REVIEW ARTICLE
Febrile Ulceronecrotic Mucha-Habermann Disease: A Case Report and Review of Literature in the Paediatric Population
Jue LIU1#, Jianbo ZHONG1#, Qiaowei WANG2, Yinglian CAI1 and Jian CHEN1
1Department of Dermatology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine and 2Department of Dermatology, Yes Skin Care Center, Hangzhou, China
#These authors contributed equally to this article.
Febrile ulceronecrotic Mucha-Habermann disease (FUMHD) is a rare fulminant variant of pityriasis lichenoides et varioliformis acuta (PLEVA) that is characterized by a large ulceronecrotic appearance with high fever and a variety of systemic symptoms. We report here a case of FUMHD in a 17-year-old male Chinese patient who was treated successfully with a combination therapy of methotrexate, methylprednisolone, and intravenous immunoglobulin. In addition, a literature review was conducted to summarize the key characteristics of paediatric FUMHD cases.
Key words: febrile ulceronecrotic Mucha-Habermann disease; FUMHD; pityriasis lichenoides et varioliformis acuta; PLEVA.
SIGNIFICANCE
A 17-year-old male patient was diagnosed with febrile ulceronecrotic Mucha-Habermann disease (FUMHD), a rare and severe form of pityriasis lichenoides et varioliformis acuta (PLEVA). The patient was treated with a combination of methotrexate, methylprednisolone, and intravenous immunoglobulin, and a favourable outcome was achieved. A literature review of paediatric cases of FUMHD was performed to summarize the characteristics of this condition in the paediatric population and to emphasize the importance of prompt recognition and appropriate treatment.
Citation: Acta Derm Venereol 2023; 103: adv4806. DOI: https://doi.org/10.2340/actadv.v103.4806.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Mar 15, 2023; Published: Apr 19, 2023
Corr: Jian Chen, Department of Dermatology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China. E-mail: janejane819@sina.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Pityriasis lichenoides et varioliformis acuta (PLEVA), or Mucha-Habermann disease, is the acute form of pityriasis lichenoides characterized by erythematous, scaly papules often accompanied by haemorrhagic and papulonecrotic lesions. First described by Degos in 1966 (1), febrile ulceronecrotic Mucha-Habermann disease (FUMHD), also known as PLEVA fulminans, is considered a rare and severe variant of PLEVA. Patients with FUMHD usually present with typical PLEVA lesions, but progress rapidly into a large ulceronecrotic appearance, accompanied by high fever and a variety of systemic symptoms. FUMHD can be life-threatening.
This paper presents a destructive case of FUMHD in a 17-year-old male, which was treated successfully with a combination therapy of methotrexate (MTX), methylprednisolone and intravenous immunoglobulin (IVIG). FUMHD can occur in individuals of almost any age. To the best of our knowledge, over 100 cases of FUMHD have been published in the literature, with the youngest patient being a 9-month-old boy and the oldest being an 82-year-old woman (2, 3). A previous systemic review demonstrated distinct outcomes between paediatric and adult populations with FUMHD. Adults had a significantly higher mortality rate than children, and mortality rates increased with the age of the patients (4). However, an up-to-date comprehensive evaluation of FUMHD in the paediatric population is lacking. To gain a better understanding of the disease characteristics in this population, a literature review was performed of previously reported paediatric FUMHD cases.
CASE REPORT
A 17-year-old Chinese male was admitted to our department with a 12-day history of an abrupt onset of rapidly spreading erythematous macules, papules and vesicles that gradually developed into central ulceronecrotic and crusting lesions. The eruption initially appeared on the abdomen, with subsequent extension to the chest, back and extremities. The patient reported mild pain and itching at the affected sites and had symptoms of general malaise. He was diagnosed with erythema multiforme at the local hospital and treated with methylprednisolone and compound glycyrrhizin injection. Despite the treatments, the patient’s eruption consecutively disseminated, and he developed fever 1 day prior to admission, with a maximum body temperature of 38.7°C. There was no personal or family history of dermatological diseases. He denied receiving any medications or having an infection before the eruption. He also reported no recent vaccination history.
Physical examination revealed abundant erythematous macules, papules, papulovesicles and papulopustules with central haemorrhagic necrosis. Some of the haemorrhagic necrotic centres were covered with thick crusts, while others had formed erosions and ulcers (Fig. 1). Although different evolutionary stages were present at the same time, older lesions were distributed on the trunk, whereas more fresh lesions developed on the extremities, indicating a progression of the lesions distally. Enlarged lymph nodes were palpated in both armpits.
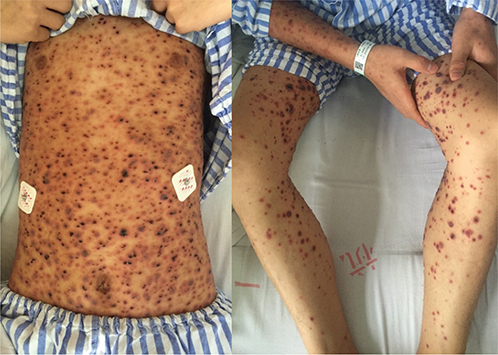
Fig. 1. Generalized ulceronecrotic papules and plaques on hospital day 5.
The laboratory findings at admission showed elevated levels of inflammatory markers, including an erythrocyte sedimentation rate (ESR) of 21 mm/h, a C-reactive protein (CRP) of 47 mg/L, and leukocytosis (10.6×109/L) with 76.8% neutrophils. Repeated blood cultures were sterile. The skin swab cultures, however, tested positive for methicillin-resistant Staphylococcus aureus (MRSA). Other significant laboratory parameters included hypoalbuminemia (33.4 g/L) and occult stool (+). Screening tests for infectious diseases (human immunodeficiency virus, syphilis, hepatitis viruses, Epstein‒Barr virus (EBV) and parvovirus B19) and autoimmunity were negative. Gastroscopy showed superficial atrophic gastritis and gastric ulcers. Skin biopsy from the abdomen was performed on the first day of admission, and histology revealed epidermal parakeratosis, focal epidermal oedema, liquefactive degeneration of basal cells, and perivascular lymphocyte infiltration (Fig. 2). The immunofluorescence test was negative.
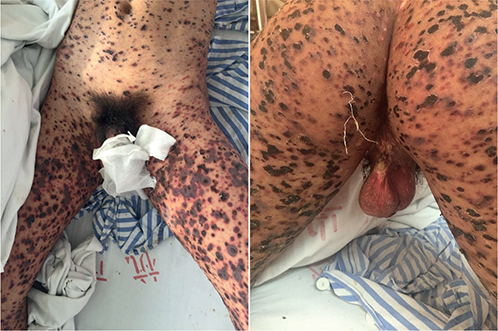
Fig. 2. Histology revealed epidermal parakeratosis, focal epidermal oedema, liquefactive degeneration of basal cells, and perivascular lymphocyte infiltration. HE staining, original magnification ×40.
PLEVA was considered the initial diagnosis, and the patient was treated with intravenous methylprednisolone 60 mg daily and piperacillin sodium and tazobactam sodium. The diagnosis was replaced by FUMHD based on rapid progression, the development of ulceronecrotic lesions and histological examination. Antibiotics were subsequently adjusted to vancomycin and levofloxacin according to skin swab culture of MRSA and the patient’s treatment response. A course of ribavirin for 5 days was also added to treat potential viral infection. Ibuprofen was prescribed for fever. Wound care was provided with topical mupirocin and wet dressing. Despite prompt treatment, the patient’s condition deteriorated, new lesions continued to develop (Fig. 3), and his temperature suddenly jumped to 40.2°C on hospital day 25. His inflammatory markers, such as CRP and procalcitonin (PCT), also peaked. Thus, we administered methylprednisolone pulse therapy (300 mg daily on the first day, 200 mg daily on the second day and 100 mg on the third day), together with 2 doses of methotrexate (15 mg weekly) and IVIG for 7 days. At the same time, the antibiotics were converted to imipenem/cilastatin and minocycline. Major treatments and body temperature are shown in Fig. 4. After the initiation of methylprednisolone pulse therapy, methotrexate and IVIG, the patient’s cutaneous condition stabilized. Although the patient’s skin eruption has demonstrated stabilization, he experienced hepatic impairment on hospital day 37, with an alanine aminotransferase (ALT) level of 533 U/L and an aspartate aminotransferase (AST) level of 80 U/L. Glutathione and polyene phosphatidylcholine were added to protect his liver function, which returned to normal in 1 week. On hospital day 50, most lesions on his trunk became atrophic scars, and lesions on the extremities were either crusted or left with erosion from the crusts peeling off (Fig. 5). The patient was discharged with oral methylprednisolone 24 mg daily on hospital day 52. After discharge, methylprednisolone was gradually tapered off within 6 weeks. There were no new eruptions or any signs of relapse.
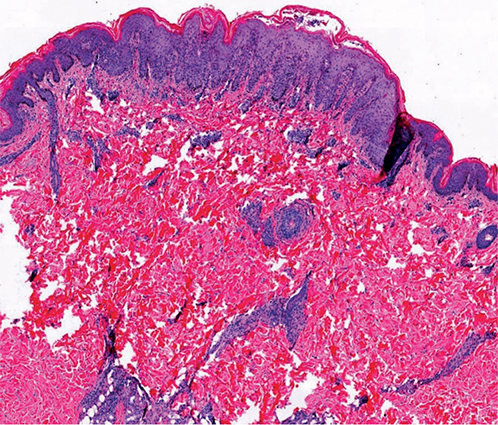
Fig. 3. New ulceronecrotic lesions continued to develop with ulcers in the genital area.
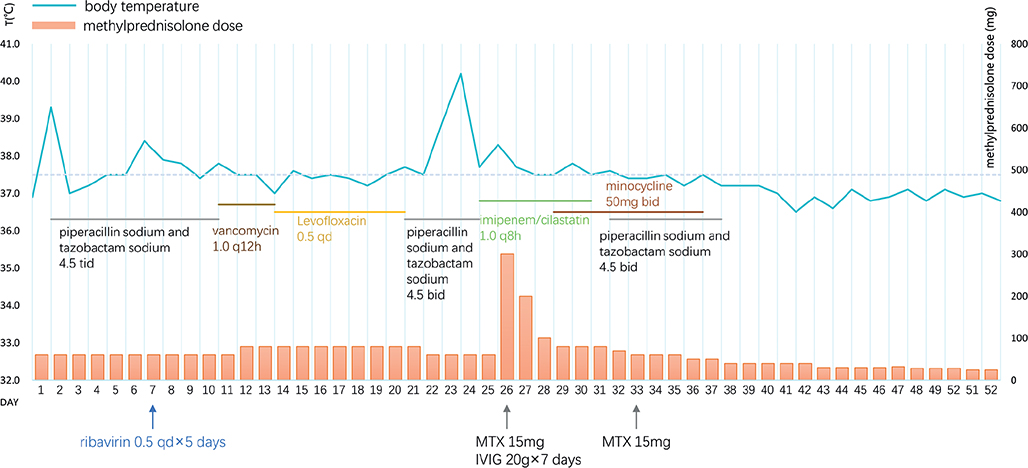
Fig. 4. Major treatments and body temperature. bid: twice daily; tid: three times daily; qd: once daily.
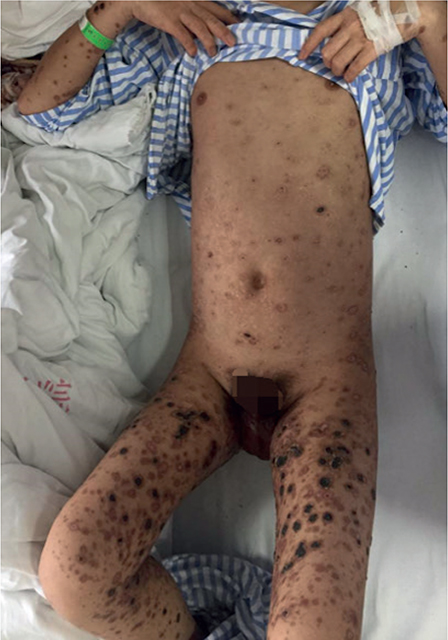
Fig. 5. On hospital day 50, most lesions on the patient’s trunk were atrophic scars, and lesions on extremities were either crusted or left with erosion due to crusts peeling off.
MATERIALS AND METHODS
Search strategy
To further investigate the characteristics of FUMHD in paediatric patients, a comprehensive literature search was conducted in 3 electronic databases: MEDLINE, PubMed and Web of Science. The search covered all available data from the inception of the databases to January 2023 (Appendix S1). A combination of medical subject headings (MeSH) and free-text terms related to FUMHD were used, such as “febrile ulceronecrotic Mucha-Habermann disease”, “ulceronecrotic Mucha-Habermann disease” and “PLEVA fulminans” to create a search strategy finalized for PubMed. This was adjusted for use in other databases. Finally, the reference lists of all relevant articles were reviewed thoroughly to identify any additional potential cases.
Inclusion and exclusion criteria
Cases diagnosed with FUMHD in patients under the age of 18 years were included. Reviews, conference abstracts or posters were excluded. Duplicate cases in different reports were also excluded. Cases not diagnosed with FUMHD or diagnosed with other diseases that looked like FUMHD, or cases that did not reach a distinctive diagnosis were excluded. Cases in which the patient’s age was not precise were excluded. Only reports published in English language were included.
Study selection and data extraction
The process of study selection and data extraction was conducted by 2 review authors (JL and JC).
Duplicate and irrelevant reports were initially screened out based on title and abstract. The full texts of the remaining studies were obtained and thoroughly evaluated by both authors for eligibility criteria. Independent data extraction was carried out by both reviewers, who used Excel to capture relevant information, including demographic information, such as age and sex, aetiology, bacterial culture results, mucosal lesion characteristics, immunohistochemistry and/or cytokines, systemic manifestations and/or laboratory finds, therapeutic interventions, and outcomes. Any discrepancies or disagreements in case selection or data extraction were discussed and resolved through consensus between the 2 reviewers. A flow diagram summarizing the selection process is shown in Fig. 6.
Fig. 6. Selection process. FUMHD: febrile ulceronecrotic Mucha-Habermann disease.
RESULTS
An initial search found 269 references, and an additional citation search identified another 8 records. After removing duplicated records as well as references that did not fulfil the inclusion criteria, 38 records were included for data retrieval. A total of 41 cases of FUMHD in paediatric patients were extracted from the included literature. The clinical features, therapies, and outcomes are summarized in Table I along with a case. Out of the 42 cases, 76.2% (32/42) were male patients, while 23.8% (10/42) were female. The distribution of cases by age group is depicted in Fig. 7. The majority of cases (88%) occurred among children 6 years or older. Five cases of FUMHD were reported in children under 3 years of age, an absence of documented cases in the age group spanning 3 to 5 years old. Two fatal cases were reported, both in male patients, one 9 years old and the other 10 years old.
| Reference | Age/Sex | Possible aetiology | Culture | Mucosal | Immunohistochemistry and/or cytokines | Systemic manifestations and/or laboratory finds | Therapy | Out-come | |
| Skin | Blood | ||||||||
| Burke 1969 (16) | 15 years/M | Uk | E. coli S. aureus Pseudomonas |
– | + | Uk | Lymphadenopathy, anaemia | ATB | Cure |
| Burke 1969 (16) | 12 years/F | Uk | Staphylococci P. mirabilis |
– | – | Uk | – | SS | Cure |
| Auster 1979 (17) | 7 years/F | Adenovirus | S. aureus C. albicans |
– | Uk | Uk | Pulmonary involvement | ATB | Cure |
| Lubertil 1991 (18) | 12 years/M | Uk | S. aureus | S. aureus | – | Uk | Arthritis, sepsis. | ATB, SS, ACV | Cure |
| Fink-Puches 1994 (19) | 16 years/M | Uk | – | – | + | Uk | – | SS, ATB, MTX | Cure |
| Maekawa 1994 (20) | 16 years/M | Uk | E. faecalis Candida spp. |
– | – | Uk | Eosinophilia | ATB, ACV | Cure |
| Romani 1998 (21) | 12 years/F | Uk | Uk | Uk | Uk | Uk | Lymphadenopathy | MTX | Cure |
| Hsieh 2001 (22) | 8 years/F | Uk | Uk | Uk | Uk | Uk | Uk | ATB, UVB, ACV | Cure |
| Ricci 2001 (23) | 10 years/F | Varicella | – | – | – | Uk | – | ACV | Cure |
| Yang 2003 (24) | 14 years/M | EBV | S. aureus | – | + | CD3 + lymphocytic infiltrate | – | SS, ATB | Cure |
| Ito 2003 (25) | 12 years/M | Uk | P. aeruginosa MRSA | – | Uk | CD8 + lymphocytic infiltrate | Abdominal pain, lymphadenopathy, liver dysfunction | DDS, ATB, SS, MTX | Cure |
| Tsianakas 2005 (9) | 9 years/M | Enteritis (no pathogen identified) | Uk | Uk | Uk | Elevated TNF-α, CD8 + lymphocytic infiltrate | Lymphadenopathy, Liver dysfunction | SS, ATB, MTX | Cure |
| Herron 2005 (26) | 8 years/F | Uk | + (pathogen unknown) | P. aeruginosa S. epidermidis C. parapsilosis |
+ | CD3 + T-cell predominant, CD30 +, Elevated sIR-2R | Sepsis, DIC, GI haemorrhage, SIRS, ARDS | ATB, MTX, CyA | Cure |
| Kim 2007 (27) | 8 years/M | Uk | Uk | Uk | Uk | – | Abdominal pain, Liver dysfunction | SS, CyA, ATB | Cure |
| Pyrpasopoulou 2007 (28) | 17 years/F | Uk | Uk | A. baumannii P. aeruginosa S. aureus Candida |
Uk | – | Sepsis, anaemia, diarrhoea | SS, MTX, ATS, IVIG acyclovir | Cure |
| Helbling 2009 (29) | 17 years/M | EBV | – | – | + | – | Lymphadenopathy | ATB, MTX | Cure |
| Zhang 2010 (30) | 12 years/M | Measles vaccine | S. aureus E. coli P. aeruginosa |
– | + | Predominantly CD3 + and CD8 + T cells infiltrate | Pulmonary involvement | SS, ATB, and MTX | Cure |
| Kaufman 2012 (31) | 21 months/F | Uk | Uk | Uk | + | – | Laryngeal oedema | SS, MTX | Cure |
| Kaufman 2012 (31) | 22 months/F | Uk | Uk | Uk | – | – | Anorexia | SS, ATB, ACV, MTX | Cure |
| Perrin 2012 (32) | 34 months/M | Uk | MRSA | – | – | IL-2R at the ULN | Hepatosplenomegaly | SS, ATB, ACV, dapsone, IVIG, MTX | Cure |
| Lin 2012 (33) | 10 years/M | Uk | Uk | – | – | – | Arthritis | SS, MTX | Cure |
| Rosman 2013 (34) | 11 years/M | Uk | Uk | S. aureus | – | Anorexia, sepsis, abdominal pain, CNS vasculitis | SS, ATB, MTX, CP | Cure | |
| Nanda 2013 (35) | 12 years/M | Parvovirus B19 | Uk | Uk | + | CD3 + and CD8 + lymphocytic infiltrate | – | ATB, IVIG, SS, NB-UVB | Cure |
| Luo 2013 (36) | 15 years/F | Uk | Uk | Uk | – | CD8 cells were predominant | Abdominal pain, diarrhoea | SS, ATB, MTX | Cure |
| Hervas 2013 (37) | 9 years/M | Varicella | Normal flora | – | – | – | – | ATB, ACV, SS | Cure |
| Uzoma 2014 (38) | 13 years/M | Uk | Uk | Uk | – | – | Sepsis, anemia. liver dysfunction | SS, ATB, CyA, NB-UVB | Cure |
| Yamada 2014 (4) | 7 years/M | Uk | – | – | – | – | – | ATB, SS | Cure |
| Lode 2015 (8) | 20 months/M | – | – | – | + | Increase in TNF-α and sIL2R, infiltration with CD8 + T cells | – | ACV, ATB, IVIG, CyA, SS | Cure |
| Griffith-Bauer 2015 (15) | 7 years/M | Uk | S. aureus E. coli | – | + | – | – | SS, ATB, and MTX | Cure |
| Griffith-Bauer 2015 (15) | 6 years/M | Uk | – | – | – | – | – | ATB, MTX | Cure |
| Bulur 2015 (39) | 11 years/M | Uk | Uk | Uk | Uk | – | Liver dysfunction | SS, ATB, MTX | Cure |
| Nofal 2016 (12) | 9 years/M | Uk | P. aeruginosa | P. aeruginosa | + | – | Septicaemia | SS, ATB, IVIG, CyA | Fatal |
| Alratrout 2016 (40) | 8 years/M | Uk | Pseudomonas | Uk | Uk | – | Anaemia | MTX, ATB, SS | Cure |
| Kilgallon 2018 (41) | 13 years/M | Uk | S aureus | S aureus | Uk | – | Abdominal pain, diarrhoea, anaemia | MTX, ATB, SS | Cure |
| Wang 2020 (42) | 11 years/M | Uk | A. baumannii | A. baumannii | Uk | – | Sepsis | Lymphoplasma--pheresis, MTX, ATB, SS | Cure |
| Weins 2020 (2) | 9 months/M | – | – | – | + | – | Lymphadenopathy, conjunctivitis | SS, MTX | Cure |
| Souza 2021 (43) | teenager/M | Uk | Uk | S aureus K. pneumoniae |
– | – | Sepsis | SS, MTX, ATB, CyA, IVIG | Cure |
| Wu 2021 (10) | 7 years/M | Streptococcal, influenza A | Uk | – | – | Elevated IL-6, IL-8, IL-10, interferon γ, and TNF-α | Respiratory failure | MTX, CyA, Etanercept, IVIG, Ruxolitinib, Tocilizumab | Cure |
| Blohm 2021 (14) | 13 years/M | Mycoplasma | Uk | – | + | Predominance of CD8+ T cells | Leukopenia, thrombocytopaenia | Antivirals, IVIG, SS, ATB | Cure |
| Tang 2022 (3) | 11 years/M | Uk | MRSA, P. aerogenes | – | – | – | Liver dysfunction | ATB, SS, MTX | Cure |
| Singh 2022 (13) | 10 years/M | Uk, exacerbate by neem and turmeric | P. aeruginosa | P. aeruginosa | – | – | Sepsis, ARDS | ATB, SS, IVIG, MTX, CyA, antifungal | Fatal |
| Present case | 17 years/M | Uk | MRSA | – | + | – | Liver dysfunction | ATB, SS, MTX, IVIG | Cure |
| A. baumannii: Acinetobacter baumannii; ACV: acyclovir; ATB: antibiotics; C. albicans: Candida albicans; CNS: central nervous system; C. parapsilosis: Candida parapsilosis; CyA: cyclosporine; CP: cyclophosphamide; EBV: Epstein‒Barr virus; E. coli: Escherichia coli; E. faecalis: Enterococcus faecalis (then called Streptococcus faecalis); F: female; IVIG: intravenous immunoglobulin; K. pneumoniae: Klebsiella pneumoniae; M: male; MTX: methotrexate; MRSA: methicillin-resistant Staphylococcus aureus; P. aerogenes: Pasteurella aerogenes; P. aeruginosa: Pseudomonas aeruginosa; P. mirabilis: Proteus mirabilis; S. aureus: Staphylococcus aureus; sIL2R: serum soluble interleukin-2 receptor; S. epidermidis: Staphylococcus epidermidis; SS: systemic steroids; TNF-α: tumour necrosis factor alpha; Uk: unknown; ULN: upper limit of normal. | |||||||||

Fig. 7. Number of febrile ulceronecrotic Mucha-Habermann disease cases reported, by age group.
DISCUSSION
In this study of 42 paediatric cases of FUMHD reported in the English language literature, including the current case, it was observed the that, although 5 cases of children under 3 years of age were diagnosed with FUMHD, 4 of these initially presented with a different admitting diagnosis, including Stevens-Johnson syndrome, Kawasaki disease and chickenpox infection. In the case detailed here, as in most cases of children above 6 years of age, the patients predominantly presented with generalized ulceronecrotic skin lesions with flexural accentuation, fever and sometimes mucous membrane and/or systemic involvement. In this age group, the clinical features were more typical and consistent with the diagnostic criteria proposed by Nofal et al. (5). Thus, the results showed that, in the paediatric population, FUMHD is most commonly seen in children over 6 years of age, with a more typical and consistent presentation. In contrast, in those younger than 6 years old, FUMHD is less common, and skin lesions are less typical. The exact aetiology and pathogenesis of FUMHD remain unestablished. However, a possible association with hypersensitivity to an infectious agent, particularly a virus, has been implicated in several reports. In the current review, 21.4% (9/42) of cases presented a potential causative aetiology, which included adenovirus, EBV, parvovirus, varicella zoster virus, measles vaccine, and mycoplasma. Whereas in adults, EBV, CMV (6), and HSV-2 (7) have been suspected as triggers for FUMHD.
Secondary infection was frequently observed in patients with FUMHD. In this review, 50% (21/42) of the paediatric FUMHD population reported positive skin and/or blood cultures, mainly with Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii, Candida, etc. These organisms could exacerbate the condition and may lead to septicaemia and death (5). The underlying mechanism of how infectious agents could trigger or exacerbate FUMHD at the cellular and cytokine levels is not clear. An anecdotal study found that CMV exerts a direct effect on endothelial cells with subsequent dermal necrosis (6). Other studies have observed a predominant CD8+ and/or CD3+ T-cell infiltration in lesional skin, indicating a dysregulated T-cell response following a possible infection trigger (8). Tsianakas’ study associated an increasingly dense perivascular lymphocytic infiltrate and high serum levels of tumour necrosis factor alpha (TNF-α) with the transition from PLEAVA to FUMHD (9). Wu et al. (10) revealed elevated interleukin (IL)-6, IL-8, IL-10, interferon-γ, and TNF-α levels in patients with FUMHD. T-cell clonality has also been implicated in the pathogenesis of FUMHD. It has been proposed that FUMHD with clonality represents a cutaneous T-cell lymphoma entity, and T-cell clonality may be related to disease severity (11). Monoclonal T cells were detected in several fatal cases in adults. However, to the best of our knowledge, only polyclonal T cells have been reported in children, and both fatal paediatric cases did not test T-cell clonality (12, 13). Thus, the prevalence and meaning of T-cell clonality in children with FUMHD require further investigation.
The patient described here presented with extensive mucosal involvement in the genital area. He also had liver dysfunction, lymphadenopathy and secondary infection. In the current literature review, mucous membrane involvement was found in 33.3% (14/42) of cases, and systemic manifestations and/or abnormal laboratory findings were observed in 76.2% (32/42) of cases. Systemic symptoms reported in the paediatric population include abdominal pain, diarrhoea, anaemia, liver dysfunction, lymphadenopathy, pulmonary involvement, arthritis, sepsis, disseminated intravascular coagulation, gastrointestinal (GI) haemorrhage and acute respiratory distress syndrome. Sepsis accounted for 21.4% (9/42) of cases in the paediatric population. Sepsis is considered an independent risk factor for poor prognosis (14). Both fatal cases reported in children were associated with pseudomonas septicaemia (12). In the current patient, high fever with elevated PCT and CRP raised the possibility of sepsis, even though blood culture results were negative. He also experienced an episode of liver dysfunction, which developed after initiation of MTX and a high-dose of corticosteroids highlighting the need to consider both systemic involvement and drug-induced liver impairment. Febrile dermatosis with denuded skin and possibly mucus membrane involvement should be considered in the differential diagnosis, such as Stevens-Johnson syndrome, Kawasaki disease, chickenpox infection, lymphoma, syphilis, vasculitis, and pyoderma gangrenosum.
In the current patient, despite initial treatment with systemic steroids, antibiotics and antiviral therapy, new lesions continued to develop and his overall condition also deteriorated, with high fever and severe pain at the ulceronecrotic sites. Therefore, a treatment regimen was implemented that included methylprednisolone pulse therapy, methotrexate and intravenous immunoglobulin. Within days, the patient became afebrile, new lesions development ceased, and old lesions began to heal. Although no consensus about first-line therapy for FUMHD has been reached as it is a rare condition, a wide variety of treatment modalities have been suggested to be effective, including systemic steroids, MTX, cyclosporine, dapsone, and IVIG. As treatment response differ among patients and patient usually treated with a combination therapy, it is difficult to reach a conclusion on a single treatment. However, as in the current case and other cases that failed initial systemic steroid therapy, MTX showed excellent results (15). Based on the finding of elevated cytokines in FUMHD, biologics have been applied in recent years. Wu et al. (10) successfully treated a FUMHD patient with tocilizumab, an IL-6 inhibitor, and ruxolitinib, a JAK 1/2 inhibitor, according to elevated cytokine levels, suggesting that the use of serum cytokine measurements may be beneficial in the targeted treatment of patients with FUMHD. Relapse was not observed in the current patient, although recurrences that are less severe than the primary episode and recurrent PLEVA have been reported in the literature (10).
In summary, we present here a typical FUMHD case in a 17-year-old boy and summarize the age distribution, superinfection rate, mucosal and systemic involvement, and treatment of paediatric FUMHD cases previously reported in the literature. This emphasizes the crucial role of early diagnosis, thorough evaluations, and appropriate treatment selection for these patients.
ACKNOWLEDGEMENTS
Informed consent for publication of their details was obtained from the patient.
REFERENCES
- Degos R DB, Daniel F. Le parapsoriasis ulcéro-nécrotique hyperthermique. Ann Dermatol Syphiligr (Paris) 1966; 93: 481–496.
- Weins AB, Theiler M, Bogatu B, Kerl K, Pleimes M, Pachlopnik-Schmid J, et al. Febrile ulceronecrotic Mucha-Habermann disease mimicking Kawasaki disease. J Dtsch Dermatol Ges 2020; 18: 140–142.
- Tang P, Chen JS, Wang H, Yang H. Febrile ulceronecrotic Mucha-Habermann disease: a case report and a systematic review. Case Rep Dermatol 2022; 14: 169–177.
- Yamada K, Motegi S-i, Matsushima Y. Febrile ulceronecrotic Mucha-Habermann disease in a young boy: a case report and review of the literature. Acta Derm Venereol 2014; 94: 603–604.
- Nofal A, Assaf M, Alakad R, Amer H, Nofal E, Yosef A. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol 2016; 55: 729–738.
- Tsai KS, Hsieh HJ, Chow KC, Lin TY, Chiang SF, Huang HH. Detection of cytomegalovirus infection in a patient with febrile ulceronecrotic Mucha-Habermann’s disease. Int J Dermatol 2001; 40: 694–698.
- Smith JJL, Oliver GF. Febrile ulceronecrotic Mucha-Habermann disease associated with herpes simplex virus type 2. J Am Acad Dermatol 2009; 60: 149–152.
- Lode HN, Döring P, Lauenstein P, Hoeger P, Dombrowski F, Bruns R. Febrile ulceronecrotic Mucha-Habermann disease following suspected hemorrhagic chickenpox infection in a 20-month-old boy. Infection 2015; 43: 583–588.
- Tsianakas A, Hoeger PH. Transition of pityriasis lichenoides et varioliformis acuta to febrile ulceronecrotic Mucha-Habermann disease is associated with elevated serum tumour necrosis factor-α. Br J Dermatol 2005; 152: 794–799.
- Wu R, DiLorenzo A, Lotke M, Habeshian K, Brooks J, Keller MD, et al. Evaluation and treatment of febrile ulceronecrotic mucha-habermann disease with ruxolitinib and tocilizumab as guided by cytokine profile. JAMA Dermatol 2021; 157: 1381–1383.
- Cozzio A, Haffner J, Kempf W, Haffner A, Palmedo G, Michaelis S, et al. Febrile ulceronecrotic Mucha-Habermann disease with clonality: a cutaneous T-cell lymphoma entity? J Am Acad Dermatol 2004; 51: 1014–1017.
- Nofal A, Alakad R, Assaf M, Nofal E. A fatal case of febrile ulceronecrotic Mucha-Habermann disease in a child. JAAD Case Rep 2016; 2: 181–185.
- Singh G, Arora S, Das P, Gupta A. A fatal case of febrile ulceronecrotic mucha-habermann disease in a 10-year-old boy. Indian Dermatol Online J 2022; 13: 535–538.
- Blohm ME, Ebenebe CU, Rau C, Escherich C, Johannsen J, Escherich G, et al. Mucha-Habermann disease: a pediatric case report and proposal of a risk score. Int J Dermatol 2021; 61: 401–409.
- Griffith-Bauer K, Leitenberger SL, Krol A. Febrile ulceronecrotic Mucha-Habermann disease: two cases with excellent response to methotrexate. Pediatr Dermatol 2015; 32: e307–e308.
- Burke DP, Adams RM, Arundell FD. Febrile ulceronecrotic mucha Habermann’s disease. Arch Dermatol 1969; 100: 200–206.
- Auster BI, Santa Cruz DJ, Eisen AZ. Febrile ulceronecrotic Mucha-Habermann’s disease with interstitial pneumonitis. J Cutan Pathol 1979; 6: 66–76.
- Luberti AA, Rabinowitz LG, Ververeli KO. Severe febrile Mucha-Habermann’s disease in children: case report and review of the literature. Pediatr Dermatol 1991; 8: 51–57.
- Fink-Puches R, Soyer HP, Kerl H. Febrile ulceronecrotic pityriasis lichenoides et varioliformis acuta. J Am Acad Dermatol 1994; 30: 261–263.
- Maekawa Y, Nakamura T, Nogami R. Febrile ulceronecrotic Mucha-Habermann’s disease. J Dermatol 1994; 21: 46–49.
- Romani J, Puig L, Fernandez-Figueras MT, de Moragas JM. Pityriasis lichenoides in children: clinicopathologic review of 22 patients. Pediatr Dermatol 1998; 15: 1–6.
- Hsieh CG CY, Ho JC, Huang SC. Febrile ulceronecrotic Mucha-Habermann’s disease. Dermatoogical Sinica 2001; 19: 233–242
- Ricci G, Patrizi A, Misciali D, Masi M. Pathological case of the month. Febrile Mucha-Haberman disease. Arch Pediat Adol Med 2001; 155: 195–196.
- Yang CC, Lee JY, Chen W. Febrile ulceronecrotic Mucha-Habermann disease with extensive skin necrosis in intertriginous areas. Eur J Dermatol 2003; 13: 493–496.
- Ito N, Ohshima A, Hashizume H, Takigawa M, Tokura Y. Febrile ulceronecrotic Mucha-Habermann’s disease managed with methylprednisolone semipulse and subsequent methotrexate therapies. J Am Acad Dermatol 2003; 49: 1142–1148.
- Herron MD, Bohnsack JF, Vanderhooft SL. Septic, CD-30 positive febrile ulceronecrotic pityriasis lichenoides et varioliformis acuta. Pediatr Dermatol 2005; 22: 360–365.
- Kim HS, Yu DS, Kim JW. A case of febrile ulceronecrotic Mucha-Habermann’s disease successfully treated with oral cyclosporin. J Eur Acad Dermatol Venereol 2007; 21: 272–273.
- Pyrpasopoulou A, Athyros VG, Karagiannis A, Chrysomallis F, Zamboulis C. Intravenous immunoglobulins: a valuable asset in the treatment of a case of septic febrile ulceronecrotic Mucha-Habermann disease. Dermatology 2007; 215: 164–165.
- Helbling I, Chalmers RJ, Yates VM. Febrile ulceronecrotic Mucha-Habermann disease: a rare dermatological emergency. Clin Exp Dermatol 2009; 34: e1006–1007.
- Zhang LX, Liang Y, Liu Y, Ma L. Febrile ulceronecrotic Mucha-Habermann’s disease with pulmonary involvement. Pediatr Dermatol 2010; 27: 290–293.
- Kaufman WS, McNamara EK, Curtis AR, Kosari P, Jorizzo JL, Krowchuk DP. Febrile ulceronecrotic Mucha-Habermann disease (pityriasis lichenoides et varioliformis acuta fulminans) presenting as Stevens-Johnson syndrome. Pediatr Dermatol 2012; 29: 135–140.
- Perrin BS, Yan AC, Treat JR. Febrile ulceronecrotic Mucha-Habermann disease in a 34-month-old boy: a case report and review of the literature. Pediatr Dermatol 2012; 29: 53–58.
- Lin CY, Cook J, Purvis D. Febrile ulceronecrotic Mucha-Habermann disease: a case with systemic symptoms managed with subcutaneous methotrexate. Australas J Dermatol 2012; 53: e83–e86.
- Rosman IS, Liang L-C, Patil S, Bayliss SJ, White AJ. Febrile ulceronecrotic Mucha-Habermann disease with central nervous system vasculitis. Pediatr Dermatol 2013; 30: 90–93.
- Nanda A, Alshalfan F, Al-Otaibi M, Al-Sabah H, Rajy JM. Febrile ulceronecrotic Mucha-Habermann disease (pityriasis lichenoides et varioliformis acuta fulminans) associated with parvovirus infection. Am J Dermatopathol 2013; 35: 503–506.
- Luo DQ. Febrile ulceronecrotic Mucha-Habermann disease. Cutis 2013; 92: E9–E12.
- Hervas JA, Martin-Santiago A, Hervas D, Gomez C, Duenas J, Reina J. Varicella precipitating febrile ulceronecrotic Mucha-Habermann disease. Pediatr Dermatol 2013; 30: e216–217.
- Uzoma MA, Wilkerson MG, Carr VL, Westhoven GS, Raimer SA. Pentoxifylline and cyclosporine in the treatment of febrile ulceronecrotic Mucha-Habermann disease. Pediatr Dermatol 2014; 31: 525–527.
- Bulur I, Kaya Erdoğan H, Nurhan Saracoglu Z, Arık D. Methotrexate treatment in children with febrile ulceronecrotic Mucha-Habermann disease: case report and literature review. Case Rep Dermatol Med 2015; 2015: 357973.
- Alratrout J, Alshammasi F, Ansari N. Febrile ulceronecrotic Mucha–Habermann disease in an 8-year-old boy responding to methotrexate. Int J Dermatol 2016; 55: 1205–1209.
- Kilgallon K, Urs J, Fernandez Faith E. Visual diagnosis: severe ulceronecrotic eruption with systemic symptoms. Pediatr Rev 2018; 39: e54–e56.
- Wang B, Li J, Xie H-f, Chen M, Li B, Shi W. Striking case of febrile ulceronecrotic Mucha-Habermann disease responding to lymphoplasmapheresis and methotrexate. J Dermatol 2020; 47: E430–E431.
- Schaan de Souza M, Perinazzo Pauvels LS, Martins Costa Jappur D, Poy Dondonis F, da Silva M, da Silva Cartell A, et al. Combination therapy with cyclosporine and intravenous immunoglobulin for febrile ulcerative Mucha-Habermann disease. Dermatol Ther 2021; 34: e14655.