a board-certified dermatologist were interviewed in person by well-trained interviewers during regular follow-up visits to the outpatient clinics at 3 participating hospitals located in northern, central, and southern Taiwan. A stratified sampling design was adopted to ensure sufficient recruitment of participants in each of the 3 disease severity groups. Disease severity was assessed by dermatologists using Scoring Atopic Dermatitis (SCORAD). Among patients who completed the interview, the final numbers of patients with mild, moderate, and severe AD were 70, 72, and 58, respectively. A more detailed description has been reported elsewhere (7).
Estimation of out-of-pocket costs incurred by atopic dermatitis
Detailed information regarding AD-associated OOP costs paid by the patient or their family but not reimbursed by the NHI was collected using questionnaires. OOP costs were then categorized into 3 types: general OOP costs, transportation-related OOP costs, and OOP costs for complementary medical materials (1). General OOP costs include the costs of registration fees, co-payments, or other expenses not covered by the NHI incurred when seeking outpatient and inpatient care for the treatment of AD. General OOP costs per visit were estimated by asking patients to report the OOP costs associated with the most recent outpatient visit during the last 3 months. General OOP costs per hospitalization were estimated by asking patients to report the OOP costs associated with the most recent hospitalizations due to AD during the last year (2). Patients were asked to report the OOP costs associated with transportation to attend the most recent outpatient visit for AD treatment within the past 3 months. OOP transportation costs per visit were estimated by asking patients to report the 1-way transportation fares associated with follow-up visits. Patients who travelled by private car or motorcycle were asked to report the time spent driving, and transportation costs were approximated by estimating the cost of fuel for each visit. Assuming a driving speed of 60 km/h, the time spent driving was equal to the distance travelled to reach the clinic (in km). Motorcycles were assumed to be able to travel 40 km per litre of fuel, and cars were assumed to be able to travel 10 km per litre of fuel. The cost of travel was estimated by determining the amount of fuel needed to make a one-way trip to the clinic and multiplying this amount by the cost of fuel (2018 New Taiwan dollars (NT $30.58 = US$1); NT$29.28 per litre) (3). Patients were asked to report OOP expenses associated with complementary medications, such as herbal medicines, folk medicines, dietary supplements, and health foods, as well as medical materials associated with AD, during the previous year.
Annual general OOP costs associated with medical treatments for AD were estimated for each AD severity level by multiplying the mean general OOP costs per visit and per hospitalization by the respective number of previously reported annual AD-associated outpatient visits and hospitalizations (16). Similarly, annual transportation costs were estimated for each AD severity level by first doubling the mean transportation cost per visit (to reflect travel to and from the clinic) and multiplying the result by the number of previously reported annual AD-associated outpatient visits (16).
Estimation of productivity loss due to AD
Annual productivity losses due to AD were estimated as follows. First, annual incomes were estimated for each AD severity level based on the 2018 mean annual incomes reported for each age group in the working population (15–24 years, NT$318,408; 25–44 years, NT$457,104; 45–64 years, NT$510,528; and 65 years or older, NT$406,812) (21). Incomes were then weighted by the percentages of patients with AD in each age group, according to the NHIRD, as described in a previous study (16). The resulting estimated annual incomes used for this study were NT$434,892 for mild AD, NT$433,169 for moderate AD, and NT$438,007 for severe. Secondly, the estimated productivity loss due to work impairment associated with both absenteeism and presenteeism was estimated by multiplying the mean annual income by the corresponding mean Work Productivity and Activity Impairment (WPAI) scores for each severity level (mild AD: 24.7%; moderate AD: 44.8%; and severe AD: 65.0%) reported previously by Chan et al. (7).
Estimation of the economic burdens associated with atopic dermatitis
The total economic burden associated with AD was estimated by adding total direct medical costs financed by NHI, as reported previously (16); total OOP costs; and the estimated costs of productivity loss.
Statistical analysis
Descriptive statistics for study participants who completed patient interviews were reported previously by Chan et al. (7). Crude values for each OOP category are presented according to disease severity, based on the unadjusted proportions of costs incurred and unadjusted OOP costs for those with positive values. To account for the zeroness and right-skewing nature of the distribution of costs, OOP costs were analysed using a 2-part model consisting of logistic regression to predict the probability of costs incurred and a generalized linear model to predict costs among those with positive values. Covariates were entered into the models to predict OOP costs, including sex, age, body mass index, education, marital status, and household income. Differences in mean adjusted values for each OOP cost category among severity levels were assessed by analysis of variance. All costs were reported in 2018 New Taiwan dollars (NT $30.58 = US$1).
Findings with a 2-sided p-value of ≤ 0.05 were considered significantly different between groups. All analyses were performed using SAS/Stat system for Windows, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Out-of-pocket costs
Table I lists OOP payments according to disease severity, including transportation costs, outpatient costs, inpatient costs, and other costs. OOP outpatient costs per visit were NT$419.5 for patients with mild AD, NT$411.2 for patients with moderate AD, and NT$437 for patients with severe AD, with no significant differences (p = 0.27).
| Crude values | ||||||
| Proportion of non-zero costs | Among those with non-zero costs | Regression-adjusted values | ||||
| N | n (%) | Mean ± SD | Median [IQR] | Mean ± SD | p-value | |
| OOP costs incurred for outpatient visits | 0.27 | |||||
| Mild | 70 | 54 (77.1) | 555.1 ± 217 | 520 [80] | 419.5 ± 87.6 | |
| Moderate | 72 | 64 (88.9) | 539.3 ± 230 | 520 [130] | 411.2 ± 90.7 | |
| Severe | 58 | 50 (86.2) | 544.4 ± 255.6 | 520 [80] | 437 ± 94.2 | |
| All | 200 | 168 (84) | 545.9 ± 232.6 | 520 [100] | 421.6 ± 90.8 | |
| OOP costs incurred for hospitalizationa | – | |||||
| Mild | 70 | 0 (0) | – | – | – | – |
| Moderate | 72 | 1 (1.4) | 3,500.00 | – | – | – |
| Severe | 58 | 2 (3.4) | 10,550.00 ± 13,364.30 | – | – | – |
| All | 200 | 3 (1.5) | 8,200.00 ± 10,289.30 | 3,500.00 [18,900.00] | – | – |
| Transportation costs | 0.02 | |||||
| Mild | 140 | 106 (151.4) | 121.2 ± 220.4 | 48 [64.8] | 89.8 ± 54.4 | |
| Moderate | 144 | 110 (152.8) | 135.6 ± 185.2 | 60 [132] | 111.2 ± 65.6 | |
| Severe | 116 | 94 (162) | 181.8 ± 401 | 60 [136] | 133.4 ± 124.8 | |
| All | 400 | 310 (155) | 144.6 ± 277.6 | 60 [136] | 110.2 ± 85.6 | |
| Other OOP costsb | < 0.0001 | |||||
| Mild | 70 | 51 (72.9) | 7,051.00 ± 8,568.50 | 3,000.00 [8,000.00] | 5,248.20 ± 3,955.50 | |
| Moderate | 72 | 58 (80.6) | 11,681.00 ± 18,386.40 | 5,000.00 [10,600.00] | 8,794.10 ± 5,710.20 | |
| Severe | 58 | 49 (84.5) | 20,944.90 ± 38,079.50 | 8,000.00 [16,300.00] | 18,015.70 ± 19,387.80 | |
| All | 200 | 158 (79) | 13,059.50 ± 24,925.50 | 5,750.00 [10,000.00] | 10,227.30 ± 12,323.50 | |
| aRegression-adjusted values were not computed due to the small number of patients who received inpatient care. bIncluding complementary medication, such as herbal medicine, folk medicine, dietary supplements, and health food, as well as medical materials associated with atopic dermatitis. IQR: interquartile range; SD: standard deviation. |
||||||
Other costs were NT$5,248.2, NT$8,794.1, and NT$18,015.7 in patients with mild, moderate, and severe AD, respectively, reaching statistical significance (p<0.0001). The regression-adjusted values for inpatient costs could not be computed due to the small number of patients who received inpatient care; therefore, costs involving inpatient care were not included in the calculation of the economic burden of AD.
Costs of productivity loss
Table II shows the final costs associated with work productivity loss according to AD severity, which were NT$107,418, NT$194,060, and NT$284,705 for patients with mild, moderate, and severe AD, respectively.
Total atopic dermatitis-associated economic burden
Fig. 1 displays the annual costs per patient with mild, moderate, and severe AD are NT$126,938, NT$218,453, and NT$327,741, respectively. The most significant economic burdens experienced by patients with AD were associated with the costs of work productivity loss across all 3 AD severity levels, contributing 84.62%, 88.83%, and 86.87% of the total expenses associated with mild, moderate, and severe AD, respectively. Costs reimbursed by the NHI were the smallest components of the total cost, representing only 7.49%, 4.44%, and 4.81% of the costs associated with mild, moderate, and severe AD, respectively. For details of itemized costs, please refer to Table S1.
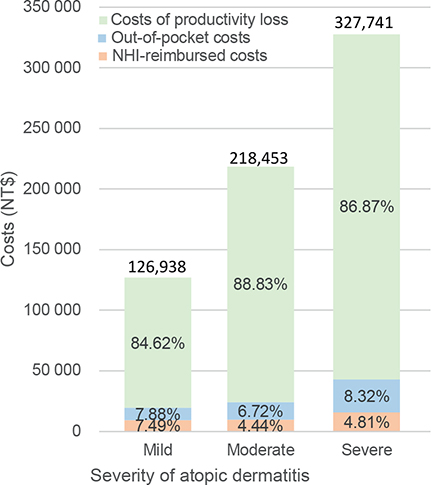
Fig. 1. Annual economic burden of atopic dermatitis per patient in Taiwan by disease severity. NHI: National Health Insurance; NT$: 2018 New Taiwan dollars.
DISCUSSION
Out-of-pocket costs and associations with disease severity
Using a questionnaire designed specifically for this study, the OOP costs were calculated as representing 10–15% of the total costs associated with AD, with estimated means of NT$10,008, NT$14,688, and NT$27,274 reported by patients with mild, moderate, and severe AD, respectively (equivalent to US$327.3, US$480.3, and US$891.9, respectively, using a conversion rate of NT$30.58 = US$1). Chinese medicines, moisturizers, and dietary supplements were the major contributors to OOP costs. In a cross-sectional study conducted in 9 European countries and analysing 1,189 patients receiving systemic treatments or phototherapy, the mean annual OOP expenses per patient were reported to be US$1047.65 (€927.12, using a conversion rate of €1 = US$1.13), which was much higher than any of the AD groups in our study (17). The patients in the European study tended to have higher education levels, which may indicate an improved ability to pay for extra costs, and the overall disease severity was high among these patients due to the applied inclusion criteria for this study. Fivenson et al. (11) estimated mean annual OOP costs of US$147, with greater than 75% of total OOP expenses associated with household items and medications. The much lower cost reported by Fivenson et al. (11) relative to the current study may be due to differences in the study years (2002 vs 2018), which may have contributed to differences in the disease prevalence and the exchange rates. In addition, complementary and alternative medicines are less popular in the USA than in Taiwan.
Cost of work productivity loss
In the current study, the costs associated with work productivity loss were US$3512.7, US$6346.0, and US$9310.2 for patients with mild, moderate, and severe AD, respectively, constituting 84.62%, 88.83% and 86.87% of total expenses. Fivenson et al. (11) calculated productivity loss by multiplying work days lost due to AD by residence-adjusted gross annual income, reporting annual costs per patient of US$181.51, US$280.82 and US2,159.65 for mild, moderate, and severe AD, respectively. These results indicate that costs increase with disease severity. However, the much lower results reported by Fivenson et al. (11) compared with the costs calculated for the current study might be due to the lack of consideration for presenteeism in that study. A study from the USA in 2017 also utilized the WPAI score to calculate the costs of productivity loss, considering both work absenteeism and presenteeism, resulting in an estimated annual cost of US$8907 per patient associated with work productivity loss, which is similar to our results (5).
Total costs of atopic dermatitis-associated economic burden in Taiwan
This study attempted to provide a thorough evaluation of the AD-associated costs in Taiwan by integrating healthcare-reimbursed costs, which were calculated in a previous study. The results showed that the annual economic burden combining NHI-reimbursed costs, OOP costs, and loss of productivity were NT$126,938, NT$218,453, and NT$327,741, respectively, per patient with mild, moderate, and severe AD. The total costs of AD in Taiwan, computed by multiplying the direct costs per patient by the number of patients with AD and adding the total costs associated with productivity loss across all patients with AD of working age, were estimated to be NT$37.90 billion, equivalent to approximately 0.207% of Taiwan’s gross domestic product (GDP) for 2018 (Taiwan’s GDP in 2018 was NT$18342.89 billion) (22). AD accounted for a larger proportion of GDP than psoriasis, which is a less prevalent skin disease. In 2009, the annual total costs of psoriasis, without accounting for the costs associated with presenteeism, were estimated to be equivalent to approximately 0.013% of Taiwan’s GDP (23). These findings indicate that AD places considerable economic burdens on patients, families, the national healthcare system, and society.
Atopic dermatitis-associated economic burdens in Taiwan compared with other countries
A 3-month prospective study from South Korea estimated that the annual total costs of AD at the national level, including both direct and indirect costs, was South Korean won (KRW) 5.8 trillion, representing approximately 0.35% of South Korea’s GDP in 2015 (12). The higher percentage of GDP associated with AD may be due to the higher prevalence of AD in South Korea (29.2%) compared with Taiwan. In addition, the South Korean study used a small sample size (n = 38), and differences in socioeconomic status may have contributed, as all of the patients in the South Korean study were enrolled from university hospitals. Another comprehensive study conducted in the US in 2002 calculated the total AD-related costs of mild, moderate, and severe AD to be US$435.35, US$578.79, and US$3,229.05 per patient, respectively, which accounted for 1.1%, 1.5%, and 8% of the US GDP per capita in 2002 (US’s GDP per capita in 2002=US$38,023.20) (24). In the current study, the total AD-related costs associated with mild, moderate, and severe cases were 16.09%, 27.69%, and 41.55% of Taiwan’s GDP per capita in 2018, respectively (Taiwan’s GDP per capita in 2018 = US$25,792) (25).
Study limitations
This study has several limitations. First, to calculate the overall costs, the study combined data from the NHIRD and questionnaire, which utilized differing strategies for classifying severity. Only 200 patients, aged 20 years and older, completed the study questionnaires, which may not be a representative sample of the total AD population in Taiwan. This may result in underestimation of the costs associated with work loss, by not capturing the lost productivity of caregivers for children with AD, who represent a substantial portion of the AD population. In addition, impaired quality of life and comorbidities may confer additional economic burden. However, both were difficult to quantify and were incorporated consistently into the current findings when direct costs were estimated using National Health Insurance database with no information on quality of life, whereas productivity loss and out-of-pocket costs were estimated using patient interview data with incomplete information on comorbidities. Furthermore, recall bias is an inevitable risk associated with the use of self-reported questionnaires and interviews.
Conclusion
AD imposes a significant financial burden, costing an estimated NT$37.90 billion in 2018, representing approximately 0.207% of Taiwan’s GDP. In addition to the considerable burden placed on the NHI system, OOP costs and costs associated with the loss of work productivity are large contributors to the burdens placed on patients and their families. These costs should not be overlooked when evaluating and managing this disease. This substantial economic burden also indicates an existing need for the development of more effective and equitable AD treatments.
ACKNOWLEDGEMENTS
Institutional Review Board (IRB) approval: The study was approved by the National Taiwan University Hospital Research Ethics Committee (201802007RINA).
REFERENCES
- Torres T, Ferreira EO, Goncalo M, Mendes-Bastos P, Selores M, Filipe P. Update on atopic dermatitis. Acta Med Port 2019; 32: 606–613.
- Ahn C, Huang W. Clinical presentation of atopic dermatitis. Adv Exp Med Biol 2017; 1027: 39–46.
- Stander S. Atopic dermatitis. N Engl J Med 2021; 384: 1136–1143.
- Cho Y-T, Hsieh W-T, Chan TC, Tang C-H, Chu C-Y. Prevalence of baseline comorbidities in patients with atopic dermatitis: a population-based cohort study in Taiwan. JAAD Int 2020; 1: 50–58.
- Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: An analysis using the National Health and Wellness Survey. J Am Acad Dermatol 2017; 77: 274–279 e273.
- Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol 2017; 137: 26–30.
- Chan TC, Lin YC, Cho YT, Tang CH, Chu CY. Impact of atopic dermatitis on work and activity impairment in Taiwan. Acta Derm Venereol 2021; 101: adv00556.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015; 66: 8–16.
- Mei-Yen Yong A, Tay YK. Atopic dermatitis: racial and ethnic differences. Dermatol Clin 2017; 35: 395–402.
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006; 55: 490–500.
- Fivenson D, Arnold RJ, Kaniecki DJ, Cohen JL, Frech F, Finlay AY. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm 2002; 8: 333–342.
- Kim C, Park KY, Ahn S, Kim DH, Li K, Kim DW, et al. Economic impact of atopic dermatitis in Korean patients. Ann Dermatol 2015; 27: 298–305.
- Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol 2002; 46: 361–370.
- Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: analysis of Healthcare Claims Data in the Commercial, Medicare, and Medi-Cal Databases. Adv Ther 2017; 34: 1989–2006.
- Ngamphaiboon J, Kongnakorn T, Detzel P, Sirisomboonwong K, Wasiak R. Direct medical costs associated with atopic diseases among young children in Thailand. J Med Econ 2012; 15: 1025–1035.
- Lee EM, Cho YT, Hsieh WT, Chan TC, Shen D, Chu CY, et al. Healthcare utilization and costs of atopic dermatitis in Taiwan. J Formos Med Assoc 2022; 121: 1963–1971.
- Zink AGS, Arents B, Fink-Wagner A, Seitz IA, Mensing U, Wettemann N, et al. Out-of-pocket costs for individuals with atopic eczema: a cross-sectional study in nine European countries. Acta Derm Venereol 2019; 99: 263–267.
- Jenner N, Campbell J, Marks R. Morbidity and cost of atopic eczema in Australia. Australas J Dermatol 2004; 45: 16–22.
- Launois R, Ezzedine K, Cabout E, Reguai Z, Merrhand S, Heas S, et al. Importance of out-of-pocket costs for adult patients with atopic dermatitis in France. J Eur Acad Dermatol Venereol 2019; 33: 1921–1927.
- Andersen L, Nyeland ME, Nyberg F. Increasing severity of atopic dermatitis is associated with a negative impact on work productivity among adults with atopic dermatitis in France, Germany, the U.K. and the U.S.A. Br J Dermatol 2020; 182: 1007–1016.
- Table 50 from Manpower Utilization Survey, 2018. National Statistics, R.O.C. (Taiwan). [cited 2020 Jul 9]. Available from: https://www.stat.gov.tw/ct.asp?xItem=43604&ctNode=3579&mp=4.
- Latest Indicators of GDP at current prices, 2018. National Statistics, R.O.C. (Taiwan). [cited 2020 May 30]. Available from: https://eng.stat.gov.tw/point.asp?index=1.
- Chen KC, Hung ST, Yang CW, Tsai TF, Tang CH. The economic burden of psoriatic diseases in Taiwan. J Dermatol Sci 2014; 75: 183–189.
- GPD per capita – United States (2002). The World Bank. [cited 2020 May 30]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=US.
- Latest Indicators of GDP per capita, 2018. National Statistics, R.O.C. (Taiwan). [cited 2020 May 30]. Available from: https://eng.stat.gov.tw/point.asp?index=1.