ORIGINAL REPORT
Psoriasis in Norway: A Prescription-based Registry Study of Incidence and Prevalence
1Department of Dermatology, Haukeland University Hospital and 2Department of Clinical Medicine, University of Bergen, Bergen, Norway
Epidemiological data on psoriasis in Norway are limited. The aim of this study was to provide objective national data on the incidence and prevalence of psoriasis. Patients registered in the Norwegian Prescription Database with a diagnostic code indicating psoriasis vulgaris on prescriptions were included. During the period 2004 to 2020, 272,725 patients received prescription for psoriasis vulgaris in Norway. During the period 2015 to 2020, 84,432 patients received prescription for psoriasis vulgaris for the first time. In 2020, 71,857 (97.7%) patients received topical, 7,197 (9.8%) conventional systemic and 2,886 (3.9%) biological medication for psoriasis vulgaris. In the period 2015 to 2020, the point prevalence of psoriasis was 3.8–4.6% and the incidence was 0.29–0.25%. Norway is divided into 4 geographical health regions. A latitudinal difference was observed between the 4 regions, highest in Northern Norway. In the incidence population, median age was 47–53 years and males comprised 46–50%. In this study of psoriasis vulgaris, prevalence in Norway was higher than in earlier reports from other countries. There was a small female predominance regarding incidence and prevalence; however, men had more prescriptions for systemic treatment. Prescriptions for psoriasis vulgaris showed a stable level, with a trend of increasing use of biological medication during the study period.
Key words: psoriasis; incidence; prevalence; Norway; latitudinal difference
SIGNIFICANCE
The aim of this study was to investigate how many people in Norway get psoriasis each year (incidence) and how many live with the disease (prevalence). Data were collected from the Norwegian Prescription Database. For the period 2015 to 2020, 3.8–4.6% of the Norwegian population had a prescription for psoriasis vulgaris. Each year 0.29–0.25% received a prescription for psoriasis vulgaris for the first time. People living in the Northern region of Norway had a higher prevalence of psoriasis than those in the other health regions. Even though slightly more women have psoriasis, men use more systemic medication; tablets and injections. Prescription for psoriasis vulgaris was stable over time, but with increasing use of modern medication (biologics) in recent years.
Citation: Acta Derm Venereol 2023; 103: adv4591. DOI: https://doi.org/10.2340/actadv.v103.4591.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 21, 2023; Published: Apr 19, 2023
Corr: Silje Michelsen Solberg, Department of Dermatology, Haukeland University Hospital, Jonas Lies vei 73, NO-5021 Bergen, Norway. E-mail: solberg_silje@hotmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Epidemiological measures of disease frequency, incidence and prevalence, are the foundation for monitoring disease, defining and evaluating healthcare policy and conducting scientific research (1). Such epidemiological data for psoriasis vulgaris (PsO) in Norway are limited to studies with relatively short duration in the Middle and Northern regions, with self-reported and, to a lesser extent, physician-reported, study data (2–4).
The Norwegian Prescription Database (NorPD; http://www.norpd.no/) is a valid and reliable national data source for studying the use of prescription drugs, based on mandatory pharmacy reporting (5).
The aim of this study is to provide high-quality registry-based estimates of incidence and prevalence of PsO in Norway, in order to determine the epidemiology of PsO in Norway, trends over time and regional differences.
MATERIALS AND METHODS
This registry study was based on data from NorPD, regarding pharmacy-dispensed prescriptions for PsO and the medical specialty of prescribers (dermatologist, rheumatologist or primary practitioner). NorPD includes national data on all pharmacy-dispensed medications on an individual level, dispensing dates, Anatomical Therapeutic Chemical (ATC) code from 2004, International Classification of Disease version 10 (ICD-10) diagnosis codes (secondary care) and International Classification of Primary Care (ICPC) diagnosis codes (primary care) from 2008. It is mandatory for pharmacies to provide monthly reports to NorPD regarding dispensed medications; hence coverage is likely to be complete. In addition, data on birth and death, and data on the general population, were collected from Statistics Norway (www.ssb.no).
The study was approved by the Southeastern Regional Committee for Medical and Health Research Ethics (2021/173689) in Norway.
Study population and data extraction
The study population included all patients in Norway registered in NorPD during the data extraction period 2004 to 2020, who met at least 1 of 4 identification criteria (Table I), e.g. diagnostic code or ATC code indicating PsO on 1 or more prescriptions for topical or systemic treatment (Tables SI and SII). NorPD contains in general 3 characters from ICD-10. In addition, NorPD contains 4 characters (e.g. L40.0) for biological treatment from 2017. Since L40 includes L40.5 used for psoriatic arthritis (PsA), 1 of the 4 criteria was prescription L40 by a dermatologist. In addition, since vitamin D analogues, starting with ATC code D05AX, are prescribed solely for PsO, it was possible also to identify patients with PsO for the period prior to registration of ICD-10 and ICPC in NorPD (2004 to 2008). Similar selection criteria have been used by others (6, 7).
| Identification criteria and prescription/diagnostic codes for PsO | Number of patientsa n (%) |
| Ib: ICD-10 code of L40 prescribed by a dermatologist | 99,839 (36.6) |
| IIc: ICD-10 code of L40.0, L40.8 or L40.9 | 2,446 (0.9) |
| IIId: ICPC-code S91 | 233,906 (85.8) |
| IVe: ATC code starting with D05AX (calcipotriol) | 134,962 (49.5) |
| Number of patients registered with the different criteria and percentage of the total number (%) are listed. aNumber of patients who met 1 of the 4 inclusion criteria. Patients had at least 1 filled prescription with the given codes. bFrom 1 March 2008 NorPD data includes International Classification of Disease version 10 (ICD-10) diagnosis codes on prescriptions from secondary care, 3 characters (e.g. L40). cFor biological treatment, ICD-10 codes with 4 characters (e.g. L40.0) were available in NorPD from 2017. dInternational Classification of Primary Care (ICPC) diagnosis codes are included on prescriptions from primary care from 1 March 2008. eATC codes are available in NorPD from 2004. | |
Norway, located in Northern Europe, spans latitudes 58o to 71o N. The country is divided into 4 geographical health regions. The Northern region covers approximately half of the country’s length, which makes it relevant for studying differences within this long country.
Selection criteria and definitions
Index date was defined as the date a patient met 1 of the identification criteria (Table I).
Incidence population was defined as all patients registered with a PsO prescription for the first time during 2015 to 2020, except for those who had pre-existing PsO prescriptions during 2004 to 2014 (wash-out period), divided by the estimated mid-year general population of Norway in that year.
Prevalence population was defined as all patients registered with a PsO prescription from the first prescription year until the end of follow-up (31 December 2020 or death).
Point prevalence was defined as patients registered with a PsO prescription during the study period until 31 December of the relevant year, divided by the general population at the end of that year.
Actively treated patients were defined as those who collected a PsO prescription in that year.
Statistical analysis
Data are presented descriptively; categorical variables are described as number (n) and percentages (%), and continuous variables as medians with confidence intervals (CI). Incidence and prevalence are presented as total numbers (n), percentages (%) and per 100,000 inhabitants. Python 3.X (Python Software Foundation, Virginia, USA) was used for data handling and analysis. GraphPad Prism 7 (Dotmatics, Boston, USA) was used to plot Fig. 3.
RESULTS
The PsO prevalence population comprised 272,725 patients; most of whom met criterion III, i.e. received a prescription for ICPC S91 (Table I).
The point prevalence for PsO in Norway varied in the period 2015 to 2020 from 3.8% to 4.6%. In 2020, most patients in the prevalence population were in the age range 45–75 years; and there was a female predominance, except for the age groups 40–54 years, which had slightly more males (Fig. 1).
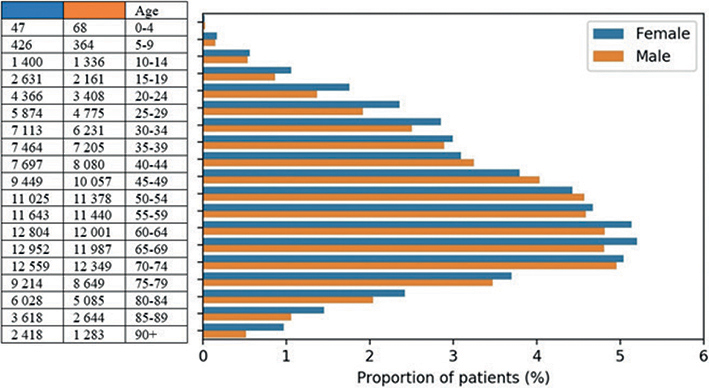
Fig. 1. Age and sex distribution of prevalent patients with psoriasis vulgaris in 2020 (n=249,586). Proportion of patients is shown in the figure and total numbers per age group (years) in the adjacent table.
Differences were observed between the 4 health regions. In 2020, the prevalence of PsO varied between 4.3% in the Middle and West up to 6.1% in the North (Fig. 2).
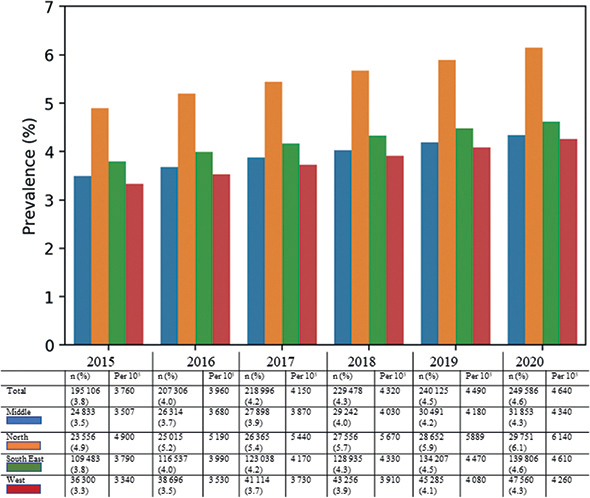
Fig. 2. Point prevalence of psoriasis vulgaris (PsO) by year and health region. Number of patients (n) and percentage of population (%) with prescription for PsO in that region, also listed as patients per 100,000 inhabitants.
Approximately 1.4% of the total population in Norway received prescription for PsO each year; i.e. were actively treated patients (Table II). In 2020, 71,857 (97.7%) patients received topical treatments, 7,197 (9.8%) received conventional systemics, and 2,886 (3.9%) biological medications for PsO. For actively treated patients, the prevalence was also highest in the Northern region (1.8–1.9%) (Table III).
The PsO incidence population consisted of 84,432 patients, with incidence 0.29% in Norway in 2015 with a declining trend to 0.25% in 2020 (Table IV). The incidence in 2020 varied between health regions in the range 0.24–0.32%, being highest in Northern Norway. Interestingly, the incidence, stratified by age and sex in the 4 health regions, showed the same pattern, as shown in Fig. 3.
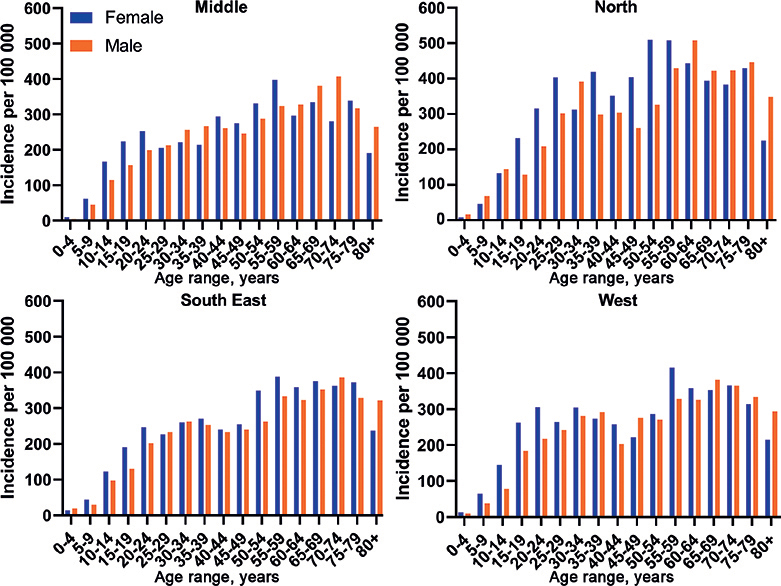
Fig. 3. Incidence of psoriasis vulgaris in the 4 health regions of Norway, stratified by age and sex, displayed per 100,000 inhabitants for 2020. Female: blue; male: orange.
In 2015 to 2020, the median age in the incidence population for the 4 health regions was 47–53 years, and the incidence of PsO for males was a little lower than for females (46–50%) (Table IV). Incidence of PsO, stratified by index year and age, is shown in Fig 4.
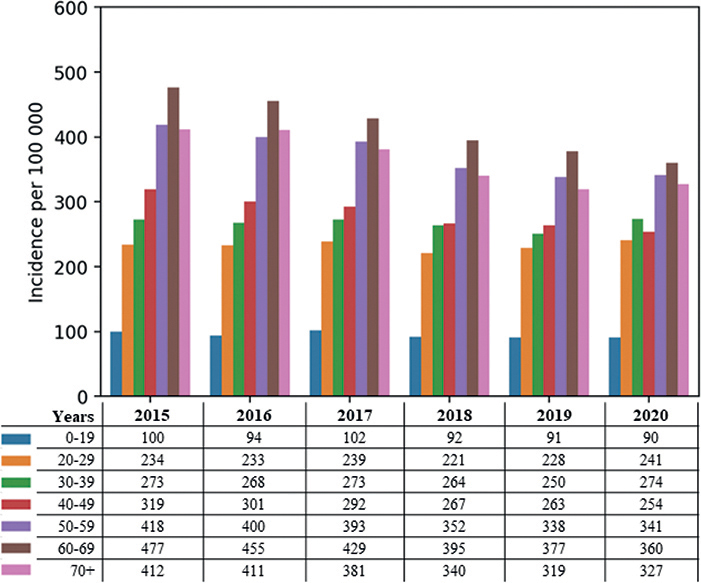
Fig. 4. Incidence of psoriasis vulgaris (PsO) per 100,000 inhabitants by index year, stratified by age.
DISCUSSION
Epidemiological data on PsO in Norway to date are based mainly on health surveys and interviews. PsO incidence data and national prevalence data with objective measurements, including the regions in the West and South East of Norway have not been reported previously. Prescription data can be more reliable than questionnaires, as self-reporting does not always reflect exact diagnosis or treatment, even though validated in selected populations (2, 8). Self-reported use of prescription-only medications has been validated in relation to gold-standard pharmaceutical claims (i.e. dispensing) data, and is found to be useful for identifying exposure to prescription medications for chronic use, but less so for intermittent use (8). Psoriatic skin lesions can be in remission for months or years, hence self-reported use of prescription-only medications may be of less use in these patients (9). Administrative registry data, however, are optimal for studying disease epidemiology over long time-periods. The mandatory monthly reporting from pharmacies to NorPD on collected prescriptions, including diagnosis codes and ATC codes, provides high-quality data with completeness and national coverage. The nationwide population-based design of the current study, from a country with equal access to healthcare for the entire population, is likely to provide highly generalizable results and reflects a strong method for epidemiological studies on PsO.
This study presents analyses of prevalence data from the national prescription database, collected over a period of 17 years. The use of population data results in minimal sampling uncertainty in the point estimates (10). A further strength of the current study is the relatively long wash-out period used (2004 to 2014), in order to exclude from incidence estimates those patients who already had a PsO diagnosis.
The worldwide prevalence of PsO is reported to be 2–3% (11–13). In this Norwegian study, the overall point prevalence for PsO in 2020 was 4.6%. A few studies from the 1980s and 1990s in Norway found the prevalence of PsO to be 1.4–4.2 % (14–16). However, this is lower than self-reported data in the Middle and Northern regions of Norway, on 5.8% and 11.4%, respectively (2, 3). The NorPaPP study found that 11.9% of study participants from Norway had psoriasis in skin and/or joints (17). However, the previously mentioned studies calculated prevalence using health interview surveys, smaller study populations and limited time-periods. In the current study, the point prevalence increased from 3.8% to 4.6% during 2015 to 2020. Nevertheless, due to the length and nature of the data, definite conclusions cannot be drawn that there was an increase in prevalence. Register data from several decades could, with more certainty, overcome variations related to fluctuation in disease activity for this chronic disease, the need for prescriptions, etc.
The incidence of PsO in Norway was 0.29% (288 per 100,000) in 2015 and 0.25% (249 per 100,000) in 2020. This apparent decrease might be explained by a shorter wash-out period for the 2015 index cohort, so that some patients with a previous history of PsO were not excluded in this group. Egeberg et al. (6) found that the incidence of PsO in Denmark fluctuated between 107.5 and 199.5 per 100,000. However, the incidence numbers for Norway are higher, which might be due to different methodology and selection of study population, in addition to geographical differences.
As PsO can be in remission for long time-periods, data on actively treated patients were also included, showing that approximately 1.4% of the Norwegian population collects prescriptions for PsO every year, and this proportion is highest in the Northern region (1.8%).
Occurrence of PsO varies according to geographical region, being more frequent in countries more distant from the equator (11). The Northern region of Norway constitutes approximately half of the country’s length, hence enabling the study of regional differences within this long country. The current study found a latitudinal difference, in which the Northern region had constantly higher prevalence (4.9–6.1%) than the other 3 regions (3.3–4.6%). A previous study found increasing prevalence of PsO in the Northern region (3). The current study found, however, a declining incidence of PsO in all health regions from 2015 to 2020. The incidence of PsO varied between Norway’s 4 health regions and was also highest in the Northern region. However, the distribution of age and sex at incidence was well balanced among the different health regions. Hence, demographic data could not explain the regional discrepancies.
PsO is a chronic disease and onset may occur at any age. For instance, a tendency of step-formation can be seen in the data at the beginning of age group 20–29 years and age group 50–59 years in Figs 3 and 4, which could potentially correspond to age at debut for PsO type 1 and 2, with different genetic susceptibility (12). However, a lower threshold of seeking medical attention and a higher diagnostic “intensity” in younger subjects cannot be ruled out and could explain the first plateau. Higher incidence of PsO in the elderly population, the second plateau, is in line with previous reports (6).
There was a small female predominance for incidence and prevalence in most age groups. In addition to biological factors, it is possible that men do not seek medical treatment for their PsO and therefore go undiagnosed to a greater extent than women. In this study, men received more systemic treatment than women, which might be explained by less use of topical treatment or more severe disease. The fact that men have more severe PsO and receive more systemic treatment than women is well documented (7, 12, 18). The current data on age and sex in PsO also support earlier studies (6, 7, 15, 19).
In the period 2015 to 2020, prescription for topical treatment for PsO was almost stable, with a small decline (99.2–97.7%). Since patients in Norway contact their general practitioner first when they have a medical problem, it was expected that most of the patients had prescriptions with an ICPC code for topical treatment. Systemic treatment, both conventional and biological, is often used as a proxy for moderate to severe disease in PsO. Many newly launched systemic medications, with more specific immunomodulation, have been implemented in the guidelines for treatment (7, 20). Prescription of systemic treatment in the period 2015 to 2020 increased; and biological treatment especially showed a steady increase (2.2–3.9%). This might not reflect that the disease burden of PsO is changing, but rather that new treatment options have the potential to clear skin lesions to a greater extent than topical treatment, phototherapy and conventional systemic medications and is therefore prescribed more frequently (21). The NORPAPP health survey found higher numbers for systemic treatment of PsO, but selection bias and different methodology might explain some of this difference (21). The current study found that patients in the Northern health region received nearly twice as much systemic treatment per 100,000 inhabitants as patients in the 3 other health regions. One possible explanation could be that PsO is more severe in the North due to less sunlight and a colder climate. This limits the use of natural sunlight to reduce skin inflammation, and, in addition, may result in lower levels of vitamin D, which can be of importance in PsO. In addition, genetic predisposition and lifestyle factors may be involved. However, longer travel distance to phototherapy may potentially favour the use of systemic treatment.
Study limitations
In this study, extra care was taken to make inclusion criteria specific for PsO rather than PsA. However, prescription for systemic treatment of PsO could be used to relieve symptoms of arthritis. On the other hand, prescriptions for PsA could mask the need for treatment for skin lesions, resulting in a lower rate of prescription for PsO. Inaccuracy in coding, and misclassification of diagnosis, is expected to be low, with minor variation over the years, as symptoms of PsO are typical and there is often family history associated with onset of the disease. People with very mild and limited PsO, may not collect prescriptions for their skin disease and might therefore not be included in the data. NorPD does not include data from hospitalized patients and nursing homes. Therefore, intravenous administered drugs, i.e. infliximab, are not included, but data from Norwegian Patient Registry (NPR; helsedata.no) revealed that only approximately 150 patients received intravenous medical infusion for PsO in Norway in 2020. Patients receiving phototherapy usually received prescriptions for topical treatment before or adjunctively, and therefore these patients are most likely registered in NorPD. Regarding health regions, only approximately 9% of Norway’s inhabitants live in the Northern health region, hence these data may have more uncertainty than data from larger health regions. In 2020, the COVID-19 pandemic altered doctor-seeking behaviour, medical examination, and healthcare services. It cannot be ruled out that the pandemic affected prescription data. In 2020, the total number of prescriptions was higher than in previous years, but the percentage of prescriptions for PsO for the entire population was the same as in previous years.
Conclusion
This registry study on PsO, based on data from NorPD, found the prevalence in Norway to be 3.8–4.6% and incidence 0.29–0.25% for the period 2015 to 2020. There was a small female predominance regarding the incidence and prevalence of PsO; however, men had more prescriptions for systemic treatment. A latitudinal difference with higher frequencies was observed in the Northern health region. Prescriptions for PsO showed a stable level, with a trend of increasing use of biological treatment during the study period.
ACKNOWLEDGEMENTS
Fredrik Arneberg, project lead for Psoriasis in Norway project (PIN). Sandre S. Lirhus, ML Analyse AS for analyses. Kari Skinningsrud, Limwrick, for valuable input to the writing process.
PIN is a public-private collaborative project between Helse Bergen HF with MD, PhD Silje Michelsen Solberg, Psoriasis and Eczema Association (PEF), 9 pharmaceutical companies (UCB, Novartis, Janssen, Abbvie, Bristol Meyers Squibb, Leo, Lilly, Pfizer, Boehringer Ingelheim) and the Pharmaceutical Industry Association (LMI).
REFERENCES
- Spronk I, Korevaar JC, Poos R, Davids R, Hilderink H, Schellevis FG, et al. Calculating incidence rates and prevalence proportions: not as simple as it seems. BMC Public Health 2019; 19: 512.
- Modalsli EH, Snekvik I, Asvold BO, Romundstad PR, Naldi L, Saunes M. Validity of self-reported psoriasis in a general population: the HUNT Study, Norway. J Invest Dermatol 2016; 136: 323–325.
- Danielsen K, Olsen AO, Wilsgaard T, Furberg AS. Is the prevalence of psoriasis increasing? A 30-year follow-up of a population-based cohort. Br J Dermatol 2013; 168: 1303–1310.
- Kavli G, Forde OH, Arnesen E, Stenvold SE. Psoriasis: familial predisposition and environmental factors. Br Med J (Clin Res Ed) 1985; 291: 999–1000.
- Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol 2010; 106: 86–94.
- Egeberg A, Skov L, Gislason GH, Thyssen JP, Mallbris L. Incidence and prevalence of psoriasis in Denmark. Acta Derm Venereol 2017; 97: 808–812.
- Lindberg I, Lilja M, Geale K, Tian H, Richardson C, Scott A, et al. Incidence of psoriatic arthritis in patients with skin psoriasis and associated risk factors: a retrospective population-based cohort study in Swedish routine clinical care. Acta Derm Venereol 2020; 100: adv00324.
- Gnjidic D, Du W, Pearson SA, Hilmer SN, Banks E. Ascertainment of self-reported prescription medication use compared with pharmaceutical claims data. Public Health Res Pract 2017; 27: 27341702.
- Svedbom A, Mallbris L, Larsson P, Nikamo P, Wolk K, Kjellman P, et al. Long-term outcomes and prognosis in new-onset psoriasis. JAMA Dermatol 2021; 157: 684–690.
- Desbiens NA. The reporting of statistics in medical educational studies: an observational study. BMC Med Res Methodol 2007; 7: 35.
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385.
- Boehncke WH, Schon MP. Psoriasis. Lancet 2015; 386: 983–994.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496–509.
- Braathen LR, Botten G, Bjerkedal T. Prevalence of psoriasis in Norway. Acta Derm Venereol 1989; Suppl 142: 5–8.
- Olsen AO, Grjibovski A, Magnus P, Tambs K, Harris JR. Psoriasis in Norway as observed in a population-based Norwegian twin panel. Br J Dermatol 2005; 153: 346–351.
- Falk ES, Vandbakk O. Prevalence of psoriasis in a Norwegian Lapp population. Acta Derm Venereol 1993: 182: 6–9.
- Danielsen K, Duvetorp A, Iversen L, Ostergaard M, Seifert O, Tveit KS, et al. Prevalence of psoriasis and psoriatic arthritis and patient perceptions of severity in Sweden, Norway and Denmark: results from the Nordic patient survey of psoriasis and psoriatic arthritis. Acta Derm Venereol 2019; 99: 18–25.
- Hagg D, Sundstrom A, Eriksson M, Schmitt-Egenolf M. Severity of psoriasis differs between men and women: a study of the clinical outcome measure psoriasis area and severity index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol 2017; 18: 583–590.
- Snekvik I, Nilsen TIL, Romundstad PR, Saunes M. Psoriasis and cardiovascular disease risk factors: the HUNT study, Norway. J Eur Acad Dermatol Venereol 2018; 32: 776–782.
- Nast A, Smith C, Spuls PI, Valle GA, Bata-Csorgo Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol 2020; 34: 2461–2498.
- Tveit KS, Duvetorp A, Ostergaard M, Skov L, Danielsen K, Iversen L, et al. Treatment use and satisfaction among patients with psoriasis and psoriatic arthritis: results from the NORdic PAtient survey of Psoriasis and Psoriatic arthritis (NORPAPP). J Eur Acad Dermatol Venereol 2019; 33: 340–354.



