ORIGINAL REPORT
Malassezia restricta-mediated Lipoperoxidation: A Novel Trigger in Dandruff
Roland JOURDAIN1, Alain MOGA2, Prokopios MAGIATIS3, Maxime FONTANIÉ4, Aristea VELEGRAKI5, Chrysanthi PAPADIMOU3, Valérie RAHOUL2, Audrey GUÉNICHE1, Tarun CHOPRA6 and George GAITANIS7,8
1L’Oréal Research & Innovation, Aulnay-sous-Bois, 2Synelvia SAS, Labège, France, 3National and Kapodistrian University of Athens, Department of Pharmacognosy and Natural Products Chemistry, Faculty of Pharmacy, Athens, 4VibioSphen SAS, Labège, France, 5Bioiatriki SA, Athens, Greece, 6L’Oréal Research & Innovation, Singapore, 7University of Ioannina, Department of Dermatology, Ioannina, Greece and 8DELC Clinic, Biel, Switzerland
Dandruff is a common scalp disorder with multiple microbial and host-related factors contributing to its aetiology, including alterations in scalp sebum. Despite existing evidence that the yeast Malassezia restricta plays a key role in the onset of dandruff, the interplay of these factors is poorly understood. Recently, squalene monohydroperoxide and malondialdehyde were established as biomarkers of dandruff-afflicted scalp, highlighting the role of sebum lipoperoxidation in the triggering and maintenance of dandruff, although its mechanism of action is unknown. The current study provides evidence that M. restricta mediates sebum peroxidation, leading to production of squalene monohydroperoxide and malondialdehyde. Furthermore, in vitro data show that these lipoperoxidation products act on epidermal cells and alter the skin barrier. These results support the role of Malassezia restricta-induced lipoperoxides as triggers of dandruff, which suggests that blocking their production could be a novel anti-dandruff treatment approach.
Key words: barrier function; dandruff; lipoperoxidation; Malassezia restricta; malondialdehyde; sebum; squalene monohydroperoxide.
SIGNIFICANCE
Using different in vitro methods, this study showed the ability of the yeast Malassezia restricta to oxidize squalene to squalene monohydroperoxide and produce malondialdehyde. These results are linked with previous in vivo findings that demonstrated higher quantities of M. restricta and higher levels of squalene monohydroperoxide and malondialdehyde on dandruff scalp surface. In addition, the current study showed that squalene monohydroperoxide and malondialdehyde induce dandruff-linked damage on skin models. These findings add to the understanding of a causal role of Malassezia restricta in dandruff and point to the possibility of blocking Malassezia restricta-induced lipoperoxidation as a novel treatment approach for dandruff.
Citation: Acta Derm Venereol 2023; 103: adv00868. DOI: https://doi.org/10.2340/actadv.v103.4808.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jan 19, 2023; Published: Feb 15, 2023
Corr: Roland Jourdain, L’OREAL Research & Innovation – Advanced Research, 1 avenue Eugène Schueller, FR-93600 Aulnay sous Bois, France. E-mail: roland.jourdain@loreal.com
Competing interests and funding: RJ, AG and TC are L’OREAL SA employees. AM and VR work for Synelvia SAS. MF works for VibioSphen SAS. PM, and CP work at National and Kapodistrian University of Athens. AV works for Bioiatriki SA. GG works at University of Ioannina and at DELC Clinic.
All funding was provided by L’OREAL Research & Innovation. National and Kapodistrian University of Athens, University of Ioannina, Synelvia SAS and VibioSphen SAS received funding from L’OREAL SA for this study.
INTRODUCTION
Dandruff is a common scalp disorder that affects almost half of the adult human population at some time in their lives (1). Dandruff is often considered to be a mild form of scalp seborrhoeic dermatitis. The appearance of flakes is a key clinical characteristic of dandruff and is a result of structural and functional alterations in the epidermis, including altered corneocyte cohesion, abnormal desquamation, hyperproliferation, hyperkeratosis and presence of parakeratotic cells in the upper stratum corneum (SC) (2–5). While the mechanisms that lead to the onset of dandruff are not entirely understood, proteomics studies indicate that dandruff is associated with alterations in proteins related to structural maturation and epidermal differentiation, such as transglutaminase 3 (TGM3) and filaggrin (FLG), as well as proteins involved in antioxidant defence mechanisms, such as catalase and [Cu–Zn] superoxide dismutase (6). Furthermore, compared to dandruff-free scalp areas, dandruff zones also present a dysregulated microbiome, characterized by a lower abundance of Cutibacterium acnes and higher abundance of Malassezia restricta and Staphylococcus spp. as observed in several populations around the world (7–9). Current efficacious anti-dandruff treatments target Malassezia as the main effector of dandruff (3). These observations are in line with the suggestion that dandruff and its more severe and inflammatory counterpart, scalp seborrhoeic dermatitis, are, at least in part, caused by chemical triggers potentially released by Malassezia (2).
In this area of research, squalene monohydroperoxide (SQOOH) and malondialdehyde (MDA) are of particular interest as they have been found in higher amounts on dandruff scalp areas (10). Squalene (SQ) is a lipid species unique to human skin and constitutes up to 12% of the sebum lipids (11). It comprises part of the antioxidant reservoir of the skin, and its relative amount is characteristically reduced in dandruff plaques (10), with higher amounts converted to SQOOH and an associated decrease in antioxidant reserves. Apart from being an early biomarker of lipoperoxidation, SQOOH induces the production of inflammatory mediators in HaCaT cells, which results in cellular hyper-proliferation (12) and leads to cutaneous alterations, such as hyperkeratosis and hyperpigmentation, when applied to guinea pig skin in vivo (13). MDA, on the other hand, is a late biomarker of lipoperoxidation present in higher amounts on both dandruff and scalp seborrhoeic dermatitis (10, 14) and has been linked, amongst others, to atopic dermatitis, neurological diseases (Alzheimer’s and Parkinson’s diseases), diabetes, cancer and cardiovascular diseases (15, 16).
While these findings provide important insights into dandruff aetiology, they also raise questions about the chain of events that lead to this scalp disorder and how the interplay of these factors leads to the appearance and perpetuation of dandruff. Using 2-dimensional nuclear magnetic resonance (2D NMR) and mass spectrometric approaches, this study examined whether M. restricta, which is abundantly present on dandruff scalp, leads to the production of SQOOH and MDA. Using a reconstructed human epidermis (RHE) model, the study further examined whether SQOOH and MDA cause dandruff-like scalp barrier alterations. The findings reported here broaden the preliminary data we have previously presented as a poster (17). The aim of the study is to elucidate the pathobiological role of Malassezia restricta in dandruff, which could lead to a novel targeted anti-dandruff approach involving blocking the damage to the skin due to Malassezia restricta-induced lipoperoxidation.
MATERIALS AND METHODS
Two-dimensional nuclear magnetic resonance experiments
Synthesis and purification of squalene monohydroperoxide. NMR experiments were conducted in the Department of Pharmacognosy and Natural Products Chemistry, Athens, Greece. Squalene hydroperoxides were prepared by photo-oxidation according to a previously reported method with minor modifications (18). A flask containing a mixture of organic solvents with SQ (1.287 g, ≥98%, Sigma Aldrich, Burlington, MA, USA) and rose Bengal (10 mg, >95%, ref: 330000, Merck, Darmstad, Germany) was closed with an elastic SUBA-SEAL® stopper (Sigma Aldrich, Burlington, MA, USA) permitting continuous air bubbling in the reaction mixture through a stainless-steel needle. The reaction mixture was stirred for 8 h under continuous mixed (ultraviolet, visible, and infrared) irradiation from a halogen lamp (400 Watt) placed at 50 cm distance. At the end of the reaction, the solvents were evaporated under reduced pressure and the residue was dried with a vacuum pump. The dried residue was subjected to thin-layer chromatographic purification on aluminium plates coated with silica (Silica gel 60 F254, Merck, Darmstad, Germany), normal phase, and cyclohexane–ethyl acetate (9:1) as mobile phase. The production of squalene peroxides was confirmed by the characteristic pink colour produced after spraying with 1% N,N-diethyl-1,4-phenylenediamine sulphate (DEPD) solution and exposure to heat (150°C) (19). The major isomer that was isolated was 2-SQOOH (the chemical structure is shown in Fig. 1). The complete NMR spectrum of 2-SQOOH is shown in Fig. S1. Fig. S2 shows the characteristic cross peaks of 2-SQOOH. In particular, the characteristic squalene cross peaks for 2-SQOOH appeared at 5.67 and 2.72 ppm. 2-SQOOH was the isomer used for evidencing the ability of M. restricta to transform SQ into SQOOH.
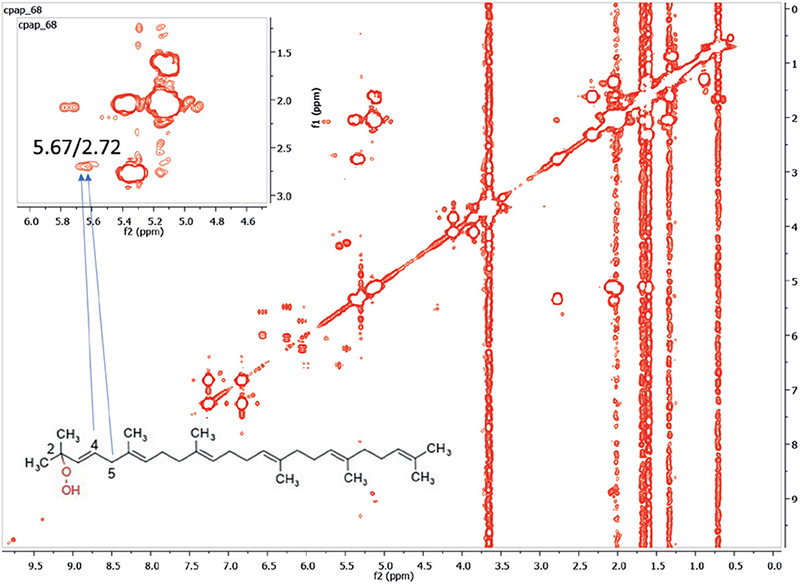
Fig. 1. Two-dimensional correlation spectroscopy (COSY) spectrum (CDCl3) of Malassezia restricta culture with linoleic acid and squalene. X and y axes labelled as ppm, the standard measurement unit for chemical shift in NMR. The cross-peaks at 5.67/2.72 ppm between H-4 and H-5 are proof of the presence of squalene 2-monohydroperoxide (2-SQOOH). A negligible amount of 2-SQOOH was observed in the control condition without M. restricta (Fig. S3).
Culture medium and extraction protocol. The strain M. restricta CBS 7877 was used for all experiments. To produce the required biomass for the subsequent experiments, a loopful from a fresh culture was incubated in 9-cm Petri dishes containing modified Dixon’s medium (mDixon, ATCC Medium 2693) for 7 days at 32°C. M. restricta CBS 7877 was cultured in a 200 ml conical flask containing 3.6% malt extract (Oxoid ref.: LP0039) / 0.6% mycological peptone (Millipore ref.: 77199) / 2% bile salts (BD Biosciences ref.: DF0130-17-4) / 1% Tween 40 (Sigma ref.: P1504) / 0.2% glycerol (Sigma ref.: G9012) / 0.2% oleic acid (Sigma ref.: 75096) / 0.4% Triton-X 100 (Sigma-Aldrich ref.:T8787) / 1 L distilled water / ±lipid combination. The lipid combination included 1% SQ with 1% linoleic acid (LA) (Sigma-Aldrich ref.: L1376). The conical flask was inoculated with the content of 3 Petri dishes after 7 days of incubation at 32°C. Dichloromethane (100 ml) was added to the conical flask, sealed and placed in an ultrasonic bath for 30 min. Then, the flask content was filtered, and the filtrate was concentrated in vacuum using a rotary evaporator. The concentrate was exhaustively dried in a vacuum pump; then a portion was dissolved in deuterated chloroform and analysed by 1D and 2D NMR.
Liquid Chromatography coupled to tandem Mass Spectrometry (LC-MS/MS) and Gas Chromatography-Mass Spectrometry (GC/MS) experiments
Preparation of M. restricta supernatants. The LC-MS/MS and GC/MS experiments and those on RHE models described below were conducted by Synelvia SAS and VibioSphen SAS (Labège, France). M. restricta CBS 7877 stock was transferred in 100 ml of mDixon medium and incubated, in a dark chamber, for 1 day at 32°C under agitation (250 rpm) until the culture reached an optical density (OD) of ~12 (read at 570 nm). At this point, the culture was in the exponential phase, with ~1.108 cells/ml. To check Malassezia growth, 100 µl liquid culture was plated on modified Leeming & Notman agar (MLNA, ATCC Medium 2737). At this stage, SQ and LA acid were diluted in DMSO before the addition of olive oil. A 100 µl volume of this lipidic solution containing 20% SQ and 2% LA were added to 900 µl M. restricta culture. This lipid solution without SQ was added to the control mDixon + M. restricta. After 2, 6 and 24 h, M. restricta supernatants were prepared by centrifugation at 3,500 rpm for 5 min at 4°C and frozen until quantification of SQOOH and MDA in supernatants, which was carried out within 1 week of sample preparation. In order to independently confirm this key part of the study, 2 sets of M. restricta supernatants (supernatants A then supernatants B) were successively produced with the aforementioned procedure from 2 independent cultures of M. restricta (cultures A and B). SQOOH and MDA were quantified by mass spectrometry in M. restricta supernatants A, obtained by centrifugation of culture A 2, 6 and 24 h after addition of SQ. They were also quantified in M. restricta 24 h supernatant B, obtained by centrifugation of culture B 24 h after addition of SQ. Both M. restricta 24 h supernatants A and B were used in the 2 different sets of experiments on RHE models and are mentioned accordingly below.
Squalene monohydroperoxide quantification by Liquid Chromatography coupled to tandem Mass Spectrometry (LC-MS/MS). Samples were extracted using a proprietary double liquid/liquid extraction method, evaporated under nitrogen at 60°C, and the residue was resuspended in 100 µl methanol. As no commercially available SQOOH standards were available for chromatographic analysis, an in-house standard was synthesized using a procedure based on SQ photo-oxygenation in the presence of Rose Bengal (Sigma-Aldrich, ref.: 330000-5G) as photosensitizer (18). An UltiMate 3000 (Dionex, Sunnyvale, CA, USA) liquid chromatography system coupled to a MSQ Plus detector (Fisher Scientific, Waltman, MA, USA) was used for the detection of SQOOH. In the used Agilent 1100 Series LC-MSD system (Agilent Technologies, Avondale, PA, USA), 2 mobile phases [M1, water] and [M2, acetonitrile] were used at a flow rate of 0.2 ml/min. The mobile phases were programmed consecutively, as follows: an isocratic elution of 50% M1 from 0 to 15 min, a linear gradient of 50–0% M1 between 15 and 20 min, an isocratic elution of 0% M1 for 45 min, and an isocratic elution of 50% M1 from 45 to 60 min for column equilibration. Injection volume was 20 µl, and column temperature was maintained at 40°C. For MS detection, atmospheric pressure chemical ionization was used as the ion source, with the probe temperature set at 500°C, and a cone voltage of 50 V. Positive ion spectra were recorded in the range 50–450 m/z.
Malondialdehyde quantification by Gas Chromatography-Mass Spectrometry (GC/MS). Samples were extracted into a buffer lysis and centrifuged at 20,000 g for 5 min. Acid hydrolysis and pentafluorobenzyl (PFB) derivatization was performed, followed by liquid/liquid extraction, with the organic phase dried under nitrogen at 60°C. Residues were resuspended in 50 µl hexane. Gas spectrometry and separation was achieved using a 30 m × 0.25 mm × 0.10 µm Zebron ZB-5HT Inferno GC (Phenomenex, Torrance, CA, USA) capillary column, and helium was used as a carrier gas at a constant flow of 1 ml/min. Both injector and transfer line temperatures were set at 250°C. Pulsed splitless mode (25 psi pulse) was used (20). Oven temperatures started with a 30 s hold at 50°C, increased to 180°C at 25°C/min; then to 250°C at 5°C/min; to 300°C at 25°C/min, with a final hold for 10 min. Negative ion chemical ionization with methane reagent gas was performed using selected ion monitoring (SIM).
Reconstructed human epidermis morphology and permeability
Reconstructed human epidermis treatment. SkinEthicTM RHE model (Episkin, Lyon, France) at day 17 was used, corresponding to a high stage of maturity. RHE 3D skin models reproduce epidermal morphology, are a validated alternative to animal testing for certain toxicological assessments and represent the state-of-art for pre-clinical testing (21, 22). To assess the effects of M. restricta supernatant, SQOOH and MDA on RHE morphology and permeability, 50 µl of the following solutions diluted at 20% in sterile water were topically applied on RHE: mDixon + SQ 2% (culture medium of M. restricta used as control), M. restricta 24 h supernatant A, SQOOH at 180 ng/ml in mDixon + SQ 2%, MDA at 50 ng/ml in mDixon + SQ 2%, SQOOH at 180 ng/ml + MDA at 50 ng/ml in mDixon + SQ 2%. RHEs were treated the day after their reception, which corresponds to day 18 of tissue reconstruction. Three RHEs were used for each condition tested and 3 others were treated with sterile water as control. After 24 h of contact with the different solutions, RHE were processed for assessment of their morphology and permeability.
Hematoxylin and eosin (H&E) staining for morphology. RHE were fixed with 4% formaldehyde containing PBS buffer. Sections of paraffin-embedded RHE were used for H&E staining (21).
Lucifer yellow diffusion. For analysis of lucifer yellow (LY) permeability, 200 µl of 1 mmol/L lucifer yellow (Sigma-Aldrich) were added onto the reconstructed human epidermis at day 19. After incubation at 37°C for 0.5, 1, 2, 4 up to 6 h, dye concentration in the culture medium corresponding to data not shown in the paper was measured fluorometrically in a Fluoroskan Ascent (Thermo Scientific, Waltham, MA, USA) with excitation and emission at 425 and 550 nm, respectively (23). After 6 h of incubation, the RHE were fixed in 4% formaldehyde and embedded in paraffin. Nuclei were stained with DAPI (Sigma-Aldrich), and the slides were mounted with Mowiol 4-88 (Merck). Sections were inspected under the confocal microscope (Zeiss, 710).
Reconstructed human epidermis expression of filaggrin and transglutaminase 3
To quantify the level of expression of the proteins filaggrin and transglutaminase 3, the Protein Simple Wes capillary-based automated immunoassays platform was used (24). RHE were treated as previously described for morphology and permeability experiments (see above Materials and Methods, Reconstructed human epidermis treatment). For these experiments, in addition to sterile water and mDixon + SQ 2%, M. restricta 24 h supernatant B was used and the SQOOH concentration was adapted accordingly to 193 ng/ml in mDixon + SQ 2%, MDA at 60 ng/ml in mDixon + SQ 2%, and SQOOH at 193 ng/ml + MDA at 60 ng/ml in mDixon + SQ 2%. Each solution was applied in triplicate. Samples were lysed using a specific buffer with Precellys® lysing kits (Bertin Technologies) designed for soft tissue homogenizing and dosed by bicinchoninic acid (BCA) assay. Diluted protein lysate was mixed with fluorescent master mix and heated at 37°C for 10 min. The plate was loaded into the instrument (WES, Protein Simple) and protein was drawn into individual capillaries on a 25-capillary cassette (12-230 kDa or 66-440 kDa) provided by the manufacturer (SM-W001). Proteins of interest were immunodetected with primary antibodies targeting FLG or TGM3 and peroxidase-conjugated secondary antibodies (Goat anti-Mouse or Rabbit IgG-HRP). Signals were normalized to cofilin immunodetection.
RESULTS
First evidence of squalene monohydroperoxide production by M. restricta: 2-dimensional nuclear magnetic resonance experiment
NMR spectroscopy is one of the most powerful analytical techniques to identify biomolecules and elucidate their chemical structures. It also enables the study of chemical reactions by comparing fingerprints of reactions at different time-points or through comparison with controls. For complex mixtures, 2-dimensional correlation spectroscopy (COSY) is often employed, as it allows better resolution of overlapping peaks, thereby providing a more accurate comparison and estimation.
To demonstrate that M. restricta was able to peroxide SQ, M. restricta was cultured with pure SQ in the presence of linoleic acid, which was essential for its growth. Control spectra without M. restricta were firstly analysed and cross peaks corresponding to SQ (at 5.10 and 2.07 ppm), Triton X-100, which was used as an internal standard (7.22 and 6.79 ppm) were identified in the COSY spectrum (Fig. S3). A negligible amount of squalene peroxide (2-SQOOH) was observed, with cross peaks at 5.54 and 2.64 ppm (Fig. S3). COSY spectrum of the M. restricta culture with linoleic acid and SQ is shown in Fig. 1. Cross peaks for SQ can be seen at 5.10 and 2.07 ppm, and that of its peroxide (2-SQOOH) at 5.67 and 2.72 ppm. The cross peak at 7.22 and 6.79 ppm belongs to Triton X-100 (internal standard). The cross peak of 2-SQOOH was integrated and compared with the corresponding cross peak of the control sample. In both cases, the cross peak of the internal standard (Triton-X) was set to the same value. The significant increase in the integration value of the cross peak of 2-SQOOH in the presence of M. restricta in comparison with the control is direct proof of the role of this microorganism on the production of 2-SQOOH.
Quantification of squalene monohydroperoxide and malondialdehyde produced by M. restricta
Table I depicts the quantities of SQOOH and MDA present in M. restricta supernatant A at different culture time-points. The yeast was grown in mDixon medium in the presence of 2% SQ (32°C, in the dark). The quantity of SQOOH in M. restricta supernatant increased over time, from an undetectable level up to 179.4 ng/ml after 24 h. However, the rate of increase slowed over time. Thus, 50% and 62.5% of the total measured quantity at 24 h was, respectively, produced at 2 and 6 h. Likewise, the quantity of MDA increased from an undetectable level up to 51.2 ng/ml within 24 h. Interestingly, 90% of the total quantity of MDA measured at 24 h was produced within the first 2 h. The production of SQOOH and MDA in M. restricta supernatant required the presence of both SQ and M. restricta (Table I). In the absence of either of these, only traces of SQOOH and smaller amounts of MDA could be detected. In the identical second experiment, SQOOH was quantified at 197.5 ng/ml and MDA at 61.2 ng/ml in the 24 h supernatant B in the experimental setting of mDixon + SQ 2% + M. restricta. These results are similar and confirmatory to those obtained with 24 h supernatant A: 179.4 ng/ml for SQOOH and 51.2 ng/ml for MDA (Table I). Thus, it was clearly proven with 2 temporally independent experiments that M. restricta induces SQ peroxidation and stimulates MDA production in the above culture conditions.
Effects of M. restricta supernatant, squalene mono-hydroperoxide and malondialdehyde on the morphology and permeability of reconstructed human epidermis
For this first set of experiments, the topically applied concentrations of SQOOH (180 ng/ml) and MDA (50 ng/ml) were selected to equal those present in the M. restricta supernatant A after 24 h incubation (179.4 ng/ml for SQOOH and 51.2 ng/ml for MDA; Table I).
Reconstructed human epidermis morphology. Fig. 2 shows the morphological changes induced by the solutions applied on RHE model for 24 h. mDixon + SQ did not induce significant changes compared with sterile water and is used for morphological comparison purposes with the other combinations tested. Thus, the M. restricta supernatant as a whole impacted RHE morphology as depicted by the decrease in keratohyalin granules at stratum granulosum. In contrast, individual application of SQOOH and MDA also did not induce significant morphological changes, although their combination profoundly affected the morphology of the RHE, as shown by the resulting absence of keratohyalin granules, the appearance of apoptotic cells and the disorganization of the basal layer. Taken together, these results indicate that M. restricta supernatant is able to impair the skin barrier. At least partially this harmful action can be attributed to the production of SQOOH and MDA.
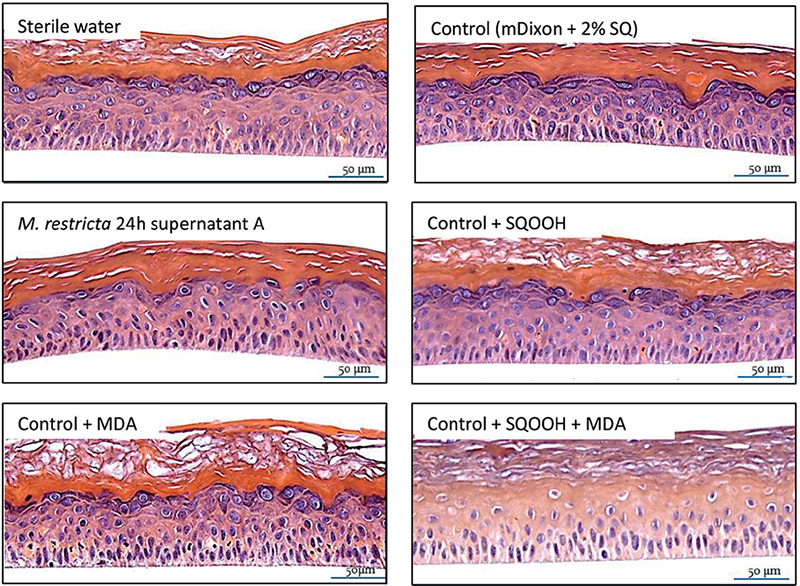
Fig. 2. Reconstructed human epidermidis (RHE) morphology assessments by haematoxylin and eosin stain. Morphological changes induced by different solutions topically applied on RHE model for 24 h. 20X magnification. Malondialdehyde (MDA) and squalene monohydroperoxide (SQOOH) assessed at their respective concentrations present in Malassezia restricta 24 h supernatant A. Control (mDixon + 2% SQ) did not induce significant changes compared with sterile water. M. restricta supernatant impacted RHE morphology as depicted by the decrease in keratohyalin granules at stratum granulosum. When applied alone, SQOOH and MDA did not induce significant morphological changes, although their combination (SQOOH + MDA) profoundly affected the morphology, as shown by the resulting absence of keratohyalin granules, the appearance of apoptotic cells and the disorganization of the basal layer.
Reconstructed human epidermis permeability. Fig. 3a illustrates the penetration of LY into RHE models treated for 24 h with the different test solutions. As illustrated, the LY did not penetrate the skin model treated with sterile water. This reflects the absence of any significant impact on the barrier function. Compared with sterile water, the control solution (mDixon + SQ) barely increased tissue permeability. M. restricta supernatant strongly increased LY penetration compared with control. The same effect on RHE permeability was observed with SQOOH and a slightly weaker effect with MDA. The strongest effect was observed when MDA and SQOOH were applied simultaneously. In that case, LY reached viable skin layers. The effects of the different solutions on RHE permeability were also quantified. The quantitative results are illustrated in graph form (Fig. 3b), which illustrates the observations of Fig. 3a.
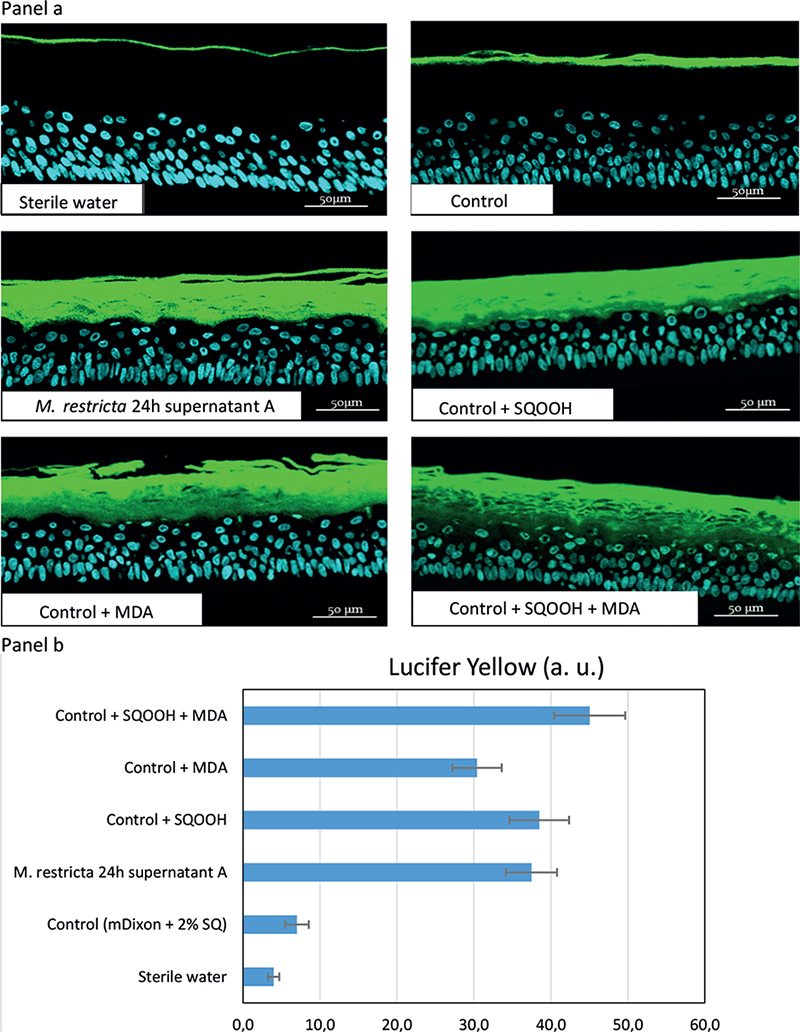
Fig. 3. Panel a: Reconstructed human epidermidis (RHE) permeability illustrated by penetration of lucifer yellow (LY) into RHE models topically treated for 24 h with different test solutions. 20X magnification. Malondialdehyde (MDA) and squalene monohydroperoxide (SQOOH) assessed at their respective concentrations present in M. restricta 24 h supernatant A. As illustrated, the LY did not penetrate the skin model treated with sterile water. This reflects the absence of any significant impact on the barrier function. Compared with sterile water, control (mDixon + 2% SQ) barely increased tissue permeability. M. restricta supernatant strongly increased LY penetration compared with control. The same effect on RHE permeability was observed with SQOOH and a slightly weaker effect with MDA. The strongest effect was observed when MDA and SQOOH were applied simultaneously with LY reaching viable skin layers. Panel b: Quantification of LY into RHE models. Histograms showing the mean for triplicates in arbitrary units (a.u.), with bars indicating standard deviations (SD).
Effects of M. restricta supernatant, squalene monohydro-peroxide and malondialdehyde on reconstructed human epidermis expression of transglutaminase 3 and filaggrin
To further investigate and illustrate barrier function impairment, in a second set of experiments, this study assessed the level of expression of 2 proteins involved in barrier function and known to be downregulated in dandruff: FLG and TGM3. Experiments were conducted with M. restricta 24 h supernatant B containing SQOOH at 197.5 ng/ml and MDA at 61.2 ng/ml. SQOOH was tested at 193 ng/ml and MDA at 60 ng/ml in mDixon + SQ 2%. Three RHE were used per condition. Figs 4a and b, respectively, display the results for FLG and TGM3 quantification by WES. As illustrated by Fig. 4a, mDixon + SQ did not have any impact on the level of FLG expression compared with sterile water. M. restricta supernatant significantly reduced expression of FLG compared with mDixon + SQ. MDA had a slightly weaker effect than M. restricta supernatant. SQOOH also tended to reduce FLG expression compared with mDixon + SQ. When associated in mDixon + SQ, SQOOH and MDA had the same negative impact as M. restricta supernatant on FLG expression. As shown by Fig. 4b, the impact of the different treatments was weaker on TGM3 expression than on FLG expression. As for FLG, mDixon + SQ did not have any impact on the level of TGM3 expression compared with sterile water. M. restricta supernatant only tended to reduce the expression of TGM3 compared with mDixon + SQ. This slight effect of M. restricta supernatant was observed when SQOOH and MDA were applied together. Reductions observed with either MDA or SQOOH were even weaker.
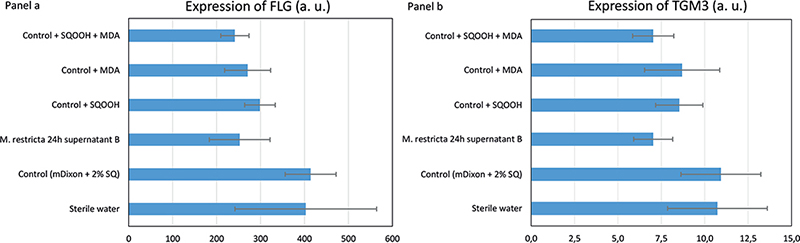
Fig. 4. Reconstructed human epidermidis (RHE) expression of filaggrin (FLG) and of transglutaminase 3 (TGM3). Quantification of FLG (Panel a) and of TGM3 (Panel b) into RHE models topically treated for 24 h by different solutions. MDA and squalene monohydroperoxide (SQOOH) assessed at their respective concentrations present in Malassezia restricta 24 h supernatant B. Histograms showing the mean for triplicates in arbitrary units (a. u.) and bars indicating standard deviations (SD).
DISCUSSION
Using different methods (NMR, LC-MS/MS, and GC/MS), this study showed the ability of M. restricta to oxidize SQ to SQOOH and produce MDA, when cultured in the dark. SQ peroxidation, not MDA production, has been described previously (25) from 2 strains designated at that time as Pityrosporum orbiculare, which under the new taxonomy would probably correspond to M. globosa species (26). The current in vitro results with M. restricta are strongly linked with previous in vivo findings that demonstrated higher quantities of M. restricta cells/cm2 (7–9) and higher levels of SQOOH and MDA on dandruff scalp surface (10). The herein presented in vitro data highlight M. restricta as a possible source of the elevated amounts of SQOOH and MDA on dandruff scalp. However, some other sources of the in loco SQ peroxidation could be sun exposure and C. acnes. Indeed, ultraviolet (UV) exposure induces SQ peroxidation on hairless human skin (18, 27). However, dandruff scalp surfaces are generally covered by hair, and UV radiation reaches them only minimally because human hair acts as a natural sun protection agent (28). In addition, the dandruff condition is exacerbated during the winter months (9). Therefore, it is unlikely that UV exposure participates in the increased SQ peroxidation observed in dandruff-affected skin. Likewise, the induction of SQ peroxide formation by C. acnes through coproporphyrin action also requires UV radiation, especially UVA (29). Moreover, unlike M. restricta, C. acnes density is not increased on dandruff scalp areas (7–9). Therefore, our in vitro results show that M. restricta is able to produce SQOOH and MDA by lipoperoxidation of sebaceous lipids, especially SQ. In accordance with the in vivo data (10), it is reasonable to argue that this also happens on the scalp surface. Thus, the recorded higher quantities of SQOOH and MDA on dandruff scalp areas could be, at least partially, attributed to the action of the increased populations of M. restricta on these scalp areas (7–9).
In experiments with RHE, 3 classes of results are observed, from lower to higher biological effects, as measured by alterations in the RHE morphology, barrier function and the level of FLG and TGM3 expression: application of SQOOH and MDA individually, application of M. restricta supernatants and combined application of SQOOH and MDA. The results with M. restricta supernatant were more or less expected, as they were observed in a recent study in which the same RHE model at a lower level of maturity was treated with an inoculum of M. restricta (5 × 105 CFU) for 72 h (22). What is new and notable is the observation that concurrent exposure to MDA and SQOOH impacts RHE integrity more in comparison with M. restricta supernatant. This could be attributed to a dual action of M. restricta, with the production of the damaging SQOOH and MDA, on the one hand, and the synthesis of substances with ameliorating effects, such as aryl-hydrocarbon receptor ligands (30, 31). Likewise, SQOOH and MDA could interact with other lipids, proteins or structural components linked to M. restricta biomass, restricting their oxidative ability. Along this line, it is impressive that SQOOH and MDA alone did not impact RHE morphology compared with their joined action. This observation confirms a previous result obtained with similar concentrations of MDA and of SQOOH topically applied on another RHE model (17). This finding is suggestive of a combinational damaging effect (32) of these 2 substances, which could result from exhaustion of the available antioxidant reserves (33) present on the RHE employed. More intriguingly, it could underlie the activation of different genetic or epigenetic responses to oxidative stress. SQ is a natural expendable protective factor to UV radiation and oxidative stress, that leads initially to the production, among other peroxides, of SQOOH and, subsequently, to MDA (34). Thus, concurrent exposure to SQOOH and MDA corresponds to real-life intense oxidative stress compared with either SQOOH or MDA alone, and thus a different biological response would be anticipated.
It should be stressed that the impairment of barrier function and the decrease in epidermal structural proteins are morphological and functional changes associated with dandruff (3). To our knowledge, this is the first time that the deleterious effects of MDA on the skin have been assessed, and therefore no comparison data exist. On the other hand, the current in vitro findings with SQOOH are in line with some in vivo results indicating that topically applied SQOOH on guinea pig skin resulted in cutaneous damage, including hyperkeratosis and hyperpigmentation (13). Other chemical entities produced by Malassezia genus from sebum have been previously proposed as aetiological factors in dandruff, such as unsaturated free fatty acids (FFA). FFA are released from hydrolysis of sebaceous triglycerides by fungal lipases. Malassezia was described to preferentially consume saturated FFA for its needs, and to induce the accumulation of irritating unsaturated species on the surface of the scalp (35). For the time being, this hypothesis could not be confirmed by in vivo (10) or by subsequent in vitro experiments (36).
The current experiments with RHE observed some dandruff-related features, such as increased permeability and alterations of proteins involved in barrier function such as TGM3 and FLG (6). On the other hand, we did not record other relevant features, such as scaling and parakeratosis. This is anticipated, as the employed 3D skin model reproduces the differentiated human epidermis in terms of morphology (22), but lacks the physiological complexity of human skin. In particular, like the other RHE models, this model cannot show any desquamation. It would be interesting to reproduce this experiment with a more complex skin model, such as skin explants from human donors, which recapitulate the skin’s physiology with greater fidelity (37). The skin barrier function of the RHE models is not as efficient as the in vivo one. Hence, this deficiency in barrier function could have favoured skin penetration of SQOOH and MDA and enhanced their respective action. In the current experiments with RHE, M. restricta supernatant corresponded to 3.6 ng/cm2 of SQOOH and 1 ng/cm2 of MDA. These concentrations were also used in the experiments with SQOOH and MDA directly added to the culture medium used to grow M. restricta. Using the current clinical results on 10 dandruff and 10 non-dandruff subjects (10), it was estimated that in vivo median concentrations were, respectively, 310 ng/cm2 for SQOOH and 0.75 ng/cm2 for MDA on non-dandruff scalps and 591 ng/cm2 for SQOOH and 0.98 ng/cm2 for MDA on dandruff scalps. Hence, the amount of MDA (1 ng/cm2) topically applied on RHE was very similar to the estimated in vivo level on scalp surface. On the other hand, the applied quantity of SQOOH (3.6 ng/cm2) on RHE was one hundred times lower than the quantity estimated in vivo. It is possible that such a concentration of MDA would not induce epidermal damages on skin models with a better barrier function. However, given the damage observed on RHE with such a low concentration of SQOOH, it is highly probable that the much higher concentrations observed in vivo could harm the barrier function of the scalp skin, especially for subjects with dandruff who present the higher quantities of SQOOH (10) associated with an already impaired scalp barrier function (3).
Despite the possible limitations of the RHE model, these results strongly suggest that the elevated quantities of SQOOH and MDA on dandruff skin could elicit respective alteration through direct deleterious action on scalp barrier function. Both compounds are associated with reactive oxygen species (ROS) and may participate in the oxidative damage that accompanies or causes dandruff (3). The results of this study point to a new paradigm of dandruff origin. This also entails increased lipoperoxidation attributed to elevated numbers of M. restricta and alteration in antioxidant defence mechanisms (6). Abolishing the appearance of dandruff at the clinical level through targeted reduction in M. restricta-related lipoperoxidation could confirm the current suggested novel view of dandruff triggering. Such a possible antidandruff approach, especially using a compound of natural origin, could be a way to meet growing consumer expectations for more natural antidandruff products.
ACKNOWLEDGEMENTS
We thank Lionel Breton, Amit Jayaswal and Laurent Pavan for their constant support and helpful comments.
REFERENCES
- Park M, Cho Y-J, Lee YW, Jung WH. Understanding the mechanism of action of the anti-dandruff agent zinc pyrithione against Malassezia restricta. Sci Rep 2018; 8: 12086.
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol 2015; 3: 10.13188/2373-1044.1000019.
- Schwartz JR, Messenger AG, Tosti A, Todd G, Hordinsky M, Hay RJ, et al. A comprehensive pathophysiology of dandruff and seborrheic dermatitis – towards a more precise definition of scalp health. Acta Derm Venereol 2013; 93: 131–137.
- Singh B, Haftek M, Harding CR. Retention of corneodesmosomes and increased expression of protease inhibitors in dandruff. Br J Dermatol 2014; 171: 760–770.
- Turner GA, Hoptroff M, Harding CR. Stratum corneum dysfunction in dandruff. Int J Cosmet Sci 2014; 34: 298–306.
- Cavusoglu N, Delattre C, Donovan M, Bourassa S, Droit A, El Rawadi C, et al. iTRAQ-based quantitative proteomics of stratum corneum of dandruff scalp reveals new insights into its aetiology and similarities with atopic dermatitis. Arch Dermatol Res 2016; 308: 631–642.
- Clavaud C, Jourdain R, Bar-Hen A, Tichit M, Bouchier C, Pouradier F, et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One 2013; 8: e58203.
- Wang L, Clavaud C, Bar-Hen A, Cui M, Gao J, Liu Y, et al. Characterization of the major bacterial–fungal populations colonizing dandruff scalps in Shanghai, China, shows microbial disequilibrium. Exp Dermatol 2015; 24: 398–400.
- Grimshaw SG, Smith AM, Arnold DS, Xu E, Hoptroff M, Murphy B. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLoS One 2019; 14: e0225796.
- Jourdain R, Moga A, Vingler P, El Rawadi C, Pouradier F, Souverain L, et al. Exploration of scalp surface lipids reveals squalene peroxide as a potential actor in dandruff condition. Arch Dermatol Res 2016; 308: 153–163.
- Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res 2008; 49: 271–281.
- Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis CC, Picardo M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in acne vulgaris. J Invest Dermatol 2006; 126: 2430–2437.
- Ohsawa K, Watanabe T, Matsukawa R, Yoshimura Y, Imaeda K. The possible role of squalene and its peroxide of the sebum in the occurrence of sunburn and protection from the damage caused by UV irradiation. J Toxicol Sci 1984; 9: 151–159.
- Ozturk P, Arican O, Belge Kurutas E, Karakas T, Kabakci B. Oxidative stress in patients with scalp seborrheic dermatitis. Acta Dermatovenerol Croat 2013; 21: 80–85.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014; 2014: 360438.
- Bertino L, Guarneri F, Cannavò SP, Casciaro M, Pioggia G, Gangemi S. Oxidative stress and atopic dermatitis. Antioxidants (Basel) 2020; 9: 196.
- Jourdain R, Moga A, Rahoul V, Fontanié M, Pavan L, Breton L. Malassezia restricta-mediated lipoperoxidation: a new player in dandruff origin. Poster 1026. Skin health and disease: immune, epithelial and microbiome crosstalk. Keystone Symposia, Hannover 2019.
- Nakagawa K, Ibusuki D, Suzuki Y, Yamashita S, Higuchi O, Oikawa S, et al. Ion-trap tandem mass spectrometric analysis of squalene monohydroperoxide isomers in sunlight-exposed human skin. J Lipid Res 2007; 48: 2779–2787.
- Nazzaro-Porro M, Passi S, Picardo M, Mercantini R, Breathnach AS. Lipoxygenase activity of Pityrosporum in vitro and in vivo. J Invest Dermatol 1986; 87: 108–112.
- Strassnig S, Wenzl T, Lankmayr EP. Microwave-assisted derivatization of volatile carbonyl compounds with O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine. J Chromatogr A 2000; 891: 267–273.
- Pellevoisin C, Videau C, Briotet D, Grégoire C, Tornier C, Alonso A, et al. SkinEthic RHE for in vitro evaluation of skin irritation of medical device extracts. Toxicol In Vitro 2018; 50: 418–425.
- Meloni M, Balzaretti S, Collard N, Desaint S, Laperdrix C. Reproducing the scalp microbiota community: co-colonization of a 3D reconstructed human epidermis with C. acnes and M. restricta. Int J Cosmet Sci 2021; 43: 235–245.
- Frankart A, Malaisse J, De Vuyst E, Minner F, Lambert de Rouvroit C, Poumay Y. Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air-liquid interface. Exp Dermatol 2012; 21: 871–875.
- Harris VM. Protein detection by Simple Western™ analysis. Methods Mol Biol 2015; 1312: 465–468.
- De Luca C, Picardo M, Breathnach A, Passi S. Lipoperoxidase activity of Pityrosporum: characterisation of by-products and possible role in pityriasis versicolor. Exp Dermatol 1996; 5: 49–56.
- Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie van Leeuwenhoek 1996; 69: 337–355.
- Ekanayake Mudiyanselage S, Hamburger M, Elsner P, Thiele JJ. Ultraviolet A induces generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. J Invest Dermatol 2003; 120: 915–922.
- De Galvez MV, Aguilera J, Bernabo JL, Sanchez-Roldan C, Herrera-Ceballos E. Human hair as a natural sun protection agent: a quantitative study. Photochem Photobiol 2015; 91: 966–970.
- Ryu A, Arakane K, Koide C, Arai H, Nagano T. Squalene as a target molecule in skin hyperpigmentation caused by singlet oxygen. Biol Pharm Bull 2009; 32: 1504–1509.
- Magiatis P, Pappas P, Gaitanis G, Mexia N, Melliou E, Galanou M, et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol 2013; 133: 2023–2030.
- Uberoi A, Bartow-McKenney C, Zheng Q, Flowers L, Campbell A, Knight SAB, et al. Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe 2021; 29: 1235–1248.
- Gruber F, Marchetti-Deschmann M, Kremslehner C, Schosserer M. The skin epilipidome in stress, aging, and inflammation. Front Endocrinol 2021; 11: 607076.
- De Luca C, Valacchi G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediators Inflamm 2010; 2010: 321494.
- Dennis KJ, Shibamoto T. Production of malonaldehyde from squalene, a major skin surface lipid, during UV-irradiation. Photochem Photobiol 1989; 49: 711–716.
- Ro BI, Dawson TL. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J Investig Dermatol Symp Proc 2005; 10: 194–197.
- James AG, Abraham KH, Cox DS, Moore AE, Pople JE. Metabolic analysis of the cutaneous fungi Malassezia globosa and M. restricta for insights on scalp condition and dandruff. Int J Cosmet Sci 2013; 35: 169–175.
- Larson PJ, Chong D, Fleming E, Oh J. Challenges in developing a human model system for skin microbiome research. J Invest Dermatol 2021; 141: 228–231.