ORIGINAL ARTICLE
Increased Risk of Dementia in Patients with Atopic Dermatitis: A Nationwide Population-Based Cohort Study
Yu Ri WOO1, Minah CHO1, Kyung Do HAN2, Sang Hyun CHO1 and Ji Hyun LEE3
1Department of Dermatology, Incheon St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea, 2Department of Statistics and Actuarial Science, Soongsil University and 3Department of Dermatology, Seoul St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Atopic dermatitis (AD) is a chronic inflammatory skin disorder with bimodal incidence peaks in early childhood and middle-aged and older adults. Few studies have focused on the risk of dementia in AD. The aims of this study were to analyse the incidence, and risk factors for dementia in patients with AD. This nationwide population-based retrospective cohort study enrolled 38,391 adults ≥ 40 years of age with AD and 2,643,602 controls without AD from the Korean National Health Insurance System (NHIS) database from 2009 to 2016. The cumulative incidence probability of all-cause dementia, Alzheimer’s disease, or vascular dementia at 8 years was 50, 39, and 7 per 1,000 person-years in patients with AD, respectively. The adjusted risks of all-cause dementia (hazard ratio (HR), 1.072; 95% confidence interval (95% CI) 1.026–1.120), and Alzheimer’s disease (HR 1.051; 95% CI 1.000–1.104) were increased in patients with AD. The effect of AD on the development of all-cause dementia and Alzheimer’s dementia varied according to age and diabetes mellitus (all p for interaction, < 0.05). The risks of all-cause dementia and Alzheimer’s disease were increased in patients with AD. Management of modifiable risk factors is important for preventing dementia in patients with AD.
Key words: atopic dermatitis; Alzheimer’s disease; dementia; diabetes mellitus; vascular dementia.
SIGNIFICANCE
Little is known about the risk of dementia in patients with atopic dermatitis. This is the first population-based study in Korea identifying the risk of dementia among patients with atopic dermatitis. Atopic dermatitis is associated with an increased risk of all-cause dementia and Alzheimer’s disease. Management of modifiable risk factors is important for preventing dementia in patients with atopic dermatitis.
Citation: Acta Derm Venereal 2023; 103: adv4557. DOI: https://doi.org/10.2340/actadv.v103.4557.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 13, 2023; Published: Apr 27, 2023
Corr: Ji Hyun Lee, Department of Dermatology, Seoul St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. E-mail: yiji1@hanmail.net
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Dementia is an umbrella term for several disorders characterized by persistent, progressive, memory loss and impairment in cognitive abilities and behaviour, which can interfere with activities of daily living (1). Alzheimer’s disease is the most common form of dementia, accounting for 25–75% of cases (2). Other common forms of dementia include vascular dementia, dementia with Lewy bodies, and frontotemporal dementia (2). With population aging, the number of people with dementia is projected to increase substantially (3). In 2015, dementia affected approximately 5% of the world’s older population, which is estimated increase to 75 million by 2030 (1, 3). Although the aetiology of dementia is unclear, genetic predisposition, environmental factors, and neuroinflammation are considered pivotal drivers of the condition. Among these factors, neuroinflammation is associated with all types of neurodegenerative dementia (4).
Atopic dermatitis (AD) is a chronic inflammatory cutaneous skin disorder that affects people of any age. AD has a bimodal age distribution, with bimodal incidence peaks with the highest peak in early childhood and a second peak in middle-aged and older adults (5). Complex immunological alterations and epidermal barrier dysfunction are implicated in the development and aggravation of AD (6). In addition to T helper (Th) 2-driven responses, some AD phenotypes are characterized by Th1 or Th17 polarization (7). Patients with AD have a variety of neuropsychiatric comorbidities, including depression, anxiety disorder, sleep disturbances, and cognitive dysfunction (8–11). Considering the chronic inflammatory nature of AD, its association with diverse neuropsychiatric disorders and its increased prevalence in the middle-aged and older population, we hypothesize an association between AD and dementia. Few studies have focused on the relationship between dementia risk and AD. The risk of dementia and Alzheimer’s disease is reportedly increased in patients with AD (12). However, that study included only a small number of patients with AD and did not examine risk factors for dementia.
The aim of the current study was to evaluate the incidence rate of all-cause dementia, Alzheimer’s disease, and vascular dementia in patients with AD using a nationwide population-based dataset. In addition, the current study aimed to determine whether AD severity is associated with an increased risk of all-cause dementia, Alzheimer’s disease, and vascular dementia. Moreover, this study evaluated risk factors for all-cause dementia, Alzheimer’s disease, and vascular dementia in patients with AD.
METHODS
Study design, setting and participants
This is a nationwide population-based cohort study using the National Health Insurance System (NHIS), a government-operated mandatory social health insurance programme that contains health information on approximately 50 million South Koreans (12, 13).
A total of 4,238,820 subjects who underwent health examinations by the South Korean NHIS between 1 January 2009, and 31 December 2009, were enrolled. Subjects < 40 years of age (n = 1,342,500), those diagnosed with dementia prior to enrollment (n = 44,160), and those with missing data (n = 155,176) were excluded. Individuals with a follow-up observation period less than 1 year were also excluded (n = 14,991). Finally, a total of 2,681,993 subjects was followed up until dementia diagnosis or 31 December 2016 (Fig. 1). The study protocol was approved by the Institutional Review Board of the Catholic University of Korea (IRB approval number: KC20ZISI0511).
Demographic characteristics
Each individual was classified as non-obese (< 25 kg/m2) or obese (≥ 25 kg/m2) based on body mass index (BMI) (14). Blood samples were taken after an overnight fast and quality control procedures followed the Korean Association of Laboratory Quality Control guidelines. Using standardized self-reported questionnaires, smoking status, alcohol consumption, physical activity, and past medical history were assessed. Physical activity was divided into strenuously exercising less vs more than 3 times per week for at least 20 minutes each time. Baseline comorbidities were evaluated based on the combination of past medical history and International Classification of Diseases (ICD-10) codes with pharmacy and/or clinical values of each comorbidity. For example, hypertension was defined as a systolic/diastolic blood pressure > 140/90 mmHg or at least 1 claim per year for an antihypertensive prescription under ICD-10 codes I10–I13 or I15. Dyslipidaemia was defined as a total cholesterol of > 240 mg/dL or at least 1 claim per year for an antihyperlipidaemic prescription (ICD-10 code E78). Diabetes mellitus was defined as treatment with an antidiabetic drug and ICD-10 code E11–E14 or a fasting glucose level > 126 mg/dL. Ischaemic heart disease (IHD) or stroke was defined as ICD-10 codes I20-25 or I63 and I64.
Definitions of atopic dermatitis and dementia
AD was defined as ICD-10 code (L209) for AD more than 3 claims with the prescription of topical or systemic medications or phototherapies for AD during the same year. Mild AD was defined as ICD-10 code (L209) for AD and prescription of topical corticosteroids or calcineurin inhibitors more than 3 claims during the same year. Moderate-to-severe AD was defined as ICD-10 L209 and prescription of more than 1 systemic oral medication for AD or phototherapies more than 12 claims during the same year.
All-cause dementia was defined as an ICD-10 code F00, F01, F02, F03, G30, or G31 with prescription of anti-dementia medications (rivastigmine, memantine, galantamine, and donepezil). Alzheimer’s disease was defined as an ICD-10 code F00 or G30. Vascular dementia was defined as an ICD-10 code F01. Two or more dementia diagnosis codes for each individual could be registered together. If there were primary and secondary diagnoses for dementia, dementia subtype was determined based on the primary diagnosis. In cases in which all dementia codes were registered as secondary diagnoses, the decision was deferred until the next visit. If the dementia codes were confirmed as primary and secondary diagnoses at the next visit, the dementia subtype was defined from the primary diagnosis code.
Statistical analysis
Data are presented as means ± standard deviation, geometric means (95% confidence interval; CI), or percentages. Differences between groups were evaluated using Student’s t-test for continuous variables or χ2 test for categorical variables. The incidence of dementia was determined by dividing the total number of incident cases by the follow-up period (person-years) and is presented as the number of dementia cases per 1,000 person-years. The difference in the cumulative incidence of dementia based on the presence of AD was calculated by log-rank test. The Cox proportional hazards regression analysis was performed to examine the association between risk factors and all-cause dementia, Alzheimer’s disease, and vascular dementia. To control for confounding factors, this study used model 3, which adjusted for age, sex, BMI, smoking, drinking, regular exercise, hypertension, diabetes mellitus, dyslipidaemia, stroke, and ischaemic heart disease. A subgroup analysis and interaction testing by likelihood ratio test were performed to assess differences in the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia. Individuals with missing data for a specific mandatory parameter were excluded. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and a p < 0.05 was considered indicative of statistical significance.
RESULTS
Baseline characteristics
Demographic characteristics of the subjects are listed in Table I. A total of 2,681,993 subjects were identified, among whom 38,391 did and 2,643,602 did not have AD. Among the 38,391 patients with AD, 15,989 (41.64%) and 22,402 (58.36%) patients had mild and moderate-to-severe AD, respectively.
Incidence of all-cause dementia, Alzheimer’s disease, and vascular dementia in atopic dermatitis
During a mean follow-up of 8.07 years, 109,785 subjects developed dementia. Among them, 87,662 and 13,774 developed Alzheimer’s disease and vascular dementia, respectively. The cumulative incidence probability of all-cause dementia, Alzheimer’s disease, and vascular dementia at 8 years was 50, 39, and 7 per 1,000 person-years in patients with AD, respectively (Fig. 2). The cumulative incidence of all-cause dementia, Alzheimer’s disease, and vascular dementia was significantly higher in subjects with than in those without AD (p < 0.001).
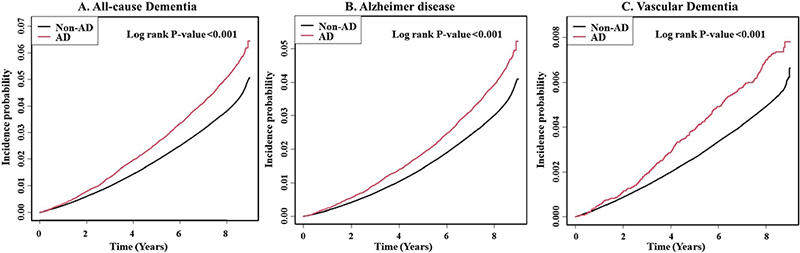
Fig. 2. Cumulative incidence probability of: (A) all-cause dementia, (B) Alzheimer’s disease, and (C) vascular dementia in individuals with and without atopic dermatitis (AD).
Risk of all-cause dementia, Alzheimer’s disease, and vascular dementia in topic dermatitis
Patients with AD had an increased risk of all-cause dementia, Alzheimer’s disease, and vascular dementia in the non-adjusted model (Table II). When adjusted for age, sex, BMI, smoking, drinking, regular exercise, hypertension, diabetes mellitus, dyslipidaemia, stroke and ischaemic heart disease, patients with AD had increased risks of all-cause dementia (hazard ratio (HR) 1.072; 95% confidence interval (95% CI) 1.026–1.120), and Alzheimer’s disease (HR 1.051; 95% CI 1.000–1.104) (Table II).
Risk of all-cause dementia, Alzheimer’s disease, and vascular dementia by atopic dermatitis severity
Patients with mild or moderate-to-severe AD had an increased risk of all-cause dementia, Alzheimer’s dementia, and vascular dementia in the non-adjusted model. When adjusted for age, sex, BMI, smoking, drinking, regular exercise, hypertension, diabetes mellitus, dyslipidaemia, stroke, and ischaemic heart disease, patients with mild AD (HR 1.083; 95% CI 1.016–1.155) or moderate-to-severe AD (HR 1.063; 95% CI 1.002–1.128) had increased risks of all-cause dementia. To determine the effect of AD severity on the risk of dementia, we further analysed the risk of dementia in patients with moderate-to-severe AD compared with mild AD. Patients with moderate-to-severe AD did not show any significant effect on the risk of all-cause dementia, Alzheimer’s dementia, and vascular dementia compared with patients with mild AD in a fully adjusted model (Table SI).
Subgroup analysis for risk of all-cause dementia, Alzheimer’s disease, and vascular dementia in atopic dermatitis
Subgroup analyses were conducted in patients with AD adjusted for age, sex, obesity, smoking status, drinking habits, exercise, diabetes, hypertension, dyslipidaemia, ischaemic heart disease, stroke, and antihistamine use. The effect of AD on the risk of all-cause dementia (p for interaction, 0.0067) and Alzheimer’s disease (p for interaction, 0.0063) differed significantly according to age. The risks of all-cause dementia (HR 1.207; 95% CI 1.095–1.331) and Alzheimer’s disease (HR 1.210; 95% CI 1.080–1.355) were higher among individuals < 65 years of age with AD than in those without AD (Table III).
| Variables | AD | All-cause dementia HR (95% CI) | p-value for interaction | Alzheimer’s disease HR (95% CI) | p-value for interaction | Vascular dementia HR (95% CI) | p-value for interaction |
| Age | 0.0067* | 0.0063* | 0.6019 | ||||
| < 65 years | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.207 (1.095–1.331) | 1.210 (1.080–1.355) | 1.168 (0.926–1.475) | ||||
| ≥ 65 years | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.038 (0.989–1.09) | 1.015 (0.96–1.072) | 1.087 (0.943–1.252) | ||||
| Sex | 0.1621 | 0.1275 | 0.7347 | ||||
| Male | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.111 (1.040–1.186) | 1.099 (1.019–1.184) | 1.135 (0.955–1.347) | ||||
| Female | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.043 (0.984–1.106) | 1.017 (0.952–1.086) | 1.088 (0.917–1.291) | ||||
| Obesity | 0.8570 | 0.6731 | 0.8961 | ||||
| BMI < 25 kg/m2 | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.068 (1.011–1.128) | 1.042 (0.979–1.108) | 1.117 (0.959–1.302) | ||||
| BMI ≥ 25 kg/m2 | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.077 (1.001–1.159) | 1.065 (0.981–1.156) | 1.099 (0.901–1.339) | ||||
| Smoking status | 0.7466 | 0.9658 | 0.4545 | ||||
| Non, Ex | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.068 (1.019–1.12) | 1.05 (0.996–1.108) | 1.086 (0.95–1.242) | ||||
| Current | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.091 (0.97–1.227) | 1.047 (0.913–1.201) | 1.224 (0.923–1.625) | ||||
| Drinking status | 0.4718 | 0.4298 | 0.6184 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.079 (1.032–1.128) | 1.058 (1.007–1.113) | 1.106 (0.976–1.253) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 0.991 (0.791–1.243) | 0.949 (0.727–1.238) | 1.264 (0.759–2.107) | ||||
| Exercise | 0.4101 | 0.7845 | 0.0747 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.081 (1.031–1.135) | 1.054 (0.999–1.113) | 1.170 (1.026–1.334) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.030 (0.928–1.144) | 1.035 (0.921–1.164) | 0.857 (0.624–1.176) | ||||
| Diabetes mellitus | 0.0404* | 0.0211* | 0.9736 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.044 (0.992–1.099) | 1.016 (0.96–1.077) | 1.112 (0.965–1.281) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.158 (1.064–1.26) | 1.159 (1.053–1.276) | 1.107 (0.875–1.4) | ||||
| Hypertension | 0.2449 | 0.2960 | 0.8438 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.034 (0.959–1.115) | 1.014 (0.932–1.103) | 1.131 (0.912–1.401) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.092 (1.036–1.152) | 1.071 (1.008–1.139) | 1.102 (0.951–1.276) | ||||
| Dyslipidaemia | 0.2115 | 0.1117 | 0.4742 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.049 (0.993–1.109) | 1.019 (0.956–1.085) | 1.149 (0.988–1.336) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.111 (1.036–1.192) | 1.106 (1.022–1.197) | 1.047 (0.855–1.284) | ||||
| Stroke | 0.3172 | 0.2334 | 0.3476 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.06 (1.01–1.114) | 1.035 (0.979–1.094) | 1.08 (0.942–1.237) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.12 (1.018–1.234) | 1.115 (1.001–1.241) | 1.246 (0.956–1.624) | ||||
| IHD | 0.4075 | 0.5084 | 0.4604 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.063 (1.012–1.116) | 1.043 (0.987–1.101) | 1.086 (0.948–1.244) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.114 (1.008–1.23) | 1.088 (0.972–1.217) | 1.217 (0.93–1.591) | ||||
| Stroke | |||||||
| No | Non-AD | 1 (Reference) | 0.2701 | 1 (Reference) | 0.3756 | 1 (Reference) | 0.5842 |
| AD | 1.061 (1.007–1.117) | 1.080 (1.031–1.131) | 1.127 (0.989–1.284) | ||||
| Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| AD | 0.966 (0.825–1.132) | 1.013 (0.887–1.157) | 1.023 (0.741–1.411) | ||||
| Antihistamine | 0.3354 | 0.3299 | 0.6175 | ||||
| No | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 0.979 (0.837–1.146) | 0.944 (0.788–1.131) | 1.008 (0.663–1.533) | ||||
| Yes | Non-AD | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| AD | 1.061 (1.014–1.111) | 1.037 (0.985–1.091) | 1.127 (0.993–1.279) | ||||
| 95% CI: 95% confidence interval; HR: hazard ratio. *p-value < 0.05. |
|||||||
The risks of all-cause dementia (HR 1.111; 95% CI 1.040–1.186) and Alzheimer’s disease (HR 1.099; 95% CI 1.019–1.184) were increased in male patients with AD compared with in those without AD. AD patients who were prescribed antihistamine had an increased risk of all-cause dementia (HR 1.061; 95% CI 1.014–1.111).
The effect of AD on developing all-cause dementia (p for interaction 0.0404) and Alzheimer’s disease (p for interaction 0.0211) differed significantly according to the presence of diabetes mellitus. Among patients with diabetes mellitus, there was a significantly higher incidence of dementia in those with AD than in those without AD (HR 1.158; 95% CI 1.064–1.260). Moreover, in diabetic patients, the risks of incident Alzheimer’s disease were higher in those with AD than in those without AD (HR 1.159; 95% CI 1.053–1.276).
DISCUSSION
The cumulative incidence of all-cause dementia, Alzheimer’s disease, and vascular dementia was significantly higher in individuals with AD than in those without AD. In addition, the risks of developing all-cause dementia and Alzheimer’s disease were increased in patients with AD. The effect of AD on the risk of all-cause dementia and Alzheimer’s dementia varied according to age and diabetes mellitus.
The mechanisms underlying dementia and Alzheimer’s disease in AD are unclear. We hypothesize that itch and chronic inflammation of AD promote neuro-immunological modulation, triggering dementia and Alzheimer’s disease. Indeed, itch-mediating factors, such as transient receptor potential (TRP) channels and protease-activated receptor-2 are also implicated in the pathogenesis of Alzheimer’s disease (13–15).
There are increased expressions of several common proinflammatory cytokines and chemokines (such as IL-33, IL-1β and eotaxin) in AD (16, 17) and Alzheimer’s disease (18–22). Indeed, proinflammatory cytokines can penetrate the blood-brain barrier during allergic events and modulate neuroimmune pathways (23). Among various chemokines, eotaxin can pass the blood-brain barrier, and stimulates microglia to induce excitotoxic neuronal cell death via producing reactive oxygen species in terminally differentiated cells of the central nervous system (21). We consider that the increased expression of proinflammatory cytokines and chemokines is involved in the persistent cutaneous inflammation in AD and cause astrogliosis, activation of microglia, and release of inflammatory factors, thereby triggering amyloidosis and neurodegeneration in Alzheimer’s disease (24).
Although the absolute risk of new-onset all-cause dementia in AD is relatively low, various subgroup analyses in this study also showed consistent results, revealing the low, but significantly increased, risk of dementia in AD. We consider the findings from this study to be meaningful.
The effect of AD on the development of all-cause dementia and Alzheimer’s disease differed significantly according to age. Compared with those without AD, individuals < 65 years of age with AD had increased risks of all-cause dementia and Alzheimer’s disease. The onset of Alzheimer’s disease at < 65 and ≥ 65 years of age is termed early-and late-onset Alzheimer’s disease, respectively (25). Early-onset and late-onset Alzheimer’s disease have different characteristics (25). Early-onset and late-onset Alzheimer’s disease is commonly characterized by an atypical clinical course, exhibiting a more impaired executive and visuospatial function, and impaired motor skills, and less memory loss than late-onset Alzheimer’s disease (25). An association between early-onset Alzheimer’s disease and AD is suggested by the increased risks of the former in younger patients with AD, which may provide insight into the pathogenesis of early-onset Alzheimer’s disease.
Males with AD had an increased risk of dementia (HR 1.111; 95% CI 1.040–1.186) and Alzheimer’s disease (HR 1.099; 95% CI 1.019–1.184) compared with males without AD. As AD is more severe in males than females (26), we suspect that the increased risk of all-cause dementia and Alzheimer’s disease in male patients with AD could be a result of more severe inflammation.
Among several risk factors for all-cause dementia and Alzheimer’s disease, this study found that the effect of AD on development of all-cause dementia and Alzheimer’s dementia varies depending on the presence or absence of diabetes mellitus. Among diabetes patients, significantly increased risks of all-cause dementia and Alzheimer’s disease were observed in AD patients compared with non-AD patients. Although further research is needed to determine the underlying shared pathomechanism among these conditions, findings from recent transcriptome analysis on AD patients identified increased expression of resistin in the lesional skin of AD (27), which is also associated with diabetes mellitus (28, 29) and Alzheimer’s disease (19, 30, 31). Resistin is a proinflammatory factor that triggers production of other inflammatory cytokines in Alzheimer’s disease and AD (32). Resistin in diabetes regulates glucose and fatty acid metabolism by targeting acetyl-CoA carboxylase, CD36, fatty acid transport protein 1, and AMP-activated protein kinase (28, 29). Mast cells are implicated in the pathogenesis of allergic, metabolic, and neurodegenerative disorders. Indeed, mast cells are involved in the pathogenesis of AD, diabetes mellitus, and Alzheimer’s disease. In patients with AD, mast cell infiltration is observed in lesional skin (33). Clinically, inhibition of mast cell degranulation in AD patients yielded clinical improvement (34, 35). Mast cell mediators are potential biomarkers of diabetes mellitus (36). In addition, mast cells are associated with neurodegeneration via the nitric oxide pathway (37), and with amyloid plaque formation during Alzheimer’s disease (38). These shared aetiological factors may explain the increased risk of all-cause dementia and Alzheimer’s disease in diabetic patients with AD.
Study limitations
This study has some limitations. First, there may be information bias, as this study used healthcare utilization databases. The definitions of AD and dementia in this study have not been validated. Although a combination of diagnostic codes and prescription codes, which could enhance the reliability of diagnosis of AD and dementia, was used in this study, there is potential for misdiagnosis or misclassification of AD and dementia. Therefore, further validation studies are necessary to determine the definition of AD and dementia using the Korea NHIS database. Secondly, this study did not consider the effect of various drug exposure and age of onset of AD on the development of dementia in AD because of the study design. Further stratified analysis utilizing data regarding the age of onset of AD could determine whether long-term cumulative AD inflammation is associated with the development of dementia. Therefore, further analysis of the effect of drug exposure and cumulative duration of AD episodes is needed. Nevertheless, the findings of the current study provide insight into the positive association between AD and dementia.
Conclusion
This study evaluated the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia in patients with AD. The findings suggest a considerable burden of dementia in South Korean patients with AD. Based on the postulated neuro-immuno-cutaneous-endocrine network, further research on the association of AD with dementia is needed in order to evaluate the common factors in their pathogenesis.
Acknowledgement
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2018R1D1A1B07044100).
REFERENCES
- Prince MJ, Guerchet MM, Prina M. The epidemiology and impact of dementia: current state and future trends. WHO Thematic Briefing. Geneva: World Health Organization, 2015.
- Choi YJ, Kim S, Hwang YJ, Kim C. Prevalence of dementia in Korea based on hospital utilization data from 2008 to 2016. Yonsei Med J 2021; 62: 948.
- Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015 – the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International, 2015.
- Pasqualetti G, Brooks DJ, Edison P. The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep 2015; 15: 17.
- Laughter M, Maymone M, Mashayekhi S, Arents B, Karimkhani C, Langan S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol 2021; 184: 304–309.
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 2011; 242: 233–246.
- Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019; 143: 1–11.
- Silverberg JI. Selected comorbidities of atopic dermatitis: atopy, neuropsychiatric, and musculoskeletal disorders. Clin Dermatol 2017; 35: 360–366.
- Bekić S, Martinek V, Talapko J, Majnarić L, Vasilj Mihaljević M, Škrlec I. Atopic dermatitis and comorbidity. Healthcare 2020; 8: 70.
- Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 2013; 131: 428–433.
- Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. Association of atopic dermatitis severity with cognitive function in adults. J Am Acad Dermatokl 2020; 83: 1349–1359.
- Pan T-L, Bai Y-M, Cheng C-M, Tsai S-J, Tsai C-F, Su T-P, et al. Atopic dermatitis and dementia risk: a nationwide longitudinal study. Ann Allergy Asthma Immunol 2021; 127: 200–205.
- Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol 2016; 3: 263–292.
- Lee K, Jo YY, Chung G, Jung JH, Kim YH, Park C-K. Functional importance of transient receptor potential (TRP) channels in neurological disorders. Front Cell Dev Biol 2021; 9: 410.
- Afkhami-Goli A, Noorbakhsh F, Keller AJ, Vergnolle N, Westaway D, Jhamandas JH, et al. Proteinase-activated receptor-2 exerts protective and pathogenic cell type-specific effects in Alzheimer’s disease. J Immunol 2007; 179: 5493–5503.
- Tamagawa-Mineoka R, Okuzawa Y, Masuda K, Katoh N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J Am Acad Dermatol 2014; 70: 882–888.
- Lyubchenko T, Collins HK, Goleva E, Leung DY. Skin tape sampling technique identifies proinflammatory cytokines in atopic dermatitis skin. Ann Allergy Asthma Immunol 2021; 126: 46–53.e42.
- Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, Zaheer A. Alzheimer’s disease: evidence for the expression of interleukin-33 and its receptor ST2 in the brain. J Alzheimers Dis 2014; 40: 297–308.
- Demirci S, Aynalı A, Demirci K, Demirci S, Arıdoğan BC. The serum levels of resistin and its relationship with other pro-inflammatory cytokines in patients with Alzheimer’s disease. Clin Psychopharmacol Neurosci 2017; 15: 59.
- Taha RA, Minshall EM, Leung DY, Boguniewicz M, Luster A, Muro S, et al. Evidence for increased expression of eotaxin and monocyte chemotactic protein-4 in atopic dermatitis. J Allergy Clin Immunol 2000; 105: 1002–1007.
- Parajuli B, Horiuchi H, Mizuno T, Takeuchi H, Suzumura A. CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 2015; 63: 2274–2284.
- Choi C, Jeong J-H, Jang JS, Choi K, Lee J, Kwon J, et al. Multiplex analysis of cytokines in the serum and cerebrospinal fluid of patients with Alzheimer’s disease by color-coded bead technology. J Clin Neurol 2008; 4: 84–88.
- Schut C, Mochizuki H, Grossman SK, Lin AC, Conklin CJ, Mohamed FB, et al. Brain processing of contagious itch in patients with atopic dermatitis. Front Psychol 2017; 8: 1267.
- Zhang Z-G, Li Y, Ng CT, Song Y-Q. Inflammation in Alzheimer’s disease and molecular genetics: recent update. Arch Immunol Ther Exp 2015; 63: 333–344.
- Tellechea P, Pujol N, Esteve-Belloch P, Echeveste B, García-Eulate M, Arbizu J, et al. Early-and late-onset Alzheimer’s disease: are they the same entity? Neurologia 2018; 33: 244–253.
- Kim MJ, Kang TW, Cho EA, Kim HS, Min JA, Park H, et al. Prevalence of atopic dermatitis among Korean adults visiting health service center of the Catholic Medical Center in Seoul Metropolitan Area, Korea. J Korean Med Sci 2010; 25: 1828–1830.
- Pavel AB, Zhou L, Diaz A, Ungar B, Dan J, He H, et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol 2020; 82: 690–699.
- Saeedi Borujeni MJ, Esfandiary E, Taheripak G, Codoñer-Franch P, Alonso-Iglesias E, Mirzaei H. Molecular aspects of diabetes mellitus: resistin, microRNA, and exosome. J Cell Biochem 2018; 119: 1257–1272.
- McTernan CL, McTernan P, Harte A, Levick P, Barnett A, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet 2002; 359: 46–47.
- Kizilarslanoğlu MC, Kara Ö, Yeşil Y, Kuyumcu ME, Öztürk ZA, Cankurtaran M, et al. Alzheimer’s disease, inflammation, and novel inflammatory marker: resistin. Turk J Med Sci 2015; 45: 1040–1046.
- Leung YY, Toledo JB, Nefedov A, Polikar R, Raghavan N, Xie SX, et al. Identifying amyloid pathology-related cerebrospinal fluid biomarkers for Alzheimer’s disease in a multicohort study. Alzheimers Dement (Amst) 2015; 1: 339–348.
- Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol 2005; 174: 5789–5795.
- Damsgaard TE, Olesen AB, Sørensen FB, Thestrup-Pedersen K, Schiøtz PO. Mast cells and atopic dermatitis. Stereological quantification of mast cells in atopic dermatitis and normal human skin. Arch Dermatol Res 1997; 289: 256–260.
- Kjellman NI, Gustafsson I. Topical sodium cromoglycate in atopic dermatitis a disappointing but informative trial. Allergy 1986; 41: 423–428.
- Yanase DJ, David-Bajar K. The leukotriene antagonist montelukast as a therapeutic agent for atopic dermatitis. J Am Acad Dermatol 2001; 44: 89–93.
- Hamdy N, Salam RF, Mohamed NAE-G. Mast cell, a new player in type 2 diabetes. Kasr Al Ainy Medical Journal 2018; 24: 59.
- Skaper SD, Facci L, Romanello S, Leon A. Mast cell activation causes delayed neurodegeneration in mixed hippocampal cultures via the nitric oxide pathway. J Neurochem 1996; 66: 1157–1166.
- Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Raikwar SP, et al. Mast cell activation in brain injury, stress, and post-traumatic stress disorder and Alzheimer’s disease pathogenesis. Front Neurosci 2017; 11: 703.