ORIGINAL REPORT
Skin Distress Screening: Validation of an Efficient One-question Tool
Tirza BLOM1, Karin B. FIETEN2, Patrick M. J. H. KEMPERMAN1, Saskia SPILLEKOM-VAN KOULIL3 and Rieky E. G. DIKMANS4
1Department of Dermatology, Amsterdam University Medical Centers, Amsterdam, The Netherlands, 2Swiss Institute of Allergy and Asthma Research (SIAF), University of Zürich, Davos, Switzerland, 3Department of Medical Psychology, Radboud University Medical Center, Nijmegen and 4Department of Psychiatry and Medical Psychology, OLVG Hospital, Amsterdam, The Netherlands
Skin diseases are often accompanied by physical, emotional and social problems, which may negatively impact health-related quality of life and result in skin-related distress. It is essential to identify patients with skin-related distress within the short time-window of an outpatient dermatological visit. Therefore the one-question screening tool, the Distress Thermometer adjusted for skin conditions, was validated in a cross-sectional questionnaire study. In 2 medical centres in Amsterdam, 214 patients with a chronic skin disease were invited to complete the Distress Thermometer and additional health-related quality of life questionnaires. To validate the Distress Thermometer, the Skindex29 was used as gold standard. To test test–retest reliability, the questionnaires were answered at 2 different time-points. Severely impaired health-related quality of life was present in 30% of respondents according to the Skindex29 using a cut-off score of 44. Receiver operating characteristic curve analyses yielded an area under the curve of 0.813 (standard error 0.04, 95% confidence interval 0.74–0.89). A cut-off score ≥ 4 on the Distress Thermometer provided the optimal ratio of sensitivity (90.7%) to specificity (56.1%). Therefore, for general practice, a cut-off score of ≥ 4 on the Distress Thermometer is advised. The Distress Thermometer seems to be a rapid, valid and reliable screening tool for identifying skin-related distress in patients with a chronic skin disease in the outpatient dermatology setting.
Key words: distress thermometer; health-related quality of life; skin diseases; validation study; depression; anxiety.
SIGNIFICANCE
Skin related distress is high among patients with a chronic skin disease and difficult to detect within the short time window of an outpatient visit. The aim of this study was to validate the distress thermometer. The distress thermometer is a one question screening tool for identifying dermatology patients with skin related distress. Our research showed that this tool is time economic and reliable.
Citation: Acta Derm Venereol 2023; 103: adv4590. DOI: https://doi.org/10.2340/actadv.v103.4590.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Mar 14, 2023; Published: May 10, 2023
Corr: Tirza Blom, Department of Dermatology, Amsterdam University Medical Centers, location AMC, Meibergdreef 9, NL-1105 AZ, Amsterdam, The Netherlands. E-mail: t.blom@amsterdamumc.nl
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Skin diseases are the fourth leading cause of disability worldwide (1, 2). Skin diseases can significantly impact all aspects of a patient’s life, including physical, emotional and social aspects. Not only functioning can be affected, but also psychosocial well-being, which negatively affects a patient’s health-related quality of life (HRQoL). Patients may experience physical problems, such as itch and pain, but also psychological adjustment problems, shame, low self-esteem and problems at work and within relationships or even symptoms of depression or anxiety (3–5). Because of the stress accompanying these problems, the skin condition may even worsen (6–8). Patients with psychosocial distress who received treatment for their skin condition had less positive treatment results compared with patients who did not experience psychosocial distress (9). Furthermore, patients with psychosocial distress have less compliance and satisfaction with care, which also leads to less positive treatment results and worsening of the skin condition (10, 11). To assess the influence of a skin disease on a patient’s life, there are several HRQoL questionnaires available within dermatology. Questionnaires, such as the Skindex29 or Dermatology Life Quality Index (DLQI), identify patients with physical, emotional and social problems that can affect HRQoL. However, assessment of current HRQoL questionnaires is not feasible within the time-window of a dermatologist consultation and therefore is insufficiently used in daily practice (12). In addition, the impact of a skin disease on patients’ lives is rarely discussed during consultations, and dermatologists tend to underestimate the negative impact of a skin disease on a patient’s life (13–15). It is of great importance to identify patients with skin-related problems at an early stage, and the negative consequences on disease severity and treatment outcomes. Including patients’ psychosocial distress in therapeutic decision-making, improves disease management and herewith clinical outcomes (16).
To address the challenge of assessing skin-related distress within the time-window of a regular dermatologist consultation, a less time-consuming screening tool is needed. In the field of oncology, a one-question tool, the Distress Thermometer (DT) is used to identify patients with physical, emotional and social health problems and diminished HRQoL (17, 18). Distress is defined as a multifactorial unpleasant emotional experience of a psychological, social or spiritual nature that can influence the ability to deal effectively with the physical condition (19). If the DT can be used to assess skin-related distress accurately in patients with a chronic skin disease, it could be a suitable solution for screening during daily practice.
The aim of this study is to validate the DT for patients with a chronic skin disease. Furthermore, an appropriate cut-off point is needed to use the DT for the detection of patients with an increased risk of physical, psychological and social problems related to their skin disease.
MATERIALS AND METHODS
Patients with a chronic skin disease aged 16 years or older, visiting the outpatient clinic of the Amsterdam UMC dermatology department or the Skin Medical Center in Amsterdam between 12 and 23 November 2018, were consecutively invited to participate in the study. Patients with insufficient mastery of the Dutch language or patients without email or digital skills were excluded. At the outpatient clinic, patients received login credentials for an online questionnaire. The questionnaire included information on age, sex and skin disease and consisted of the Dutch version of the DT and problem list (PL). The DT is a single-item, self-reported measure of psychological distress, originally designed for use in cancer patients and adjusted for patients with skin conditions. Patients were asked to rate their level of physical, emotional, social and practical distress experienced through their chronic skin disease, on a 11-point numerical rating scale, ranging from 0 (no distress) to 10 (extreme distress). The number that best represents their level of experienced distress due to their skin disease in the past week is circled (19). The accompanying PL collects information about the nature of distress-related problems rated on the DT, but does not result in a score. The final question of the PL asked whether patients wanted to receive additional professional support for their problems. Other questionnaires in the online tool were the Skindex29, DLQI, Hospital Anxiety and Depression Scale (HADS) and the Patient Global Assessment (PGA). The Skindex29 results in 3 domain scores: symptoms, emotions and functioning and an overall score. Response options range from never (0) to all the time (100) and higher scores indicate lower quality of life (QoL). Cut-off scores have been established that enable categorization of patients with mild (≥ 25), moderate (≥ 32) or severely (≥ 44) impaired QoL (20). The HADS rates the likelihood of anxiety or depression in a score by asking about the most present feelings and complaints during the last week. It is a 14-item questionnaire consisting of 2 domains: depression (HADS-D) and anxiety (HADS-A), comprising 7 items each. Response options range from not at all (0) to most of the time (3). Higher scores indicate more severe anxiety or depression, where a cut-off of 8 has been established for both domains to indicate clinically significant anxiety or depression (21). The DLQI is a widely-used and well-accepted dermatology-specific HRQoL questionnaire, designed for patients aged 16 years and older (22). The questionnaire consists of 10 questions, with 4 answer possibilities ranging from 0 (not at all) to 3 (very much). The DLQI is calculated by summing the score of each question, resulting in a maximum score of 30 and a minimum of 0. The higher the score, the more the QoL is impaired. Patients who declined to complete the information form, with login credentials, were asked for their motivation. To test consistency over time participants were asked to answer the questionnaires a second time 1 week after the first occasion. In view of this relatively short interval and the fact that during this period there were no changes in the patients’ treatment, no large changes were expected. As a reminder to complete the second questionnaire patients were sent an email. All patients provided written informed consent to participate in the study. The study was submitted for review by the institutional review board of the Amsterdam University Medical Centers location Amsterdam Medical Center in Amsterdam (AMC). The review board confirmed that the Medical Research Involving Human Subjects Act (WMO) does not apply to the study.
Statistical analysis
Descriptive statistics were used to describe the characteristics of the study population. Patients were compared with non-respondents on age and sex using Mann–Whitney U and χ2 tests. Frequency distributions were used to assess distress prevalence. Spearman’s correlation coefficients were calculated between the DT scores and the overall and domain scores of the other questionnaires. A correlation of (0.40–0.59), (0.60–0.79) and (0.80–1.0) was considered respectively moderate, strong and very strong. Receiver operating characteristic (ROC) curve analysis was used to determine whether DT scores could distinguish patients identified with skin-related distress according to the Skindex29, using a cut-off of 44. Area under the curve (AUC), sensitivity and specificity coefficients were calculated for the different DT cut-off scores. Test–retest reliability was determined by Wilcoxon’s signed rank test for a subgroup of patients who completed the questionnaire again after 1–2 weeks. Data were analysed using IBM SPSS Statistics Version 25 (Armonk, N.Y., USA), ROC analysis was performed in Graphpad Prism 6 (San Diego, CA, USA).
RESULTS
A total of 214 patients agreed to complete the information form with login credentials to participate in the study. Questionnaires were completed between December 2018 and February 2019. Sixty-seven patients did not participate and did not answer any of the questionnaires; 6 patients were excluded because of missing data, resulting in a final sample of 141 patients (response rate 70%). There were no statistically significant differences regarding age (p < 0.265) or sex (p < 0.519) between respondents and non-respondents. The median age of the study population was 48 years and 52% were female (Table I). The most prevalent skin disease was eczema (21%), followed by psoriasis (11%) and vitiligo (11%).
The median DT score of the current study sample was 5, with an interquartile range (IQR) of 5. Frequency distributions of PL are shown in Table II. The most prevalent reported problems were physical problems, such as problems with itchy/dry skin (59%), physical appearance (52%) and fatigue (52%). The most prevalent emotional problems were self-esteem (40%), tension (41%), depression (39%) and controlling emotions (36%). Practical problems were mostly related to household (27%) and work/school (28%). Family-related and spiritual problems were less prevalent. Despite the high prevalence of various problems, only 3 patients indicated a desire for additional professional support (2 patients chose a psychologist, 1 a psychotherapist, 1 a social worker and 1 someone else (the question about which additional professional support patients desired was a multiple choice question)). Thirty-three patients answered that they might want to be referred.
The levels of HRQoL, anxiety and depression are reported in Table III. Using the defined cut-off scores for severely impaired HRQoL, 30% reported severe problems according to the Skindex29, and 21% reported a large to very large effect on their QoL according to the DLQI. Using a PGA, 24% of patients rated their disease as severe or very severe. Twenty-seven percent of the study population reported clinically significant emotional distress, based on the HADS total score. Clinically significant anxiety was 26% and depression was 23% among the study population.
Correlations between the DT and other questionnaires are shown in Table IV. The highest correlation was found between the Skindex29 and the DT (0.626) and DLQI and DT (0.627). A low correlation was found between the DT and the HADS (0.421).
| Measure | DT | Skindex29 total | Skindex29 symptoms | Skindex29 emotions | Skindex29 functioning | DLQI | HADS | HADS A | HADS D | PGA |
| DT | – | 0.626* | 0.531* | 0.587* | 0.558* | 0.627* | 0.421* | 0.375* | 0.386* | 0.613* |
| Skindex29 total | 0.626* | – | 0.823* | 0.910* | 0.945* | 0.807* | 0.630* | 0.591* | 0.542* | 0.637* |
| Skindex29 symptoms | 0.531* | 0.823* | – | 0.583* | 0.697* | 0.697* | 0.445* | 0.408* | 0.377* | 0.574* |
| Skindex29 – emotions | 0.587* | 0.910* | 0.583* | – | 0.833* | 0.683* | 0.575* | 0.574* | 0.478* | 0.569* |
| Skindex29 – functioning | 0.558* | 0.945* | 0.697* | 0.833* | – | 0.794* | 0.657* | 0.587* | 0.591* | 0.570* |
| DLQI | 0.627* | 0.807* | 0.697* | 0.683* | 0.794* | – | 0.578* | 0.536* | 0.501* | 0.598* |
| HADS | 0.421* | 0.630* | 0.445* | 0.575* | 0.657* | 0.578* | – | 0.898* | 0.897* | 0.312* |
| HADS A | 0.375* | 0.591* | 0.408* | 0.574* | 0.587* | 0.536* | 0.898* | – | 0.632* | 0.259* |
| HADS D | 0.386* | 0.542* | 0.377* | 0.478* | 0.591* | 0.501* | 0.897* | 0.632* | – | 0.274* |
| PGA | 0.613* | 0.637* | 0.574* | 0.569* | 0.570* | 0.598* | 0.312* | 0.259* | 0.274* | – |
| *Correlation is significant p < 0.01. | ||||||||||
ROC curve analyses indicated that the DT was able to discriminate between clinically significant psychosocial distress, based on a Skindex29 cut-off score of 44, with an AUC of 0.813 (SE 0.04, 95% CI 0.74–0.89) (Fig. 1). A cut-off score of 4 on the DT provided the optimal ratio of sensitivity (90.7%) to specificity (56.1%). To assess the course of distress indicated with the DT, patients were grouped into low (0–3), medium (4–6), and high (7–10) DT scores at baseline and were followed over time. The second measurement in this study was 1–2 weeks after the first measurement. Only patients providing data at both time-points were included in this analysis (n = 119). There were no significant differences in DT scores between test and re-test (Wilcoxon signed-rank test, p < 0.229). At the first assessment the median (IQR) DT score was 5 (5), 52 (44%) of patients reported low distress (DT score 0–3), 31 (26%) reported medium distress (DT score 4–6) and 36 (30%) reported high distress (DT score 7–10). One to 2 weeks later, 56 (47%) patients reported low, 28 (24%) reported medium and 35 (29%) reported high distress.
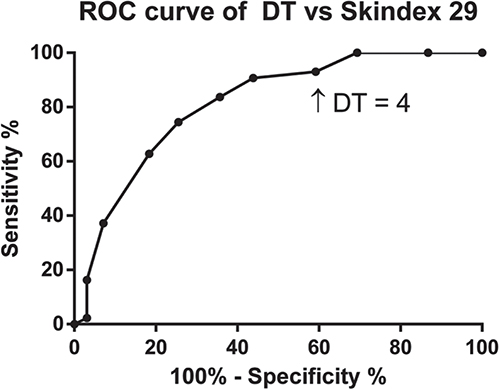
Fig. 1. Receiver operating characteristic (ROC) curve analysis comparing Distress Thermometer (DT) scores to Skindex29.
DISCUSSION
This study validated the DT to detect clinically significant physical, emotional and social distress in patients with chronic skin diseases during outpatient dermatological consultation. The statistical evaluation showed good convergent validity and test–retest reliability. The results showed a high correlation between the DT and the Skindex29. The DT was able to distinguish patients with a high score on the Skindex29 from patients with a low score on the Skindex29. This points out convergent validity with the Skindex29, which has already been validated. Correlation between the DT and the HADS was low. This may indicate that additional factors besides emotional factors, which are rated with the HADS, contributed to the distress rated on the DT. For example, physical complaints, social circumstances and functional impairment rated by the Skindex29. Since the use of a dermatology-specific HRQoL questionnaire may be more appropriate for the dermatology setting and because of the low correlation between the DT and the HADS, the Skindex29 was used as a criterion for the ROC curve analyses instead of the HADS that is commonly used in oncology studies (18). During daily practice the DT can serve as an easy and quick first screener for skin-related distress. When patients score above the threshold on the DT, a more in-depth HRQoL questionnaire can provide more detailed and specific information about the health areas affected by the skin disease and which may be susceptible through therapeutic interventions. This additional questionnaire can be used to reduce false-positives. In previous studies the Skindex29 was recommended because it encompasses all relevant dermatological aspects and domains (23, 24). The domain and the seriousness of problems indicates which kind of support the patient should and wants to receive. This study is, to our knowledge, the first to examine the validity of the DT for patients with a chronic skin disease. The AUC found in the current study shows a good diagnostic value of the DT and is comparable to the AUC found in a meta-analysis of oncology patients (25). This suggests that the DT is a valid screening instrument for skin-related distress in patients with a chronic skin disease. Several studies investigated routine screening with the DT to identify clinically significant distress (18). Values for sensitivity and specificity in this research are in line with values found in literature (26).
Reported cut-off scores on the 0–10 DT scale based on ROC analyses vary by disease, language, country, clinical setting and criterion used (18, 27, 28). Most studies identify a cut-off score of 4, as the current findings also indicate as the most appropriate for screening (18). A DT score of 4 maximizes sensitivity to detect patients with skin-related distress and specificity not to detect non-cases.
Since the current patient population consisted mainly of patients from Amsterdam, and more than half were recruited in a tertiary university hospital, generalizability of the findings may be affected. However, the included percentages of dermatological conditions in this study give a good representation of the general dermatology population (29). Furthermore, the short inclusion period and minimal resources to motivate patients to participate resulted in a modest sample size. Nevertheless, the response rate was good and comparable to similar studies (30, 31). Excluding patients without a computer or internet access could have resulted in selection bias of respondents. For future research it is advised to have a non-digital response option. Age, sex and disease-related characteristics were comparable between responders and non-responders, indicating no sign of response bias.
In order to gain more insight into the nature of the problems rated on the DT the same additional questionnaire, namely the PL, as used in oncology was used in this study. However, it appeared that many problems listed in the PL are not prevalent in patients with chronic skin disease. Therefore, it is not recommended to use the PL in patients with chronic skin diseases. Another aspect of the PL is the question regarding whether a patient wanted to be referred for additional professional support. Three patients in the study answered Yes to this question. This limited number might be explained by patients’ lack of knowledge about what psychosocial support entails, or may illustrate barriers that patients might have. Such a barrier could be the scarce availability, costs of such care or cultural background. Further research is needed to address these issues. Moreover, the referral question contains the word “professional”, but additional support could be given in a stepped care model (32). Primarily, patients can be offered psycho-education, referred to guided self-help websites or to patient associations (33). Secondarily, if needed, the patient could be referred to a professional psychosocial caregiver. Regular screening contributes to preventing unfavourable consequences associated with skin-related distress and creates awareness not only for the physical state, but also for the psychosocial state of a patient, and results in the opportunity to discuss additional support options. Screening for issues affecting HRQoL is recommended by the European Academy of Dermatology and Venereology (EADV) (34). One of the limitations of consequent screening stated by physicians is their perception of increased work burden if questionnaires are used and discussed (35, 36). However, studies show no overall increased work burden for caregivers if one-question HRQoL measures are used (37). Further research is necessary to confirm these findings for the DT in the dermatology setting.
In conclusion, the DT is a valid and reliable screening tool for detecting skin-related distress in patients with a chronic skin disease. The DT is advised to be used as a quick first-stage screener. Evaluation of specific issues of distress should be researched through other in-depth questionnaires. Further research is needed to determine the acceptance and practical feasibility of use of the DT in daily practice.
ACKNOWLEDGEMENTS
We thank the patients who participated in the study, and the staff at the Dermatology Department at Amsterdam University Medical Centers and the Skin Medical Center for cooperating with this study. We also thank the Dutch skin disease patient association (Skin Patients Netherlands) for their support.
Research grant received from the National Health Care Institute the Netherlands.
REFERENCES
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014; 134: 1527–1534.
- Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol 2017; 153: 406–412.
- Evers AW, Crijns MB, Kemperman PM. [A peek inside the field of psychodermatology]. Ned Tijdschr Geneeskd 2013; 157: A5659 (in Dutch).
- Evers AW, Duller P, van de Kerkhof PC, van der Valk PG, de Jong EM, Gerritsen MJ, et al. The Impact of Chronic Skin Disease on Daily Life (ISDL): a generic and dermatology-specific health instrument. Br J Dermatol 2008; 158: 101–108.
- Jafferany M, Pastolero P. Psychiatric and psychological impact of chronic skin disease. Prim Care Companion CNS Disord 2018; 20: 17nr02247.
- Evers AW, Verhoeven EW, Kraaimaat FW, de Jong EM, de Brouwer SJ, Schalkwijk J, et al. How stress gets under the skin: cortisol and stress reactivity in psoriasis. Br J Dermatol 2010; 163: 986–991.
- Hunter HJ, Momen SE, Kleyn CE. The impact of psychosocial stress on healthy skin. Clin Exp Dermatol 2015; 40: 540–546.
- Rodriguez-Vallecillo E, Woodbury-Farina MA. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr Clin North Am 2014; 37: 625–651.
- Fortune DG, Richards HL, Kirby B, McElhone K, Markham T, Rogers S, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol 2003; 139: 752–756.
- Renzi C, Abeni D, Picardi A, Agostini E, Melchi CF, Pasquini P, et al. Factors associated with patient satisfaction with care among dermatological outpatients. Br J Dermatol 2001; 145: 617–623.
- Renzi C, Picardi A, Abeni D, Agostini E, Baliva G, Pasquini P, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol 2002; 138: 337–342.
- Koulil S, Waalboer-Spuij R, Boonstra HE, Casteelen G, Evers AWM, Korte J, et al. [Psychodermatology in research and practice: Developments in academic centers in the Netherlands]. Nederlands Tijdschrift voor Dermatologie en Venereologie 2013; 23: 583–589 (in Dutch).
- David SE, Ahmed Z, Salek MS, Finlay AY. Does enough quality of life-related discussion occur during dermatology outpatient consultations? Br J Dermatol 2005; 153: 997–1000.
- Hermansen SE, Helland CA, Finlay AY. Patients’ and doctors’ assessment of skin disease handicap. Clin Exp Dermatol 2002; 27: 249–250.
- Picardi A, Amerio P, Baliva G, Barbieri C, Teofoli P, Bolli S, et al. Recognition of depressive and anxiety disorders in dermatological outpatients. Acta Derm Venereol 2004; 84: 213–217.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol 2019; 123: 144–151.
- Carlson LE, Bultz BD. Cancer distress screening. Needs, models, and methods. J Psychosom Res 2003; 55: 403–409.
- Donovan KA, Grassi L, McGinty HL, Jacobsen PB. Validation of the distress thermometer worldwide: state of the science. Psychooncology 2014; 23: 241–250.
- Cutillo A, O’Hea E, Person S, Lessard D, Harralson T, Boudreaux E. The distress thermometer: cutoff points and clinical use. Oncol Nurs Forum 2017; 44: 329–336.
- Prinsen CA, Lindeboom R, de Korte J. Interpretation of Skindex-29 scores: cutoffs for mild, moderate, and severe impairment of health-related quality of life. J Invest Dermatol 2011; 131: 1945–1947.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression scale. Acta Psychiatr Scand 1983; 67: 361–370.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
- De Korte J, Mombers FM, Sprangers MA, Bos JD. The suitability of quality-of-life questionnaires for psoriasis research: a systematic literature review. Arch Dermatol 2002; 138: 1221–1227; discussion 1227.
- Both H, Essink-Bot ML, Busschbach J, Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol 2007; 127: 2726–2739.
- Ma X, Zhang J, Zhong W, Shu C, Wang F, Wen J, et al. The diagnostic role of a short screening tool – the distress thermometer: a meta-analysis. Support Care Cancer 2014; 22: 1741–1755.
- Klingenstein A, Samel C, Garip-Kuebler A, Miller C, Liegl RG, Priglinger SG, et al. The national comprehensive cancer network distress thermometer as a screening tool for the evaluation of quality of life in uveal melanoma patients. Acta Ophthalmol 2019; 10.1111/aos.14277.
- Andreu Vaillo Y, Martinez Lopez P, Galdon Garrido MJ. Use of the distress thermometer in cancer survivors: convergent validity and diagnostic accuracy in a spanish sample. Oncol Nurs Forum 2019; 46: 442–450.
- Lim HA, Mahendran R, Chua J, Peh CX, Lim SE, Kua EH. The Distress Thermometer as an ultra-short screening tool: a first validation study for mixed-cancer outpatients in Singapore. Compr Psychiatry 2014; 55: 1055–1062.
- Verhoeven EW, Kraaimaat FW, van Weel C, van de Kerkhof PC, Duller P, van der Valk PG, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med 2008; 6: 349–354.
- San Giorgi MR, Aaltonen LM, Rihkanen H, Tjon Pian Gi RE, van der Laan BF, Hoekstra-Weebers JE, et al. Validation of the distress thermometer and problem list in patients with recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg 2017; 156: 180–188.
- Chambers SK, Zajdlewicz L, Youlden DR, Holland JC, Dunn J. The validity of the distress thermometer in prostate cancer populations. Psychooncology 2014; 23: 195–203.
- van Cranenburgh OD, Smets EM, de Rie MA, Sprangers MA, de Korte J. A Web-based, educational, quality-of-life intervention for patients with a chronic skin disease: feasibility and acceptance in routine dermatological practice. Acta Derm Venereol 2015; 95: 51–56.
- Lavda AC, Webb TL, Thompson AR. A meta-analysis of the effectiveness of psychological interventions for adults with skin conditions. Br J Dermatol 2012; 167: 970–979.
- Finlay AY, Salek MS, Abeni D, Tomas-Aragones L, van Cranenburgh OD, Evers AW, et al. Why quality of life measurement is important in dermatology clinical practice: an expert-based opinion statement by the EADV Task Force on Quality of Life. J Eur Acad Dermatol Venereol 2017; 31: 424–431.
- Velikova G, Awad N, Coles-Gale R, Wright EP, Brown JM, Selby PJ. The clinical value of quality of life assessment in oncology practice-a qualitative study of patient and physician views. Psychooncology 2008; 17: 690–698.
- Detmar SB, Muller MJ, Schornagel JH, Wever LDV, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 2002; 288: 3027–3034.
- Hubbard JM, Grothey AF, McWilliams RR, Buckner JC, Sloan JA. Physician perspective on incorporation of oncology patient quality-of-life, fatigue, and pain assessment into clinical practice. J Oncol Pract 2014; 10: 248–253.