QUIZ SECTION
Painless Plaques Evolving to Bullae and Painful Erosions on the Penis: A Quiz
Alberto MENEGUZZO, Roberto MAZZETTO, Annalisa LAZZAROTTO and Stefano PIASERICO
Department of Dermatology, University of Padua, Via Vincenzo Gallucci 4, IT-35121, Padova, Italy. E-mail: alberto.meneguzzo@gmail.com
Citation: Acta Derm Venereol 2023; 103: adv00871. DOI: https://doi.org/10.2340/actadv.v103.4845.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Published: Feb 16, 2023
A 71-year-old man was referred to our dermatological clinic for a 3-year history of balanoposthitis. He had diabetes mellitus, hypertension and dyslipidaemia treated with semaglutide, metformin, enalapril and metoprolol. No new drugs in the past 6 months or risky sexual behaviours were reported. On clinical examination the patient presented a painless, mildly pruritic erythematous lesion with scattered small erosions on the glans penis (Fig. 1). Clobetasol propionate 0.05% cream was prescribed once per day, resulting in improvement in the lesion. However, after 2 months he developed tense bullae, rapidly evolving to painful erosions on the same area (Fig. 2). Full blood count, kidney and liver function tests, hepatitis B and C serologies were normal. The patient tested negative for syphilis, HIV and herpes simplex virus 1 or 2 (HSV1 or HSV2). A biopsy sample was obtained for histopathological and immunofluorescence examination (Fig. 3).
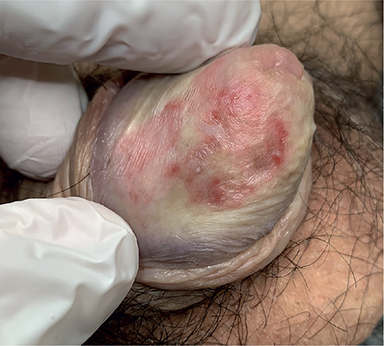
Fig. 1. Erythemato-violaceous plaque on the glans penis with small scattered erosions.
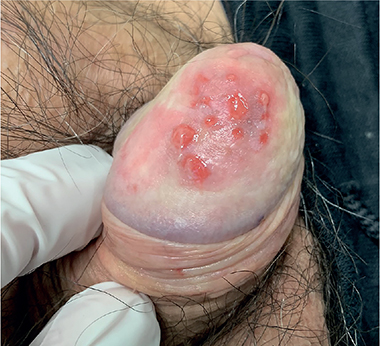
Fig. 2. Subsequent development of bullae and erosions within the plaque.
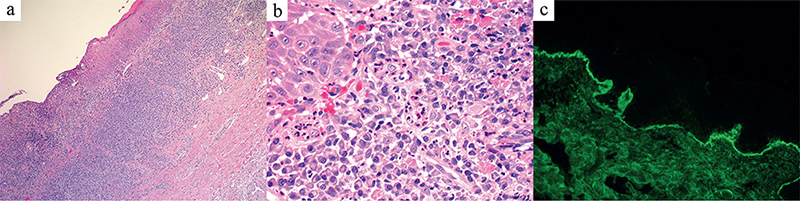
Fig. 3. (a) Hematoxylin and Eosin stained tissue section (H&E, 30x) show a band-like lymphocytic infiltrate in the dermo-epidermal junction (DEJ). (b) With an abundant infiltration of eosinophil granulocytes in the dermis (H&E, 50x). (c) Direct immunofluorescence shows linear IgG deposits along the DEJ (40x).
Histology revealed hyperkeratosis, acanthosis, vacuolar degeneration of the basal layer of the epidermis with Civatte bodies, and a band-like lymphocytic infiltrate in the dermo-epidermal junction (DEJ) (Fig. 3a), suggesting a lichenoid dermatosis, with abundant eosinophilic component in the superficial dermis (Fig. 3b). Direct immunofluorescence (DIF) showed linear IgG and C3 deposits along the DEJ (Fig. 3c). Moreover, anti-BP180 autoantibodies resulted positive (57 UI/ml, positive > 9 UI/ml).
What is your diagnosis? See next page for answer.
ANSWERS TO QUIZ
Painless Plaques Evolving to Bullae and Painful Erosions on Penis: A Commentary
Diagnosis: Lichen planus pemphigoides of the penis
Lichen planus pemphigoides (LPP) is an uncommon skin disorder, generally considered a clinical, histopathological, and immunological coexistence of lichen planus (LP) and bullous pemphigoid (BP). However, growing evidence suggests that it should be considered as a separate disease entity (1–4).
Its prevalence is unclear, with male predominance and onset mainly in the 45th decade of life.
Aetiology is idiopathic in most cases; however, onset of LPP has been associated with ultraviolet (UV) exposure, hepatitis B virus, Castleman’s disease, chronic lymphocytic leukaemia, and several drugs, such as cinnarizine, angiotensin-converting enzyme (ACE) inhibitors, simvastatin, paracetamol, and ibuprofen (1–4).
LP usually precedes the onset of the bullous dermatosis; according to this, some authors have suggested an epitope spreading phenomenon, in which a primary inflammatory damage at the level of basal cell layer during LP causes antigenic exposure and a secondary autoimmune response against hemidesmosomal unit. IgG autoantibodies to either 1 or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens have been demonstrated (5, 6). Clinical features of LPP classically include vesicles, tense bullae or erosions affecting both normal skin and skin previously affected by lichenoid dermatosis; this contrasts with bullous LP, which presents with bullae that are limited to longstanding LP lesions (1–4).
Diagnosis may be confirmed by the histopathological evidence of subepidermal bullae with a band-like leukocyte infiltrate, composed mainly of lymphocytes, plasma cells, and eosinophils in the dermis. DIF reveals a linear IgG and C3 deposit along the DEJ (1–4).
Most cases respond well to systemic corticosteroids, alone or in association with tetracyclines and nicotinamide (2–4). Other drugs include azathioprine, mycophenolate mofetil and dapsone, as used in the treatment of BP. Also, various patients with LPP presented a good response to cyclosporine or methotrexate, although they are usually poorly effective in the management of autoimmune bullous diseases (7–9). The prognosis of LPP is usually good, with a low rate of recurrence (approximately 20%). LPP of genital mucosae is uncommon and appears mostly when other sites are involved, on skin or oral mucosae. Mangin et al. (7) reported a case of LPP with erosion of the glans penis and diffuse cutaneous involvement, and Loyal et al. (8) described a case of vulvar and oral LPP. Therefore, isolated LPP could be a real challenge for clinicians, mostly in its early stage.
In particular, penile LPP should be distinguished from clinically similar conditions, such as bullous or erosive LP, mucous membrane pemphigoid, genital pemphigus, or penile fixed drug eruption (10).
Due to the patient’s poorly controlled diabetes and hypertension, systemic corticosteroids and cyclosporine were avoided, and methotrexate 15 mg/week was prescribed with significant improvement after 8 weeks.
To our knowledge, this is the first description of LPP clinically limited to the glans. This case suggests that this uncommon disease should be taken into consideration in the differential diagnosis of lichenoid or erosive balanoposthitis.
REFERENCES
- Hübner F, Langan EA, Recke A. Lichen planus pemphigoides: from lichenoid inflammation to autoantibody-mediated blistering. Front Immunol 2019; 10: 1389.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol 2017; 9: 217–224.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol 2015; 1: 140–149.
- Zaraa I, Mahfoudh A, Sellami MK, Chelly I, El Euch D, Zitouna M et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol 2013; 52: 406–412.
- Fujii M, Takahashi I, Honma M, Ishida-Yamamoto A. Bullous lichen planus accompanied by elevation of serum anti-BP180 autoantibody: a possible transitional mechanism to lichen planus pemphigoides. J Dermatol 2017; 44: e124–e125.
- Mignogna MD, Fortuna G, Leuci S, Stasio L, Mezza E, Ruoppo E. Lichen planus pemphigoides, a possible example of epitope spreading. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 837–843.
- Mangin MA, Kanitakis J, Jullien D, Lesort C. Dermpath & Clinic: lichen planus pemphigoides. Eur J Dermatol 2020; 30: 211–213.
- Loyal J, Rashtak S. Vulvar lichen planus pemphigoides. Int J Womens Dermatol 2017; 3: 225–227.
- Duong B, Marks S, Sami N, Theos A. Lichen planus pemphigoides in a 2-year-old girl: response to treatment with methotrexate. J Am Acad Dermatol 2012; 67: e154–e156.
- Gall R, Navarro-Fernandez IN. Lichen planus erosive form. [Updated 2022 Apr 18]. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022.