ORIGINAL REPORT
Diagnosis of Basal Cell Carcinoma with Ex-vivo Confocal Laser Scanning Microscopy in a Real-life Setting
Stephan FORCHHAMMER 1, Sonja GRUNEWALD 2, Matthias MÖHRLE 3, Gisela METZLER 4, Thomas EIGENTLER 5, Anne-Kristin MÜNCH 6 and Hanna OGRZEWALLA 1
1 Department of Dermatology, University Hospital Tübingen, Eberhardt Karls University, 2 Clinic and Polyclinic for Dermatology, Venerology and Allergology, Leipzig, 3 Praxisklinik Tübingen - Skin and Veins, 4 Center for Dermatohistology and Oral Pathology Tübingen/Würzburg, Tübingen, 5 Department of Dermatology, Venereology and Allergology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin and 6 Institute for Clinical Epidemiology and Applied Biometry, Eberhardt Karls University, Tübingen, Germany
Ex-vivo confocal laser scanning microscopy provides a rapid alternative to routine histological processing using haematoxylin and eosin-stained sections. Previous studies suggest high diagnostic accuracy in basal cell carcinoma. This study investigates the diagnostic accuracy of confocal laser scanning microscopy reporting of basal cell carcinoma in a real-life setting and compares reporting by dermatopathologists inexperienced in use of confocal laser scanning microscopy with reporting by an expert in confocal laser scanning microscopy. A total of 334 confocal laser scanning microscopy scans were diagnosed by 2 dermatopathologists inexperienced in the diagnosis of confocal laser scanning microscopy as well as an experienced examiner of confocal laser scanning microscopy scans. The inexperienced examiners achieved a sensitivity of 59.5/71.1% and specificity of 94.8/89.8%. The experienced examiner achieved a sensitivity of 78.5% and specificity of 84.8%. Detection of tumour remnants in margin controls showed insufficient values among inexperienced (30.1/33.3%) and experienced (41.7%) investigators. The results of this study, of real-life setting basal cell carcinoma reporting with confocal laser scanning microscopy, found a lower diagnostic accuracy than published data regarding artificial settings. A poor accuracy in tumour margin control is clinically relevant and could restrict the use of confocal laser scanning microscopy in clinical routine. Prior knowledge of haematoxylin and eosin trained pathologists can be partially transferred to the reporting of confocal laser scanning microscopy scans; however, specific training is recommended.
Key words: basal cell carcinoma; confocal laser scanning microscopy; CLSM; tumour margin control.
SIGNIFICANCE
Ex-vivo confocal laser scanning microscopy offers the possibility to scan tissue excision samples within a few minutes and to generate virtually stained pictures that correspond to conventional haematoxylin and eosin stained histological slides. This technique could revolutionize the dermatosurgical approach, by enabling immediate re-excision if tumour remnants are present at the margins. Although the use of this technique has been well studied in basal cell carcinoma in artificial study settings, it is not known whether tumour evaluation is reliable in daily routine. The current study simulates the real-life application of this method and shows the current limitations.
Citation: Acta Derm Venereol 2023; 103: adv4859. DOI https://doi.org/10.2340/actadv.v103.4859.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 27, 2023; Published: Mar 30, 2023
Corr: Stephan Forchhammer, Department of Dermatology, University Hospital Tübingen, Eberhardt Karls University, Liebermeisterstrasse 25, DE-72076 Tübingen, Germany. E-mail: stephan.forchhammer@med.uni-tuebingen.de
Competing interests and funding: SF: received personal fees from Recordati, Kyowa Kirin and Takeda Pharamceuticals (speakers honoraria) as well as institutional grants from NeraCare, SkylineDX and BioNTech outside the submitted work. SG: Mavig provided a Vivascope microscope for the duration of a separate study. MM: Mavig provided a Vivascope microscope for the duration of this study. In the past, MM had performed studies with previous models of Vivascopes (Mavig) and HistologTMScanner (SamanTree Medical SA). TE: declares speakers and advisory board honoraria from Almirall Hermal, Merck Sharp & Dome, Immunocore, Novartis, Sanofi Genzyme, Roche, Pierre Fabre and BMS. GM, AKM and HO have no conflicts of interest to declare.
INTRODUCTION
Basal cell carcinomas (BCC) are one of the most common tumours worldwide (1). The main clinically relevant subtypes are nodular, superficial, infiltrative and fibrosing BCC (1). The number of patients is particularly high in fair-skinned populations and in countries with high levels of sun exposure. Due to the high incidence of BCC, a large number of patients could benefit from rapid and safe treatment. Since BCC is a locally aggressive, but almost non-metastatic, tumour, therapy in most cases consists of complete excision of the tumour (2). For superficial BCC, topical therapy, such as imiquimod, 5-flourouracil, or photodynamic therapy, can be used instead of surgical treatment (2).
There are several methods for BCC tumour removal. Mohs surgery is used very frequently worldwide. Here, in contrast to the classic bread-loaf sectioning, the tumour excidate is not assessed in parallel lamellae, but instead from the edge of the tumour. In order to perform this as rapidly as possible, cryostat-fixed, frozen tissue sections are examined. Another technique for micrographic controlled surgery is so-called 3D-histology (“slow Mohs”). This method has one of the lowest recurrence rates for skin tumour removal (3). This low recurrence rate is achieved by a complete control of margins. If histopathological control of the margins reveals remaining tumour tissue, a second excision of the remaining tumour is performed to ensure complete tumour removal.
For evaluation by a dermatopathologist, the gold standard is preparation of formalin-fixed and paraffin-embedded (FFPE) as well as haematoxylin and eosin (H&E) stained slices. This method offers numerous advantages, such as the possibility of staining with immunohistochemical antibodies and archiving of blocks and sections. In addition, the method is widely used worldwide and is cost-effective. However, this technique has a major drawback. The reprocessing is very time-consuming and takes between 20 and 24 h in a routine setting. In margin-controlled excision, in some cases the patient’s wound is left unclosed in the meantime. In case of re-excision, such a multi-stage approach necessitates repeated operations as well as repeated anaesthesia. In addition, there is a risk of postoperative bleeding or wound infection (4).
Another method for the processing of excision samples is the preparation of cryostat sections. This method is much faster than embedding in paraffin. However, the quality of cryostat sections is sometimes not as good as that of FFPE-embedded slides. Furthermore, a very good infrastructure is needed to create the sections rapidly and at high quality.
In recent decades, a new technique has been developed to produce images of the tissue more rapidly: ex-vivo confocal laser scanning microscopy (CLSM). CLSM makes it possible to directly scan unfixed tissue with a laser and generate images within a few minutes. The latest generation of CLSM software even makes it possible to digitally stain the CLSM scans in a pink and blue fashion to resemble those created by H&E staining (5). This could make it easier for H&E-trained dermatopathologists to evaluate CLSM scans without the necessity for a long period of learning.
The use of CLSM is well established in dermatological surgery, especially in BCCs. There are numerous studies investigating the use of CLSM in BCC and proposing diagnostic criteria. Here, a high sensitivity and specificity in diagnosis could be achieved (6–8). However, these studies were mostly performed in an artificial setting: evaluators knew that the specimens they diagnosed were either BCC or the exclusion of BCC. In reality, however, the dermatopathologists making the findings usually do not know which tumour is involved. There is only a suspected clinical diagnosis. Thus, in addition to BCC, many other benign and malignant tumours must be considered in the differential diagnosis.
The aim of this study was to investigate whether the diagnosis of BCC by CLSM provides sufficient diagnostic sensitivity and specificity in a real-life setting. A further aim was to evaluate if it is possible for dermatopathologists trained on H&E sections to transfer the morphological knowledge to CLSM reporting and to diagnose BCCs in CLSM without a long training.
MATERIALS AND METHODS
Study design
All patients, regardless of the clinically suspected diagnosis, who underwent excision, shave excision or punch biopsy at the Praxisklinik Tübingen - Skin and Veins, Tübingen, Germany, from 6 April 2020 to 27 May 2020 and gave their informed consent were included in the study. During this period, a CLSM scanner, Vivascope 2500, was provided by the company Mavig GmbH (Munich, Germany) to perform the study. The indication for excision as well as the surgery itself was performed by dermatologists of the Praxisklinik Tübingen – Skin and Veins. Only patients with histological confirmation or clinical suspicion of BCC were included in this study; all other samples were evaluated in a separate study setting. From a total of 107 patients, samples of 53 patients showed clinical suspicion or histological confirmation of BCC. From these 53 patients, 61 lesions showing BCCs were excised and 334 CLSM scans were performed (Fig. 1a)
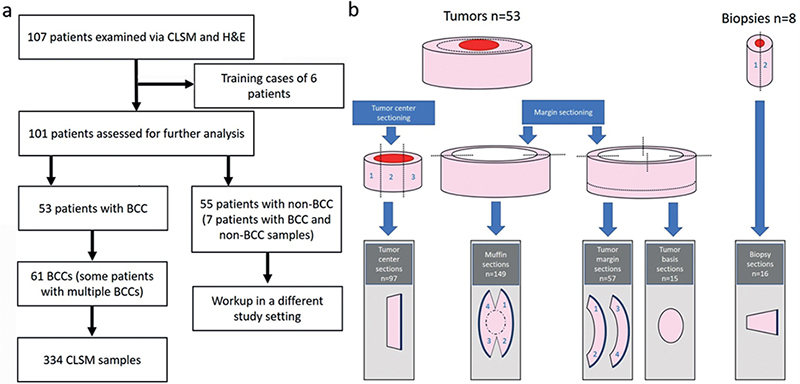
Fig. 1. (a) Study flowchart. (b) Schematic of the tissue sectioning. The tumour centres were processed in 1–5 section levels. Muffins were mostly processed in 1 piece, or sometimes, in case of large excision samples, in 2 parts. The muffin margins were virtually divided into 4 quadrants. Tumour margins were reprocessed in 1 or 2 sections; the margins were also virtually divided into 4 quadrants. Tumour bases were mostly scanned in 1 piece; or, for very large excision samples, the base was divided into up to 4 parts. Biopsies were cut in half in the middle and reprocessed in 2 planes. BCC: basal cell carcinoma; CLSM: confocal laser scanning microscopy; H&E: haematoxylin and eosin.
Preparation of CSLM sections and H&E sections
The sectioning of tumour excision samples was performed by the operating dermatologist. The preparations were cut either in bread-loaf-technique, in muffin-technique or as 3D histology with separate margin and base sections according to the “Tübinger torte” technique (9, 10). Punch biopsies were cut into 2 sections (see Fig. 1b). Depending on the size of the excision samples, tumour centre sections and tumour margins were processed into up to 5 lamellae, each of which was further evaluated as a separate specimen. The tissue samples were scanned after they were excised. If the excision samples could not be scanned immediately, they were stored on moist compresses to keep them from drying out. To prepare the scans, the excision samples were first stained with acridine orange. They were stained for 20 s in 2.5 mL Ringer’s lactate solution (B. Braun SE, Melsungen, Germany) with 20 drops of acridine orange. After staining, the excision samples were scanned with the VivaScope 2500 and digital scans were made. Afterwards, the tissue was placed in cassettes and fixed in formalin. H&E sections were then prepared from this without further cutting.
Histological reporting
The reporting of the CLSM sections was performed by 2 H&E-trained, experienced board-certified dermatopathologists (DP1 and DP2) without previous experience in the use of CLSM slides and 1 experienced reporter of CLSM slides (DP3). As training for CLSM scans, both CLSM-naive reporters (DP1, DP2) received the corresponding H&E and CLSM sections from 6 patients. These cases were excluded from further evaluation. DP1 and DP2 worked in a real-life setting with knowledge only of operation site, patient age and suspected clinical diagnosis. They diagnosed 597 CLSM sections, of which only 334 were included in the evaluation of BCC. DP3 worked in an artificial setting and was provided with the 334 CLSM sections showing BCC or exclusion of BCC. Reference findings were made by both dermatopathologists (DP1 and DP2) on the H&E sections without resorting to further immunostaining. When creating the H&E sections, no section planes were discarded. As a rule, the diagnosis was already made on the first section level. In the case of missing epidermis, deeper section levels were made. If tumour was found in these deeper sections, it was evaluated only if no tissue was found at the exact location in the corresponding previous section levels. In case of discrepancies in the diagnosis of H&E-based diagnosis by both pathologists, the sections were microscoped together and a consensus was formed. The outcome assessment of the CLSM sections was based on this consensus finding. The CLSM findings were performed at an 8-week gap from the H&E findings. This was done using the software VivaScan provided by Mavig. In addition to histological diagnosis, the parameters of time of reporting, quality of CLSM sections (with 1 as the best quality and 6 as the worst quality), and percentage of epidermis represented were recorded.
Statistical analysis
Statistical calculations were performed using IBM SPSS Statistics version 27.0 (IBM SPSS, Chicago, IL, USA). Numerical variables were described by mean values and standard deviation or median values and interquartile range (IQR).
RESULTS
Out of the total 107 patients and 597 sections examined in the whole real-life setting, 53 patients and 334 sections from BCC specimens were evaluated in this study. Patients had a median age of 68 years (range 36–89 years) and there was an almost balanced ratio of women (n = 26, 49.1%) and men (n = 27, 50.9%). The vast majority of specimens were from the head/neck area (n = 249, 74.6%). Here, the most common localization was nose (n = 86, 25.7%) and forehead (n = 75, 22.5%). The epidemiological data and tumour localization are summarized in Table I. Of the 334 excision samples, 149 were processed using the ”muffin” technique. This technique evaluates both the tumour margins and the tumour bases in a combined section. The remaining 185 excision samples were processed as punch biopsies (n = 16) or as separate tumour centres (n = 97), surgical margins (n = 57) and tumour bases (n = 15) according to the ”Tuebinger torte” technique (see Fig. 1b). The median time for making a CLSM scan was 6 min.
The majority of sections showed tumour-free margins, and 206 (61.7%) of the 334 sections showed “no tumour” or “normal skin”. A total of 128 slides (38.3%) showed BCC. Of these 128 BCCs, the majority (67 sections) belonged to the subgroup of nodular BCC. Twenty-eight sections showed superficial BCCs, 17 showed infiltrating BCCs, 13 tumours were classified as fibrosing BCC, and 3 tumours showed further subtypes of BCC (adenoid, pigmented or keratotic). The median time for evaluating the scans was 20 s for DP1 and 42.5 s for DP2. Time of evaluation was not assessed for the experienced examiner DP3. Also, the time for H&E reporting of reference findings was not collected. The median quality of the CLSM scans was rated with 2 (on a scale of 1–6 with 1 being the best and 6 being the worst quality) (see Table II). Eighty percent of the epidermis was present in tumour centre sections. In tumour margin controls (excluding tumour bases), 72% of the epidermis was shown.
Out of the 128 slides showing BCC, the 2 H&E-trained dermatopathologists gave the correct diagnosis in 72 (DP1) and 91 (DP2) cases, respectively. Of the 206 tumour-free sections, they diagnosed correctly in 200 (DP1) and 185 (DP2) cases, respectively. This resulted in a sensitivity of 59.5% (DP1) and 71.1% (DP2) and a specificity of 94.8% (DP1) and 89.8% (DP2). The reporter experienced in diagnosis of CLSM scans (DP3) correctly diagnosed 102 of 128 tumour slides and 173 of 206 tumour-free sections, resulting in a sensitivity of 78.5% and a specificity of 84.8%. In tumour centres (middle sections and punch biopsies), 61 (66.3%; DP1) and 77 (83.7%; DP2) of 92 tumours were correctly identified by the CLSM inexperienced examiners, respectively. The examiner experienced in CLSM was able to correctly identify 85 of the 92 tumours (92.4%, DP3). The subtype of BCC (in tumour centres) was correctly identified in 54.1% (n = 33 of 61) (DP1) and 74.0% (n = 57 of 77) (DP2) of all cases, respectively. The CLSM trained reporter (DP3) correctly identified the BCC subtype in tumour centres in 67.1% (n = 57 of 85) of cases.
In contrast, the findings of tumour extensions in the margin control (muffin, margin sections, base sections) showed considerably worse results. Here, only 11 (30.6%; DP1) and 12 (33.3%, DP2) of 36 margins with tumour remnants, respectively, could be correctly identified by the investigators inexperienced in CLSM. However, even the examiner experienced in CLSM was able to correctly identify only 15 of the 36 tumour extensions (41.7%, DP3). Table III shows the sensitivity and specificity of the reporting in reference to the various sectioning techniques for all 3 raters. In 12 cases, the margin controls were incorrectly classified by all 3 examiners. These CLSM sections were re-microscoped with knowledge of the tumour localization from the H&E stained slides. In 8 cases, the tumour was overseen and could be identified according to the H&E section. This was predominantly seen in sections showing numerous adnexal structures (Fig. 2) or in small remnants of superficial BCC. In 4 margins, no epidermis was present in the area of the tumour remnants in the CLSM scan, and the tumour was therefore not visualized here. This was predominantly seen in superficial BCC (Fig. 3).
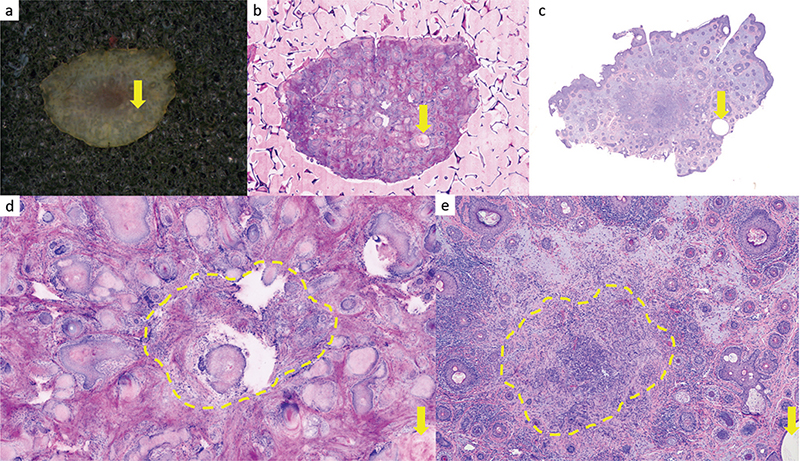
Fig. 2. Base control of a nodular basal cell carcinoma (BCC). (a–c) Overview images. (a) Macroscopic tumour base. (b) Ex-vivo confocal laser scan microscopy (CLSM) scan. (c) Formalin-fixed paraffin-embedded (FFPE) and haematoxylin and eosin (H&E)-stained slide 10 x magnification. (d, e) Magnified image with central parts of a BCC. (d) Ex-vivo CLSM scan. Base control from facial skin with numerous adnexal structures. The extensions of the BCC (circled in yellow ) were missed by all 3 examiners. The epithelial structures are surrounded by a fibrosclerotic stroma. Epithelial basaloid structures are visible and are partly “broken out” of the CLSM section. The tumour is not clearly visible in the CLSM section as there is an imaging artefact that mimics missing tissue in this area. (e) Corresponding H&E section 30 x magnification. The central area (circled in yellow ) shows remnants of a basaloid tumour cell nodule with fibrosclerotic stroma. For better orientation, the same cystic structure is marked with a yellow arrow.
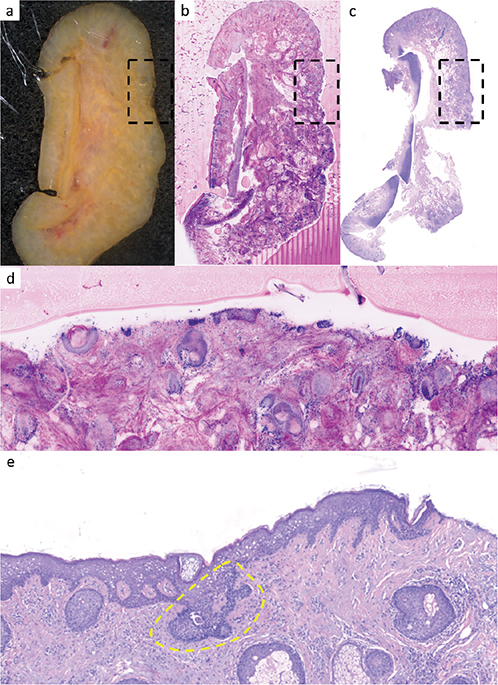
Fig. 3. Margin control of a superficial basal cell carcinoma (BCC). (a–c) Overview images. (a) Macroscopic image with central parts of the auricular cartilage. (b) Ex-vivo confocal laser scan microscopy (CLSM) scan. (c) Formalin-fixed paraffin-embedded (FFPE) and haematoxylin and eosin (H&E) stained slide 6 x magnification. (d, e) Magnified images, corresponding to the marked squares of a–c. (d) Ex-vivo CLSM scan. Marginal control with missing parts of the epidermis. The extensions of the superficial BCC carcinoma do not appear within this section 50 x magnification. (e) Corresponding H&E section. Parts of a superficially located basaloid tumour nodule originating from the epidermis are revealed (circled in yellow ).
DISCUSSION
This study demonstrates the possibilities, but also the limitations, of using ex-vivo CLSM in a real-life clinical setting. The results clearly show worse sensitivity and specificity values in CLSM reporting of BCC in a real-life setting, compared with recently published studies. The poor rate of tumour detection in the tumour margin is particularly notable and clinically relevant.
Previous studies investigating the diagnostic accuracy of CLSM in BCC included only CLSM scans showing either BCC or no tumour (4, 11–14). Nevertheless, the fact that other diagnoses besides BCC were investigated is probably not the reason for the poorer results in the current study. If the tumour is found representatively in the CLSM section, the diagnosis is simple in most cases. An exception is tumours that occur in skin that is rich in hair follicles and sebaceous glands. In the current study, this was the most common cause of incorrect diagnosis in the tumour margins. Of 12 margins that were misdiagnosed by all 3 examiners, the tumours could still be discovered in 8 sections after the tumour localization was identified in the H&E section. A major advantage of H&E diagnostics is the presence of serial sections. If, for example, an adnexal structure cannot be reliably separated from tumour extensions, this often becomes unambiguous on the following sections. This possibility is not available with CLSM diagnostics. Another reason for the poor values for the margin examination is the partly insufficient visualization of the epidermis. The epidermis was detectable in the tumour margins in only 72% of cases. It is possible, therefore, that especially small remnants of superficial BCC were not detected by CLSM. The problem of so-called flattening has been discussed in previous studies (4, 11). This was found to be the cause in 4 out of 12 margins that were classified incorrectly by all 3 reporters. Another reason for the worse results of our study is probably the way the tissue was sectioned. In the current study, the majority of tissue samples were excised as a so-called “muffin”(9). In this technique, very large pieces of tissue are created, which reflect both the edges and the base of the tumour excised into a single plane. A comparable study by Grupp et al. (11) achieved significantly better results, with 73.6% sensitivity and 96.5% specificity. With an otherwise similar study setting, the major difference was that, in this study, the preparations were processed using a bread loaf technique or the La Galette method with separate visualization of the margins and base. This resulted in smaller sections, which could be displayed with higher quality in the CLSM. In fact, 10 of the 12 specimens that were incorrectly classified by all 3 investigators were embedded as muffin. This embedding technique therefore does not seem to be suitable for diagnostics using CLSM. When cutting FFPE sections, the block is incised in 3D histology coming from the periphery of the tumour towards the centre. Each section with the microtome brings the section plane of the H&E histology closer to the centre of the tumour. Thus, it is possible that some tumour remnants are “false-positive” in H&E histology. In the current study, however, this could not be identified as a cause for the low rate of BCC detection in the tumour margin. Knowing the BCC location in the H&E histology, the corresponding tumour could also be found in the CLSM in all specimens when epidermis was present.
The second objective of the current study can be answered less clearly. There are distinct individual differences between the physicians in the reporting of CLSM scans. Previous studies have already shown that it may be possible for inexperienced examiners to evaluate CLSM scans of BCC (7). Although the highest sensitivity values of BCC diagnosis are found for the experienced reporter DP3, this is accompanied by somewhat poorer specificity. Since, nowadays, CLSM scans can be digitally stained, the scans also appear in pink and blue, analogous to H&E staining. The appearance of digitally stained CLSM scans differs very little from staining with H&E (5). Thus, it may be possible to transfer the diagnostic knowledge of H&E-stained FFPE slides to the digitally stained CLSM scans without a lot of training. It has already been published that there is a learning curve in the evaluation of CLSM scans (15). The relatively high number of specimens diagnosed accurately by the inexperienced dermatopathologists possibly resulted in part from “learning by doing”. All this could explain the relatively similar diagnostic accuracy of the experienced and novice examiners.
Between the 2 H&E-trained board-examined dermatopathologists, who showed a very high agreement in the detection of the H&E sections, there were also different values in the CLSM detection. Thus, DP1 detected less tumour remnants than DP2, resulting in a lower sensitivity rate (59.5% vs 71.1%). The classification of BCC subtypes in the tumour centre also showed individual differences with concordance rates ranging from 54.1% to 74%. The relatively high concordance rate of 74% for the CLSM inexperienced examiner DP2 shows that the morphological knowledge from the H&E examination can be transferred to the setting of CLSM examination. A recently published study investigating the grading of BCC in biopsies shows a concordance of 84%, which is slightly better than the current study (16).
One reason for the individually different results could be the duration of the assessment. DP2 required more time to diagnose CLSM scans than DP1 for all entities (median time 42.5 s vs 20 s per section). Both dermatopathologists performing the evaluation of H&E and CLSM slides had the impression that the CLSM evaluation was more time-consuming than the evaluation of H&E slides. However, the time required for H&E evaluation in this study was not recorded and therefore cannot be directly compared. In the routine setting, most BCC sections can be diagnosed in a few seconds on the H&E slide. The differentiation of artefacts or adnexal structures in CLSM scans, in particular, required more time for the inexperienced examiner, as this often requires precise zooming into the scan. It is possible that DP1, in contrast to DP2 and DP3, worked as fast as he is accustomed to in the diagnosis of H&E stained slides and therefore could not detect all tumour remnants. In comparison with the study by Grupp et al. (11), however, the time required by both physicians (DP1 and DP2) was very low. Here, a mean time of 6 min 34 s was required for microscopic diagnosis of a tumour. This significantly shorter time requirement could also be a reason for the inferior results in the current study. However, it is notable that these were different cutting methods and possibly larger samples, so that a direct comparison is not applicable. The possible increase in time requirement could be a negative point in the usability and acceptance of ex-vivo CLSM by dermatopathologists.
The current study has some limitations. These are mainly due to the small number of samples. Since this was a time-limited real-life setting, no specific tumour types were selected among the included patients. Thus, rarer subtypes of BCC may not have been representatively captured by the current study.
In summary, this study confirms that it may be possible for inexperienced, H&E-trained examiners to evaluate CLSM scans. However, since there are differences between individual examiners, a conversion in the diagnostic technique should not be recommended without thorough familiarization with CLSM reporting. The poor accuracy in tumour margin control in this real-life setting is clinically relevant and could restrict the use of CLSM in clinical routine.
ACKNOWLEDGEMENTS
We thank the medical laboratory assistants of the Center for Dermatohistology and Oral Pathology Tübingen/Würzburg for excellent technical support. We would also like to thank the practice staff and physicians of the “Praxisklinik Tübingen – Skin and Veins” for the surgical care and treatment of our patients.
The study was approved by the ethics committee of Eberhard Karls University of Tübingen (899/2019BO1).
REFERENCES
- Lang BM, Balermpas P, Bauer A, Blum A, Brölsch GF, Dirschka T, et al. S2k- Leitlinie Basalzellkarzinom der Haut–Teil 1: Epidemiologie, Genetik und Diagnostik. J Dtsch Dermatol Ges 2019; 17: 94–104.
- Lang BM, Balermpas P, Bauer A, Blum A, Brölsch GF, Dirschka T, et al. S2k- Leitlinie Basalzellkarzinom der Haut–Teil 2: Therapie, Prävention und Nachsorge. J Dtsch Dermatol Ges 2019; 17: 214–231.
- Paoli J, Cogrel O, van der Geer S, Krekels G, de Leeuw J, Moehrle M, et al. ESMS position document on the use of mohs micrographic surgery and other micrographic surgery techniques in Europe. 2019; 1–36.
- Peters N, Schubert M, Metzler G, Geppert JP, Moehrle M. Diagnostic accuracy of a new ex vivo confocal laser scanning microscope compared to H&E- stained paraffin slides for micrographic surgery of basal cell carcinoma. J Eur Acad Dermatol Venereol 2019; 33: 298–304.
- Schüürmann M, Stecher M, Paasch U, Simon J, Grunewald S. Evaluation of digital staining for ex vivo confocal laser scanning microscopy. J Eur Acad Dermatol Venereol 2020; 34: 1496–1499.
- Malvehy J, Pérez- Anker J, Toll A, Pigem R, Garcia A, Alos L, et al. Ex vivo confocal microscopy: revolution in fast pathology in dermatology. Br J Dermatol 2020; 183: 1011–1025.
- Hartmann D, Krammer S, Bachmann MR, Mathemeier L, Ruzicka T, von Braunmühl T. Simple 3- criteria- based ex vivo confocal diagnosis of basal cell carcinoma. J Biophotonics 2018; 11: e201800062.
- Bennàssar A, Carrera C, Puig S, Vilalta A, Malvehy J. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol 2013; 149: 839–847.
- Möhrle M, Breuninger H. Die Muffin- Technik – eine Alternative zur Mohs’ Chirurgie. J Dtsch Dermatol Ges 2006; 4: 1080–1084.
- Eberle FC, Kanyildiz M, Schnabl SM, Schulz C, Häfner HM, Adam P, et al. Three dimensional (3D) histology in daily routine: practical implementation and its evaluation. J Dtsch Dermatol Ges 2014; 12: 1028–1035.
- Grupp M, Illes M, Mentzel J, Simon JC, Paasch U, Grunewald S. Routine application of ex vivo confocal laser scanning microscopy with digital staining for examination of surgical margins in basal cell carcinomas. J Dtsch Dermatol Ges 2021; 19: 685–692.
- Ziefle S, Schüle D, Breuninger H, Schippert W, Moehrle M. Confocal laser scanning microscopy vs 3-dimensional histologic imaging in basal cell carcinoma. Arch Dermatol 2010; 146: 843–847.
- Schüle D, Breuninger H, Schippert W, Dietz K, Moehrle M. Confocal laser scanning microscopy in micrographic surgery (three- dimensional histology) of basal cell carcinomas. Br J Dermatol 2009; 161: 698–700.
- Longo C, Pampena R, Bombonato C, Gardini S, Piana S, Mirra M, et al. Diagnostic accuracy of ex vivo fluorescence confocal microscopy in Mohs surgery of basal cell carcinomas: a prospective study on 753 margins. Br J Dermatol 2019; 180: 1473–1480.
- Karen J, Gareau D, Dusza S, Tudisco M, Rajadhyaksha M, Nehal K. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol 2009; 160: 1242–1250.
- Bergeret B, Masset F, Bekoy YD, Roger P, Habib F, Ovtchinnikoff B, et al. Diagnostic accuracy of digital staining ex vivo confocal microscopy for diagnosing and subtyping basal cell carcinoma in fresh pretherapeutic punch biopsies: a monocentric prospective study. Dermatology 2022; 5: 1–7.