ORIGINAL ARTICLE
Cost-effectiveness and Quality of Specialized and Routine Care in a German Cohort of Patients with Chronic Pruritus
Svenja MÜLLER1,2, Sonja STÄNDER2, Mandy NAATZ3, Matthias AUGUSTIN3 and Sabine STEINKE2,4
1Department of Dermatology and Allergy, University Hospital Bonn, Bonn, 2Department of Dermatology and Center for Chronic Pruritus, University Hospital Münster, Münster, 3German Center for Health Services Research in Dermatology (CVderm), University Medical Center Hamburg-Eppendorf (UKE), Hamburg and 4Bielefeld University, Medical School Ostwestfalen-Lippe (OWL), Bielefeld, Germany
Chronic pruritus is a prevalent interdisciplinary symptom with a strong influence on health-related quality of life. Patients need extensive diagnostics and long-term treatment. This retrospective and prospective cohort study compared routine and university-based specialized care in terms of cost-effectiveness and patient benefit. Direct medical and non-medical costs and patient-reported outcomes (PRO; pruritus intensity, quality of life, treatment needs and benefits) were assessed. Data analyses were conducted using descriptive methods and non-parametric statistical tests. A total of 300 adult patients (54.3% female) participated in the study. Six months after the treatment start in a specialized German pruritus care unit, the total costs were significantly reduced (mean total costs 686 € vs 433 € per patient per half year (total cohort); p < 0.001; mean out-of-pocket costs 198 € vs 124 € per half year (total cohort), p < 0.001). Pruritus intensity (numerical rating scale 5.3 vs 3.7, p < 0.001), quality of life (Dermatology Life Quality Index 8.9 vs 5.7, p < 0.001) and patient benefit (Patient Benefit Index Pruritus 1.2 vs 2.1, p < 0.001) improved significantly (total cohort). The results of this study show, that treatment of chronic pruritus patients in a specialized itch centre leads to an improvement in patient benefit and reduces the economic burden at the same time.
Key words: cost-benefit analysis; cost of illness; patient-reported outcome measures; pruritus; quality of healthcare.
SIGNIFICANCE
Chronic pruritus is a burdensome symptom that affects 16.8% of the German working population and requires specialized, often cost-intensive diagnostics and treatment. Cost-effectiveness analyses are necessary to provide high-quality care and to reduce economic burden. This is the first retrospective and prospective cohort study that compares treatment quality and cost of a specialized university-based German itch centre with that of routine care. The results show that the treatment of patients with chronic pruritus in a specialized university itch centre improves the quality of care and patient penefits and, at the same time, reduces the economic burden.
Citation: Acta Derm Venereol 2023; 103: adv4868. DOI: https://doi.org/10.2340/actadv.v103.4868.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jan 19, 2023; Published: Apr 21, 2023
Corr: Svenja Müller, Department of Dermatology and Allergy, University Hospital Bonn, Venusberg-Campus 1, DE-53127 Bonn, Germany. E-mail: svenja2.mueller@ukbonn.de
Competing interests and funding: SM is supported by the Christine Kühne-Center for Allergy Research and Education and has been an advisor, speaker or investigator for Galderma, Incyte Inc. and Eli Lilly, outside the submitted work. SS has been an advisor, speaker or investigator for Abbvie, Almirall, Beiersdorf, Bellus Health, Benevolent, Bionorica, Cara Therapeutics, Clexio, Dermasence, Eli Lilly, Escient, Galderma, Grünenthal, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Trevi Therapeutics, Sanofi, Unna Academy and Vifor, outside the submitted work. MN reports personal fees from Lilly Deutschland GmbH and personal fees from Novartis, outside the submitted work. SS reports travel expenses and/or speaker fees and/or consultant fees from Astellas Pharma, Marpinion GmbH and Cemka. MA has served as a consultant and/or paid speaker for and/or has received research grants and/or fees for consulting and/or scientific lectures for and/or got travel expenses reimbursed and/or participated in clinical trials sponsored by companies, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene Corporation, Centocor, Eli Lilly, Galderma, Janssen-Cilag, Leo, Medac, MSD, Mundipharma, Novartis, Pfizer, Sandoz and Xenoport, outside the submitted work.
The authors have no conflicts of interests to declare.
INTRODUCTION
Chronic pruritus (CP; duration at least 6 weeks) affects approximately 16.8% of the German working population (1). Patients experience a physical and mental burden of disease, as quality of life, sleep and social interaction are highly affected (3). CP is associated with many comorbidities (e.g. neurological, psychiatric, internal) and requires extensive diagnostics and long-term, specialized treatment (2).
According to the classification of the International Forum for the Study of Itch (IFSI), patients with CP can be divided into 3 groups: CP on diseased skin (IFSI I), CP on non-diseased skin (IFSI II) and CP with chronic scratch lesions (IFSI III) (2). Patients with chronic prurigo, especially prurigo nodularis (PN), make up a large proportion of IFSI III (2).
In the context of diagnosis and treatment monitoring of CP, pruritus-specific, validated patient-reported outcome (PRO) measures are required to assess pruritus intensity, health-related quality of life (HRQoL), anxiety, depression, sleep and treatment needs and benefits.
The patient-individual diagnostic workup of CP is often complex and includes the assessment of pruritus underlying and accompanying disorders, laboratory tests and medical imaging (e.g. X-ray, ultrasound, magnetic resonance imaging (MRI), computed tomography (CT)); therapies often consist of costly off-label medication (2).
In Germany, patients with CP are treated in inpatient and outpatient settings by general practitioners and specialists (4). Diagnosis and treatment often remain unsuccessful in routine care, as therapeutic setbacks and, often, long treatment spans are described (5). Complex diagnostics and cost-intensive therapies, which are insufficiently represented in the German Diagnosis-Related Groups (G-DRG) system, mean high financial expenditure for treating physicians (6). Resident physicians’ budgets are limited and their time quota is exhausted rapidly, which makes patients feel inadequately treated and informed (6).
Specialized interdisciplinary care units, often attached to university hospitals, may improve patient treatment benefit by delivering sufficient diagnostics and treatment. This might cause additional cost to the health insurance and increase future health expenses (7). Therefore, cost-effectiveness analyses are essential to investigate costly specialized treatment and to improve quality of routine and specialized care of patients with CP (8).
The aim of the current study was to analyse the cost-effectiveness of treatment of chronic itch patients at a specialized university-based German itch centre in comparison with routine care.
MATERIALS AND METHODS
Data collection
Patients with CP were recruited when attending the competence centre for chronic pruritus (Kompetenzzentrum Chronischer Pruritus (KCP)) in Münster, North Rhine-Westphalia, Germany, for the first time (T0). Inclusion criteria were: age ≥ 18 years and the ability to give and declare consent. Data collection was carried out using paper-based methods in personal interviews and on mobile devices that were routinely used at the centre (9, 10).
At T0 and 6 months after the first consultation (T1), direct medical and non-medical costs and PRO data were calculated from the patients’ and medical records. The patients were assigned to the pruritus groups according to the IFSI classification, in order to enable subgroup comparisons.
Patient-reported outcomes
Pruritus intensity was measured with a numerical rating scale (NRS) (0–10; NRS-24 h: mean intensity of the last 24 h). The minimal clinically important difference (MCID, smallest PRO change that can be detected by the patient) for the NRS-Itch is defined as a change of 2–2.5 points (11). HRQoL was analysed using the Dermatological Life Quality Index (recommended MCID 4 points) (12) and the pruritus-specific ItchyQoL (13) (DLQI: 0–30, ItchyQol: 0–110). Patients’ needs and benefits were assessed with the Patient Benefit Index for pruritus (PBI-P: 0–4), which consists of 27 treatment goals of 5 different need dimensions. The “cut off value” for a patient relevant benefit is determined as ≥ 1 (14).
Costs
Direct medical costs include all monetary services that are provided in the context of diagnosis and treatment of a disease (inpatient and outpatient costs including medical consultations, services, medication, etc.) (15). These costs were assessed according to G-DRG (16) and the German system for reimbursement of outpatient care (“EBM – Einheitlicher Bewertungsmaßstab”, Uniform Value Scale, quarter 1/2017, respectively, GOÄ, “Gebührenordnung für Ärzte”, fees for physicians, 1 January 2002) guidelines as well as to the valid reimbursement medication price in the German Drug Directory (as referenced in the “LauerTaxe”) (reference: LAUER-TAXE® - Apotheke - Produkte - cgm.com).
Direct non-medical costs occur as a by-product of the use of medical resources (e.g. costs for transportation to the physician, costs for skin care, special food or clothing, family care costs) (15). In order to record these as precisely as possible, patients were asked to keep receipts or bank statements and to bring them to the personal interview. If these were not available, patients were asked to estimate these costs as accurately as possible.
Cost-effectiveness was calculated from the perspective of the total compulsory health insurance cost, using the formula:
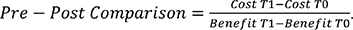
Statistical analysis
Statistical analyses were performed in SPSS Statistics for Windows (version 25.0 (IBM Corp., Armonk, N.Y., USA) using descriptive analyses.
Prior simulations showed that the data were not normally distributed. Therefore, non-parametric tests were used for analysis of significance. The Wilcoxon signed-rank test was used for post hoc analyses (comparison of cost-effectiveness and quality of specialized and routine care within each IFSI group). For intergroup comparisons (comparisons between IFSI group I–III) the Kruskal–Wallis test was used. If the Kruskal–Wallis test showed significant results between the 3 groups, the Mann–Whitney U test was added as a more specific test to investigate 2 groups more precisely.
Ethics statement
The study was approved by the Medical Ethics Committee of the University Medical Center Münster, Germany (2015-262-f-S). All patients gave written informed consent.
RESULTS
At T0, 300 adult patients with CP were recruited (mean age 57 years, 54.3% female, mean pruritus duration 92.0 ± 123.0 months). Of these, 85% (n = 255) had presented themselves to outpatient clinics 6 months beforehand. Out of all the patients, 246 (82%) attended the follow-up examination (T1). Most patients belonged to IFSI group II (CP on non-diseased skin) (see Table I). The most common underlying diseases for chronic pruritus were dermatoses (50.3%; n = 151), followed by multifactorial (22.3%, n = 67), neurological (10.7%, n = 32), systemic (8%, n = 24), psychological/psychosomatic (0.7%, n = 2) and other causes (8%, n = 24).
| Sociodemographic data | T0 (n = 300 patients, 100%) | T1 (n = 246 patients, 82%) |
| Male patients, n (%) | 137 (45.7) | 137 (55.7) |
| Female patients, n (%) | 163 (54.3) | 109 (44.3) |
| Age, years, mean ± SD (median) | 57.4 ± 17.3 (59.0) | 58.7 ± 16.8 (60.0) |
| Pruritus manifestation | T0 (n = 300 patients, 100%) | T1 (n = 246 patients, 82%) |
| Generalized pruritus, n (%) | 218 (72.7) | 177 (72.0) |
| Localized pruritus, n (%) | 82 (27.3) | 69 (28.0) |
| Classification of Itch (IFSI) | T0 (n = 300 patients, 100%) | T1 (n = 246 patients, 82%) |
| Pruritus on diseased skin (IFSI group I), n (%) | 106 (35.3) | 85 (34.6) |
| Pruritus on non-diseased skin (IFSI group II), n (%) | 122 (40.7) | 105 (42.7) |
| Chronic scratch lesions (IFSI group III), n (%) | 72 (24.0) | 56 (22.8) |
| Treatment modalities | T0 (n = 300 patients, 100%) | T1 (n = 246 patients, 82%) |
| Outpatient treatment, n (%) | 255 (85.0) | 212 (86.2) |
| Inpatient treatment, n (%) | 35 (11.3) | 28 (11.4) |
| Duration of inpatient treatment, days, mean ± SD | 14.2 ± 12.5 | 7.3 ± 6.2 |
| Systemic treatment | T0 (n = 298 patients, 99.3%) | T1 (n = 245 patients, 81.6%); p-values |
| Antihistamines n (%) | 179 (60.1) | 88 (35.9); < 0.001*** |
| Corticosteroids n (%) | 29 (9.7) | 4 (1.6); 0.003** |
| Anticonvulsants | 25 (8.4) | 28 (11.4); 0.157 |
| Immunosuppressants | 6 (2.0) | 9 (3.7); 0.564 |
| Naloxone/naltrexone | 3 (1.0) | 3 (1.2); 0.564 |
| Topical treatment | T0 (n = 298 patients, 99.3%) | T1 (n = 245 patients, 81.6%); p-values |
| Topical corticosteroids | 139 (46.6) | 74 (30.2); 0.001** |
| Topical immunomodulators | 25 (8.4) | 19 (7.8); 0.705 |
| Topical capsaicin | 7 (2.4) | 5 (2.0); 0.564 |
| Topical emollients | 91 (30.5) | 43 (17.6); < 0.001*** |
| Topical anti-infectives | 34 (11.4) | 15 (6.1); 0.480 |
| Combination treatment | 49 (16.4) | 10 (4.1); < 0.001*** |
| For systemic and topical treatment, the corresponding p-values are shown to enable post-hoc comparisons (Wilcoxon signed-rank test). *p < 0.05; **p < 0.01; ***p < 0.001; topical anti-infectives: topical antibiotics, antiseptics, antimycotics, antiparasitics, combination treatment: pharmacy-mixed creams that combine at least two active ingredient classes, e.g. topical corticosteroids and anti-infectives. SD: standard deviation; IFSI: International Forum for the Study of Itch. |
||
Systemic therapies comprised antihistamines, corticosteroids, gabapentinoids, opioid antagonists and immunosuppressants; topical therapies included corticosteroids, calcineurin inhibitors, anti-infectives (antiseptics, antimycotics, antiparasitics) and emollients. The prescription of systemic antihistamines, systemic corticosteroids, topical corticosteroids, as well as topical emollients and combination treatment was significantly reduced after the first presentation in favour of a more focused prescription, due to expert treatment (p < 0.005; see Table I).
Patient-reported outcomes
Pruritus intensity and HRQoL improved significantly according to the NRS and the DLQI/ItchyQol at T1 (p < 0.001), while the patient benefit increased significantly (PBI, p < 0.001; see Fig. 1). Significant differences were found between the IFSI groups (see Table SI): patients with diseased skin (IFSI I & IFSI III) reported a higher pruritus intensity (NRS-mean 24 h, p < 0.005) and a more impaired HRQoL (DLQI: p < 0.01; ItchyQol: p < 0.001) at T0 than patients with non-diseased skin (IFSI II), whereas no significant differences were found at T1. At T0 and T1, the highest impairment in HRQoL, as measured with the ItchyQol, was found in patients with chronic scratch lesions (IFSI III, p < 0.001).
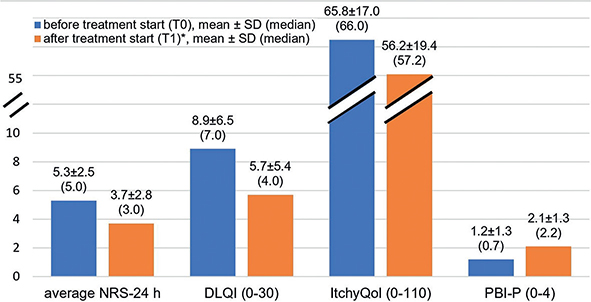
Fig. 1. Patient-reported outcomes (PRO) before and after treatment start in a specialized pruritus care unit (all p ≤ 0.001; Wilcoxon signed-rank test). *p (post-hoc difference T1–T0) < 0.001; NRS: numerical rating scale; DLQI: Dermatology Life Quality Index; PBI-P: Patient Benefit Index for Pruritus; SD: standard deviation.
Cost of illness
Six months after the treatment start in the specialized care unit total costs were significantly reduced compared with previous routine care (433.42 vs 686.4 € per patient per half year (phy) (p < 0.001). The main cost drivers at both assessment periods were costs of inpatient and outpatient treatment (composed of costs of physician visit; diagnostics including blood test, allergy and food intolerance test, MRI, CT; systemic and topical medication, lymphatic drainage, ultraviolet (UV) therapy, physiotherapy). These costs were significantly reduced, by approximately 212 € (inpatient treatment, p < 0.05) and 134 € (outpatient treatment, p < 0.001), following specialized care treatment start. Cost for topical therapy were reduced by more than 50% (outpatient treatment, total cohort; p ≤ 0.001); and patients’ out-of-pocket costs by almost 40% (total cohort; p < 0.001, see Table II).
| Costs/patient/6 months (€) | T0 | T1 | Diff. T1–T0 | p-value |
| Mean (95% CI) | Mean (95% CI) | |||
| Health insurance costs | ||||
| Inpatient treatment | 315.6 (166.4–464.8) | 103.3 (43.3–163.3) | –212.3 | 0.021* |
| Outpatient treatment | 333.9 (262.5–405.3) | 200.0(153.3–246.7) | –133.9 | < 0.001*** |
| Outpatient physician visit | 41.5 (38.0–44.9) | 17.6 (13.8–21.4) | –23.9 | < 0.001*** |
| Outpatient diagnostics | 81.5 (58.3–104.6) | 46.3 (29.4–63.2) | –35.2 | < 0.001*** |
| Systemic medication | 114.9 (67.3–162.6) | 87.4 (58.4–116.5) | –27.5 | 0.003** |
| Topical medication | 85.8 (53.9–117.6) | 40.5 (29.5–51.5) | –45.3 | < 0.001*** |
| Other therapies | 16.1 (10.4–21.9) | 5.2 (2.1–8.4) | –10.9 | 0.002** |
| Total costs to the health insurance | 548.9 (420.0–677.9) | 322.6 (230.8–414.4) | –226.3 | < 0.001*** |
| Out-of-pocket costs | 197.7 (158.6–236,7) | 123.5 (93.1–154.0) | –74.2 | < 0.001*** |
| Total costs | 686.4 (543.4–829.3) | 433.4 (315.2–551.7) | –253.0 | < 0.001*** |
| Other therapies: physical therapy, ultraviolet (UV) therapy, lymphatic drainage. Outpatient diagnostics: blood test, allergy test, food intolerance tests, magnetic resonance imaging, computed tomography; systemic medication: antihistamines, corticosteroids, anticonvulsants, opioid antagonists (naloxone/naltrexone), immunosuppressants; topical medication: corticosteroids, immunomodulators, anti-infectives, antiseptics, antimycotics, antiparasitics, emollients, combination products; other therapies: lymphatic drainage, light therapy, physiotherapy, combination therapies. *p < 0.05; **p < 0.01; ***p < 0.001 (Wilcoxon signed-rank test). 95% CI: 95% confidence interval for mean (bootstrap results). |
||||
At T0 and T1, patients with diseased skin (IFSI I, III) reported significantly higher costs (especially out-of-pocket costs) than patients with pruritus on non-diseased skin (IFSI II) (p < 0.05) (see Table III), while IFSI I patients had the highest costs for topical treatment of all (p < 0.001).
| T0 | T1 | |||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Inpatient treatment | ||||
| IFSI I | 150.6 (11.3–289.9) | 127.6 (–10.2–265.4) | ||
| IFSI II | 327.5 (45.4–609.6) | 54.6 (1.2–108.0) | ||
| FSI III | 538.4 (191.6–885.2) | 0.09 | 150.1 (30.5–269.7) | 0.29 |
| Outpatient treatment | ||||
| Physician visit | ||||
| IFSI I | 39.8 (35.0–44.7) | 19.6 (12.3–26.9) | ||
| IFSI II | 42.9 (36.5–49.4) | 14.6 (8.5–20.7) | ||
| IFSI III | 41.4 (34.8–47.9) | 0.89 | 19.7 (13.6–25.8) | 0.10 |
| Diagnostics | ||||
| IFSI I | 62.8 (38.4–87.1) | 58.7(25.9–91.4) | ||
| IFSI II | 94.6 (54.8–134.4) | 32.2 (8.1–56.2) | ||
| IFSI III | 82.9 (55.1–110.6) | 0.07 | 46.1 (20.3–71.9) | 0.01* |
| Systemic medication | ||||
| IFSI I | 101.3 (37.1–165.4) | 90.4 (32.9–147.8) | ||
| IFSI II | 110.7 (37.3–184.1) | 73.3 (41.6–105.0) | ||
| IFSI III | 114.1 (55.0–173.3) | 0.13 | 108.3 (33.7–182.9) | 0.90 |
| Topical medication | ||||
| IFSI I | 119.3 (55.3–183.2) | 57.0 (37.5–76.5) | ||
| IFSI II | 53.7 (23.5–83.9) | 27.2 (9.8–44.5) | ||
| IFSI III | 81.6 (49.7–113.4) | < 0.001*** | 38.0 (18.8–57.1) | < 0.001*** |
| Other therapies | ||||
| IFSI I | 19.9 (9.9–30.0) | 4.0 (–0.5–8.5) | ||
| IFSI II | 7.4 (1.7–13.1) | 5.9 (0.5–11.2) | ||
| IFSI III | 24.4 (13.1–35.7) | 0.01* | 5.5 (–0.8–11.9) | 0.88 |
| Patient costs | ||||
| IFSI I | 224.7 (160.6–288.8) | 139.3 (86.9–191.7) | ||
| IFSI II | 173.5 (119.5–227.6) | 84.5 (51.6–117.4) | ||
| IFSI III | 208.7 (147.1–270.4) | 0.04* | 165.5 (79.7–251.3) | 0.02* |
| Other therapies: physical therapy, ultraviolet (UV) therapy, lymphatic drainage. SD: standard deviation. *p < 0.05; **p < 0.01; ***p < 0.001 (differences between the groups). |
||||
The highest out-of-pocket costs were found for IFSI I (p < 0.05) at T0, and for IFSI III at T1 (p < 0.05). Costs for inpatient treatment, physician visits and systemic medication did not differ significantly between the groups (see Table III).
Cost-effectiveness analysis
Significant differences in cost-effectiveness between the IFSI groups could be found only for the differences in NRS-mean 24 h (p < 0.01). Patients with CP on diseased skin had a better cost-effectiveness (mean ± SD; median –66.1 ± 585.7; 0.0; Δ€/ΔNRS/phy than patients with pruritus with non-diseased skin (mean ± SD; median: 97.3 ± 276.9; 26.7; Δ€/ΔNRS/phy) and chronic scratch lesions (mean ± SD; median: –30.8 ± 681.7; –5.8; Δ€/ΔNRS/phy).
DISCUSSION
To our knowledge, this is the first cost-effectiveness analysis from a societal perspective in pruritus research in Europe. Compared with other prevalent diseases with high economic impact, such as chronic low back pain (CLBP), the mean duration of CP (current study 7.7 ± 10.3 years; other studies related to CP approximately 3–6 years (3, 17)) seems to be longer (duration of 92% of all episodes of CLBP: 6 months or less (18)) and intensity values (NRS) prior to and after interventions appear to be slightly higher for CP (3,19) . In terms of HRQoL, patients with CP are often even more affected than patients with other chronic inflammatory skin diseases, such as psoriasis or atopic dermatitis (3).
Moreover, treatment benefit (assessed with PBI) seems to be lower in CP than in other pruritic skin diseases (20). Whereas a score of 2.1 ± 1.3 was calculated for patients CP in specialized university care in the current study (T1), patients with psoriasis tend to achieve highest PBI values (e.g. 3.0 ± 1.0 (20)), followed by patients with atopic dermatitis (e.g. PBI = 2.3 ± 0.8 (20)). This reflects the high need for improved care of patients with CP, as they often do not receive sufficient antipruritic therapies (21). Recently, it has been shown that 77% of patients who received outpatient care only did not benefit from an improvement in pruritus, but still experienced pruritus frequently or permanently (21). Moreover, despite a notable improvement in all PRO after the treatment start in a specialized pruritus care unit, patients remain affected by pruritus and require care.
Despite the severe impairment of patients with CP and the extensive diagnostics and expensive long-term treatments, including over-the-counter (OTC) medication (22), the economic burden to the compulsory health insurance and to the patient, has been analysed in only a few studies.
The analysis of a nationally representative survey assessing patterns of utilization of outpatient care in the USA shows that expenditures in the USA for pruritus account for 90 billion US dollar ($) per year (23). Recently, annual median total costs of $1,067 (~880 €) for patients with CP were presented, of which $286 (~236 €) were direct costs, $662 (~546 €) opportunity costs, and $118 (~97 €) OTC costs (24). In the current study, direct costs are almost 5 times higher than in the US study, which could be due to unconsidered costs for diagnostics, which account for a quarter of the total costs of outpatient treatment in the current study. Especially in CP, diagnostics are costly and time-consuming owing to a patient-individual systematic diagnostic work-up, laboratory analyses, imaging tests and interdisciplinary cooperation (25).
Compared with the costs of CLBP (78–380 € per capita per year (26–28)), the economic burden of CP might be much higher (686 € respectively (resp.) 433 € per patient per half-year), though only direct medical and non-medical costs were focused. When treating psoriasis, total direct costs range up to 5,164 € per patient per year (29). Therefore the costs for CP are also expected to increase in the future, as new immunomodulating therapies, such as dupilumab and nemolizumab, are in the pipeline, especially for PN (30). In addition, outpatient systemic therapies have already been among the main cost drivers in the current study conducted before the advent of biologic agents in CP. Costs for inpatient stays are also usually one of the main cost drivers in pruritic dermatological diseases other than PN (31) (e.g. psoriasis (29), chronic hand eczema (32), and atopic dermatitis (33)).
Unfortunately, the duration of skin improvement after inpatient treatment is short, and a high need for outpatient biologic prescription is reported after the discharge of patients with psoriasis without decreased follow-up costs (34). This might also apply to CP due to similar inpatient treatment (UV therapy, intensified local therapy), short hospitalization periods and an increasing pressure of economizing (4).
For severely affected patients, those with multiple comorbidities who require extensive diagnostic procedures, inpatient treatment will remain an important pillar of therapy in the future. For less affected patients, the increase and strengthening of specialized outpatient care may reduce the need and costs of inpatient treatment. The alarming shift from inpatient and outpatient settings towards costly emergency room treatment of patients with CP (23) could be prevented by easier access to specialized outpatient pruritus care. As proposed by Ständer et al. (6), specialized centres should receive an additional compensation to enable provision of better care and manage increasing economic regulations as cost pressures increase.
The prescription of most drugs, except for anti-histamines, is off-label for pruritus, which could lead to uncertainty among outpatient clinics (5). Consequently, topical therapies and OTC medication are recommended predominantly, resulting in high out-of-pocket costs (6) that were more than 3 times as high as in the US comparative study (24). In the current study, patients with CP had higher annual out-of-pocket costs than patients with mild and moderate atopic dermatitis, as described by Launois et al. (35). However, out-of-pocket costs in patients with psoriasis and atopic dermatitis that were comparable and higher than in the current study, are reported as well (29, 34, 35, 36, 37).
The current data show that patients with diseased skin (IFSI I and III) are significantly more burdened than patients with CP on non-diseased skin (significantly higher pruritus intensity and impairment of HRQoL). Patients in the IFSI III group report a significantly higher pruritus intensity than IFSI I and II before treatment start, which correlates with other results (3, 38, 39). Regarding ItchyQol, IFSI III patients show significantly higher score values than IFSI I and II patients at both times of examination, which matches other data (3). It has been reported that 50% of IFSI III patients have psychiatric comorbidities and are more stressed psychologically than other patients (40). Patients with PN are severely affected, experience the highest pruritus intensities, a highly negatively affected quality of life and mental health and increased systemic diseases in comparison with patients with other inflammatory skin disorders (17).
The current data show statistically higher costs of diagnostics, topical medication, and patient expenses for patients with CP on diseased skin, closely followed by patients with chronic scratch lesions. The high financial burden on the patients themselves is underlined by the fact that patients with chronic scratch lesions, especially, rated the therapeutic need of having lower out-of-pocket treatment costs as more important than did the other groups (38).
In particular, the treatment of high-need patients (patients with chronic scratch lesions, such as PN) requires more attention.
Study limitations
Other cost analyses determine a high impact of indirect costs (costs of lost productivity, absenteeism, inability to work, presenteeism, reduced functionality in terms of quantity and quality while working, early retirement) as they account for more than 80% of total costs (26). Thus, the economic burden of pruritus may be even higher, as they were not considered in this cost-effectiveness analysis.
Moreover, the current results may have been influenced by some bias that due to the chosen methodology: (i) as some patients had to estimate their direct non-medical costs, if receipts or bank statements were not available, recall bias cannot be ruled out, which might have affected the actual costs in both directions (under- and over-estimation of costs); (ii) patients were included and prospectively observed in the study who had been diagnosed and treated in different outpatient settings previously (primary/secondary care services). One should bear in mind that the observed reduction in costs and improvement in cost-effectiveness might result from less diagnostics (which might have been done prior to the first presentation in the specialized centre) and more targeted therapies (since less effective therapies may have already been tried previously). Furthermore, it is possible that a more focused and rational treatment plan, which is predominantly worked out in a specialized centre, may also determine cost-reductions.
The generalizability of cost of illness analyses is limited, because cost calculations depend on the different national health systems in which they are conducted. The cost calculation in the current study is, for example, based on German Diagnosis Related Groups (G-DRG) and the German system for reimbursement of outpatient care (“EBM - Einheitlicher Bewertungsmaßstab”, Uniform Value Scale, quarter 1/2017 respectively GOÄ, “Gebührenordnung für Ärzte”, fees for physicians, dated 1 January 2002) guidelines. Therefore, the cost of illness analysis is of only limited significance for other countries, on the one hand. On the other hand, the data could very well mirror cost-effectiveness and quality of specialized care of itch centres throughout Germany. The study’s single-centre design and the pre-post comparison, however, impairs the generalization of results. For further evaluation, prospective long-term, multicentre cost of illness analyses are required.
Conclusion
This study shows that the treatment of patients with CP in a specialized university itch centre improves the quality of care and patient benefits and, at the same time, reduces the economic burden. Further research concerning the medical (e.g. the development of new effective antipruritic therapies) and (socio-)economic point of view (e.g. effectiveness of interventions and the economic burden of CP) is imperative to improve the outcomes and care of patients with CP.
ACKNOWLEDGEMENTS
This work was supported by the Open Access Publication Fund of the University of Bonn.
The study was reviewed and approved by the Medical Ethics Committee of the University Medical Center Münster, Germany; approval #2015-262-f-S.
REFERENCES
- Ständer S, Schäfer I, Phan NQ, Blome C, Herberger K, Heigel H, et al. Prevalence of chronic pruritus in Germany: results of a cross-sectional study in a sample working population of 11,730. Dermatology 2010; 221: 229–235.
- Weisshaar E, Szepietowski JC, Dalgard FJ, Garcovich S, Gieler U, Giménez-Arnau AM, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol 2019; 99: 469–506.
- Steinke S, Zeidler C, Riepe C, Bruland P, Soto-Rey I, Storck M, et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: results of the European Academy of Dermatology and Venereology Network on Assessment of Severity and Burden of Pruritus (PruNet) cross-sectional trial. J Am Acad Dermatol 2018; 79: 457–463.e5.
- Pereira MP, Steinke S, Bruland P, Ständer HF, Dugas M, Augustin M, et al. Management of chronic pruritus: from the dermatological office to the specialized itch center: a review. Itch (Phila) 2017; 2: e6.
- Ständer S, Zeidler C, Magnolo N, Raap U, Mettang T, Kremer AE, et al. Clinical management of pruritus. J Dtsch Dermatol Ges 2015; 13: 101–115.
- Ständer S, Ständer HF, Steinke S, Bruland P, Dugas M, Augustin M. Chronischer Pruritus: Versorgung in der Praxis. Hautarzt 2016; 67: 640–647.
- Lim HW, Collins SAB, Resneck JS, Bolognia JL, Hodge JA, Rohrer TA, et al. The burden of skin disease in the United States. J Am Acad Dermatol 2017; 76: 958–972.
- Fleßa S. Gesundheitsökonomik: Eine Einführung in das wirtschaftliche Denken für Mediziner; mit 17 Tabellen. 2., durchges. und aktualisierte Aufl. Berlin: Springer; 2007.
- Soto-Rey I, Rehr M, Bruland P, Zeidler C, Riepe C, Steinke S, et al. Electronic collection of multilingual patient-reported outcomes across Europe. Methods Inf Med 2018; 57: e107–e114.
- Storck M, Zeidler C, Rehr M, Riepe C, Dugas M, Ständer S, et al. Validation of pruritus measures gathered with the electronic patient-reported outcome system MoPat. Acta Derm Venereol 2018; 98: 38–43.
- Reich A, Riepe C, Anastasiadou Z, Mędrek K, Augustin M, Szepietowski JC, et al. Itch assessment with visual analogue scale and numerical rating scale: determination of minimal clinically important difference in chronic itch. Acta Derm Venereol 2016; 96: 978–980.
- Basra MKA, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230: 27–33.
- Krause K, Kessler B, Weller K, Veidt J, Chen SC, Martus P, et al. German version of ItchyQoL: validation and initial clinical findings. Acta Derm Venereol 2013; 93: 562–568.
- Blome C, Augustin M, Siepmann D, Phan NQ, Rustenbach SJ, Ständer S. Measuring patient-relevant benefits in pruritus treatment: development and validation of a specific outcomes tool. Br J Dermatol 2009; 161: 1143–1148.
- Héquet D, Huchon C, Soilly A-L, Asselain B, Berseneff H, Trichot C, et al. Direct medical and non-medical costs of a one-year care pathway for early operable breast cancer: results of a French multicenter prospective study. PLoS One 2019; 14: e0210917.
- Hensen P, Beissert S, Bruckner-Tuderman L, Luger TA,Roeder N, Müller ML. Introduction of diagnosis-related groups in Germany: evaluation of impact on in-patient care in a dermatological setting. Eur J Public Health 2008; 18: 85–91.
- Zeidler C, Pereira MP, Dugas M, Augustin M, Storck M, Weyer-Elberich V, et al. The burden in chronic prurigo: patients with chronic prurigo suffer more than patients with chronic pruritus on non-lesional skIn: a comparative, retrospective, explorative statistical analysis of 4,484 patients in a real-world cohort. J Eur Acad Dermatol Venereol 2021; 3: 738–743.
- Shekelle PG, Markovich M, Louie R. An epidemiologic study of episodes of back pain care. Spine (Phila Pa 1976) 1995; 20: 1668–1673.
- Suzuki H, Aono S, Inoue S, Imajo Y, Nishida N, Funaba M, et al. Clinically significant changes in pain along the Pain Intensity Numerical Rating Scale in patients with chronic low back pain. PLoS One 2020; 15: e0229228.
- Topp J, Augustin M, Usslar K von, Gosau R, Reich K, Reusch M, et al. Measuring patient needs and benefits in dermatology using the Patient Benefit Index 2.0: a Validation Study. Acta Derm Venereol 2019; 99: 211–217.
- Kopyciok MER, Ständer HF, Osada N, Steinke S, Ständer S. Prevalence and characteristics of pruritus: a one-week cross-sectional study in a German dermatology practice. Acta Derm Venereol 2016; 96: 50–55.
- Stull C, Lavery MJ, Yosipovitch G. Advances in therapeutic strategies for the treatment of pruritus. Expert Opin Pharmacother 2016; 17: 671–687.
- Tripathi R, Knusel KD, Ezaldein HH, Bordeaux JS, Scott JF. The cost of an itch: a nationally representative retrospective cohort study of pruritus-associated health care expenditure in the United States. J Am Acad Dermatol 2019; 80: 810–813.
- Luk KM, Shaw FM, Zhang C, Culler SD, Chen SC. The annual direct and indirect health care costs for patients with chronic pruritus and their determining factors. J Invest Dermatol 2020; 140: 699–701.
- Ständer S, Pogatzki-Zahn E, Stumpf A, Fritz F, Pfleiderer B, Ritzkat A, et al. Facing the challenges of chronic pruritus: a report from a multi-disciplinary medical itch centre in Germany. Acta Derm Venereol 2015; 95: 266–271.
- Olafsson G, Jonsson E, Fritzell P, Hägg O, Borgström F. Cost of low back paIn: results from a national register study in Sweden. Eur Spine J 2018; 27: 2875–2881.
- Radoičić MJ, Božović BV, Ilić KD, Janković SM, Anđelković JZ, Kostić MJ. Pharmacoeconomic Aspects of low back pain treatment: cost of illness study in the Republic of Serbia. Acta Med Port 2019; 32: 272–278.
- Ekman M, Johnell O, Lidgren L. The economic cost of low back pain in Sweden in 2001. Acta Orthop 2005; 76: 275–284.
- Jungen D, Augustin M, Langenbruch A, Zander N, Reich K, Strömer K, et al. Cost-of-illness of psoriasis – results of a German cross-sectional study. J Eur Acad Dermatol Venereol 2018; 32: 174–180.
- Müller S, Bieber T, Ständer S. Therapeutic potential of biologics in prurigo nodularis. Expert Opin Biol Ther 2022; 22: 47–58.
- Whang KA, Kang S, Kwatra SG. Inpatient burden of prurigo nodularis in the United States. Medicines (Basel) 2019; 6: 88.
- Diepgen TL, Scheidt R, Weisshaar E, John SM, Hieke K. Cost of illness from occupational hand eczema in Germany. Contact Dermatitis 2013; 69: 99–106.
- Narla S, Hsu DY, Thyssen JP, Silverberg JI. Inpatient financial burden of atopic dermatitis in the United States. J Invest Dermatol 2017; 137: 1461–1467.
- Steinke SIB, Peitsch WK, Ludwig A, Goebeler M. Cost-of-illness in psoriasis: comparing inpatient and outpatient therapy. PLoS One 2013; 8: e78152.
- Launois R, Ezzedine K, Cabout E, Reguai Z, Merrhand S, Heas S, et al. Importance of out-of-pocket costs for adult patients with atopic dermatitis in France. J Eur Acad Dermatol Venereol 2019; 33: 1921–1927.
- Sohn S, Schoeffski O, Prinz J, Reich K, Schubert E, Waldorf K, et al. Cost of moderate to severe plaque psoriasis in Germany: a multicenter cost-of-illness study. Dermatology 2006; 212: 137–144.
- Mohr N, Naatz M, Zeervi L, Langenbruch A, Bieber T, Werfel T, et al. Cost-of-illness of atopic dermatitis in Germany: data from dermatology routine care. J Eur Acad Dermatol Venereol 2021; 35: 1346–1356.
- Steinke S, Bruland P, Blome C, Osada N, Dugas M, Fritz F, et al. Chronic pruritus: evaluation of patient needs and treatment goals with a special regard to differences according to pruritus classification and sex. Br J Dermatol 2017; 176: 363–370.
- Hayani K, Weiss M, Weisshaar E. Clinical findings and provision of care in haemodialysis patients with chronic itch: new results from the German Epidemiological Haemodialysis Itch Study. Acta Derm Venereol 2016; 96: 361–366.
- Lehmann M, Cazzaniga S, Simon D, Perruchoud DL, Borradori L, Rammlmair A. Patterns among patients with chronic pruritus: a retrospective analysis of 170 patients. Acta Derm Venereol 2020; 100: adv00068.