ORIGINAL REPORT
Variable Outcome of Immunotherapy in Advanced Multiple Cutaneous Squamous Cell Carcinomas in Two Patients with Recessive Dystrophic Epidermolysis Bullosa
Laura TREFZER1, Maria E. HESS2,3, Lena SCHOLTEN1, Kristin TECHNAU-HAFSI1, Frank MEISS1, Melanie BOERRIES2–4, Cristina HAS1 and David RAFEI-SHAMSABADI1
1Department of Dermatology, 2Institute of Medical Bioinformatics and System Medicine and 3Comprehensive Cancer Centre Freiburg (CCCF), Medical Centre – University of Freiburg, Faculty of Medicine, Freiburg, and 4German Cancer Consortium (DKTK), partner site Freiburg; and German Cancer Research Centre (DKFZ), Heidelberg, Germany
Cutaneous squamous cell carcinoma (cSCC) is a major complication of recessive dystrophic epidermolysis bullosa (RDEB) that has high morbidity and mortality rates and unmet therapeutic needs. The aim of this study was to evaluate the molecular pattern of cSCC and the clinical course of immunotherapy in 2 RDEB patients with multiple advanced cSCC. Clinical course and disease staging were evaluated retrospectively. The tumour tissues were subjected to immunohistochemical staining. DNA from the blood and cSCC samples was subjected to massive parallel sequencing, and somatic mutations were determined. Patient 1 survived for over 2 years as disease control was achieved with cemiplimab and intralesional interleukin-2. The target advanced cSCC demonstrated a high rate of somatic mutations and strong expression of the immune markers, indoleamine 2,3-dioxygenase, programmed cell death protein ligand 1, and lymphocyte-activation gene 3. The patient ultimately succumbed to complications of oesophageal carcinoma. Patient 2 had an undifferentiated cSCC on the foot, which displayed a low mutational burden and did not express immune markers. The tumour progressed quickly even with cemiplimab therapy. These 2 cases underscore the challenges of cSCC treatment for RDEB. Multiple tumours with different molecular and immune profiles occur concomitantly or sequentially, and surgical excision is not always possible because of the anatomical and tissue constraints imposed by the disease itself. In conclusion, programmed cell death protein 1 inhibitors are approved and effective in treating metastatic and locally advanced cSCC. Our experience and the literature suggest that cemiplimab is an option in patients with RDEB if surgery is not. Somatic mutations and the immune microenvironment should be characterized to predict therapeutic response, particularly in aggressive undifferentiated tumours.
Key words: collagen VII; epidermolysis bullosa; squamous cell carcinoma.
SIGNIFICANCE
Recessive dystrophic epidermolysis bullosa, a rare genetic disease with skin blistering, frequently limits life expectancy in patients with cutaneous squamous cell carcinomas despite aggressive tumour resection. Surgical cure is hindered by frequent recurrence and the development of multiple cutaneous squamous cell carcinoma. We report here 2 patients with multiple cutaneous squamous cell carcinoma who received programmed cell death protein 1 inhibitor therapy. Patient 1, who had a high mutational burden, responded well, while patient 2, who had low mutational burden, experienced disease progression during treatment. We suggest that somatic mutations and the immune microenvironment should be characterized to predict therapeutic response, particularly in aggressive undifferentiated tumours.
Citation: Acta Derm Venereol 2023; 103: adv4870. DOI https://doi.org/10.2340/actadv.v103.4870.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: March 14, 2023; Published: Jun 20, 2023
Corr: Cristina Has, Department of Dermatology, Medical Centre – University of Freiburg, Hauptstrasse 7, DE-79104 Freiburg, Germany. E-mail: cristina.has@uniklinik-freiburg.de
INTRODUCTION
Recessive dystrophic epidermolysis bullosa (RDEB) is a rare genetic mechanobullous disorder characterized by skin blistering, wounds with bacterial colonisation, and a high risk of cutaneous squamous cell carcinoma (cSCC), which metastasizes early and has a severe and often lethal course (1–7). cSCC develops at sites of persistent injury with chronic inflammation, and innate sensing of microbial products are important risk factors (8, 9). Endogenous mutation processes dominated by apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC), cause RDEB skin and tumours to rapidly acquire a high burden of mutations (10). Scarring, activation of fibroblasts, increased production of transforming growth factor beta-1, and other pathways involved in tissue repair contribute to the aggressive course of cSCC in RDEB (11). Inflammation is considered an important component in the pathogenesis of RDEB, but the immunological microenvironment of RDEB-associated cSCC has not yet been characterized.
Although cSCC represents the main cause of mortality in RDEB, little is known about adjuvant, neoadjuvant, or palliative therapies for RDEB-associated cSCC (5) and only single case reports are available (12–16). We report here the immunological microenvironment and somatic mutations of advanced cSCC in 2 patients with RDEB and the clinical course of treatment with cemiplimab, an inhibitor of programmed cell death protein 1 (PD-1).
MATERIALS AND METHODS
Patients and samples
cSCC samples obtained from 2 patients with severe RDEB were studied. The patients did not receive any therapy prior to tumour excision for therapeutic purposes. Histopathological characteristics were retrieved from pathology reports. After obtaining informed consent, genetic testing was performed by whole exome sequencing (Novogene, https://en.novogene.com/) using the Agilent SureSelect All Exon V6 protocol for exome capture and Illumina HiSeq XTEN. This study was approved by the ethics committee of the University of Freiburg (EK-Freiburg 45/18 and 5/20) and was conducted according to the principles of the Declaration of Helsinki. The patients in this manuscript provided written informed consent for publication of their case details.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on FFPE sections for indoleamine 2,3-dioxygenase (IDO), PD-1 ligand 1 (PD-L1), PD-1, lymphocyte-activation gene 3 (LAG-3), and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3). AxioVision (Zeiss, Feldbach, Switzerland) was used for imaging. Three observers who were blinded to the clinical parameters independently scored the IHC-stained sections. The histology score (H-Score) was calculated for IHC markers IDO, PD-1, TIM-3, and LAG-3 as a semi-quantitative approach and was measured using 3 different scores: combined positive score (CPS), tumour proportion score (TPS), and immune cell score (IC). Further details are provided in Appendix S1; Table SI.
Whole exome sequencing and bioinformatic analysis
Whole exome sequencing (WES) was performed by Novogene (https://en.novogene.com/) using the Agilent SureSelect All Exon V6 protocol for exome capture and Illumina HiSeq XTEN. Bioinformatic analyses included quality control to filter out reads containing adapters or with low quality, alignment with the reference genome (Genome UCSC hg19), statistics of sequencing depth and coverage, single nucleotide polymorphism, insertion or deletion of bases (InDel) and copy number variation (CNV) calling, annotation, and statistics. The inclusion criteria for the variants were as follows: at least 8 reads per base and at least 2 reads supporting the variant; rare mutations (minor-allele frequency < 0.001, gnomAD); no “Black-listed” genes; and variant allele frequency > 10%.
This study only considered alterations that were recurrent in at least 2 samples, related to tumourigenesis or significantly mutated in RDEB-SCC, head and neck SCC, or UV-SCC, according to the literature.
To calculate recurrence percentages, we considered an alteration to be activating if it landed in a known oncogene and was either a known activating mutation based on a literature search or highly amplified. Similarly, alterations were considered to be inactivated if they were nonsense mutations or homozygous deletions. Missense or other nonsynonymous mutations in COSMIC (https://cancer.sanger.ac.uk/cosmic) were considered mutations of unknown functional effects but potentially associated with cancer.
RESULT
Clinical and molecular features
Patient 1 had RDEB caused by the COL7A1 homozygous mutation c.425A > G. At the age of 36 years, he developed the first cSCC lesion on the right hand (Fig. 1a, d). Histopathology revealed a well-differentiated verrucous cSCC with a tumour thickness > 12 mm. There were no loco regional or distant metastases, and the tumour was completely excised via micrographic surgery. The tumour recurred locally twice in the following 2 years (Fig. 1a, d) and could not be completely removed. The interdisciplinary tumour board recommended amputation, which was declined by the patient, who wanted to maintain his grasping capacity.
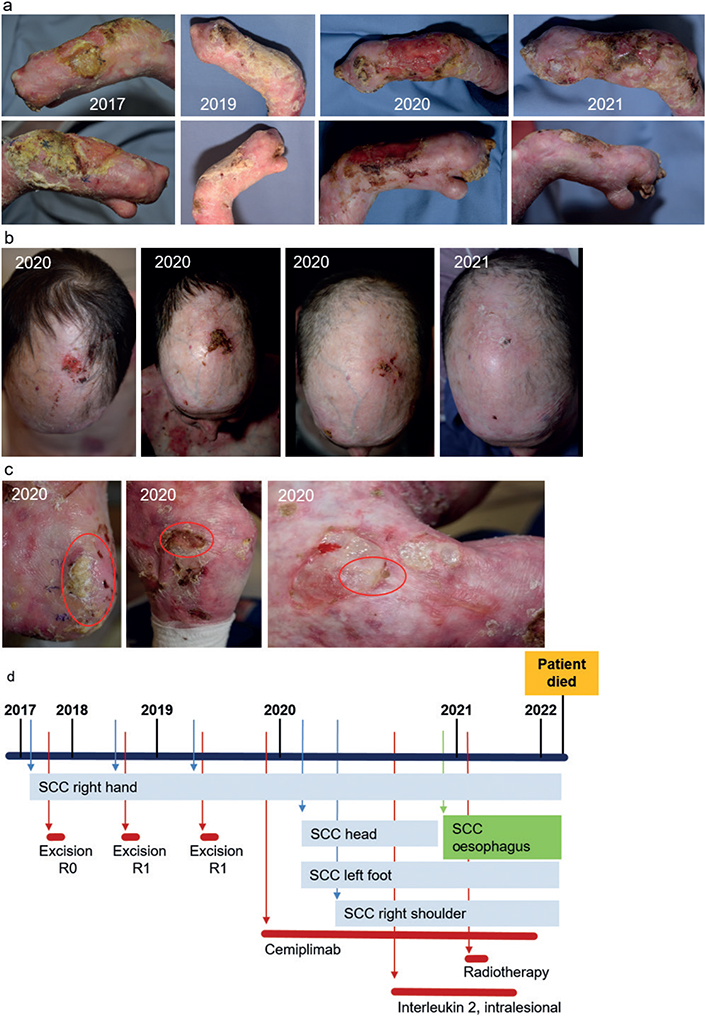
Fig. 1. Disease course in patient 1. (a) Clinical pictures of a cutaneous squamous cell carcinoma (cSCC) of the right hand, which was surgically treated 3 times, and finally improved with the use of systemic immunotherapy and cemiplimab. (b) A new cSCC on the patient’s head, which occurred while receiving cemiplimab therapy, but regressed completely with the use of additive intralesional interleukin-2 treatment. (c) Further cSCC on the left foot, right shoulder, and arm, which developed while the patient was on cemiplimab therapy. (d) Time line of the disease course of patient 1. The newly developed cSCC of the left foot und the right shoulder remained stable while the patient was on cemiplimab therapy. The patient developed a SCC of the oesophagus, which was treated with radiotherapy. At the last visit, the cSCC remained stable while the oesophageal SCC recurred.
IHC staining of cSCC (second recurrence) showed strong IDO expression in stromal immune and tumour cells (Fig. 2 and Appendix S1; Table SII). Furthermore, intense staining for PD-1 and LAG-3 and moderate staining for TIM-3 were observed in the stromal cells. Tumour and immune cells in the stroma showed strong PD-L1-expression (Fig. 2, Appendix S1; Table SII). The cSCC contained 236 somatic mutations, resulting in a mutation rate of 10.04/Mb. The main driving variants were in the oncogenes HRAS, FGFR3, and NOTCH1 and the tumour suppressor genes CTCF, FAT1, CASP8, and KMT2B, and the dominant mutational signature was UV followed by APOBEC (Table I).
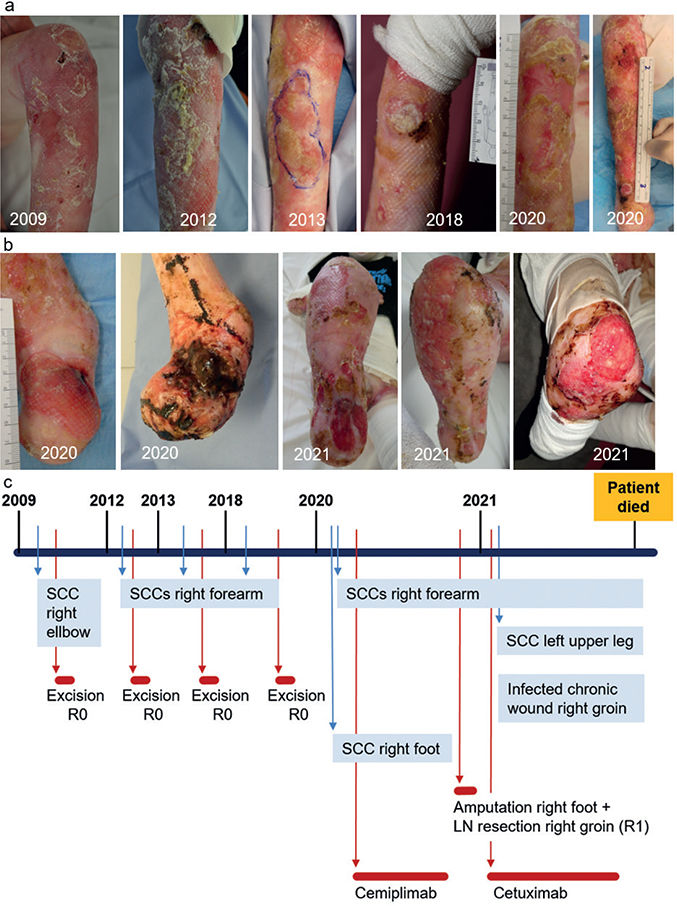
Fig. 2. Disease course in patient 2. (a) Clinical pictures of cutaneous squamous cell carcinomas of the right elbow and forearm over a time-span of 10 years. The patient underwent several surgical treatments in this area. (b) A poorly differentiated cSCC that developed on the patient’s right foot together with lymph node metastasis in the right groin. While receiving cemiplimab therapy, the cSCC progressed dramatically, which led to amputation of the right foot and R1-resection of 2 metastatic lymph node (LN) in the right groin. With subsequent systemic therapy with cetuximab and celecoxib, new poorly differentiated cSCC developed in the patient’s right leg and left upper leg. (c) Timeline of the disease course in patient 2. The patient died due to an infected non-healing deep wound of his right groin.
Based on this molecular profile, the interdisciplinary tumour board recommended anti-PD1 therapy. Cemiplimab (350 mg every 3 weeks) was initiated and administered for 2 years. In addition, intralesional interleukin-2 (IL-2) (6 MIU/every 3 weeks) was administered to a newly developed cSCC on the scalp (Fig. 1b). While receiving this regimen, the cSCC on the hand showed some reduction in hyperkeratosis and ulceration, while the scalp lesion regressed completely (Fig. 1a, b). Regular magnetic resonance imaging (MRI) scans were used to exclude metastases. The patient developed further cSCC on the foot, right shoulder, and arm but remained stable without evidence of disease progression (Fig. 1c). One year after cemiplimab initiation, oesophageal SCC with lymph node metastases was diagnosed (17). Collagen VII is expressed in mucous membranes making them prone to trauma and squamous cell carcinoma formation. This tumour responded well to radiotherapy (17), but the patient succumbed 1 year later at the age of 42 years due to complications including mucositis, scarring, and recurrence of the oesophageal tumour. At the last visit, the cSCC was stable and he had no metastasis.
Patient 2 had RDEB caused by the compound heterozygous COL7A1 variants c.425A>G and c.1837C>T, p.Arg613*. The clinical course in this patient was severe, with extensive chronic heavily bacterially colonized wounds and repeated episodes of fever, oesophageal stenosis, and cachexia (body mass index (BMI) 16.6 kg/m2). He developed his first cSCC on the right elbow at the age of 16 years, followed by several well-differentiated cSCC on his right forearm within the following years, all of which could be completely excised (Fig. 2a). At the age of 27 years, he developed recurrence of cSCC on his right forearm and a large ulcerative cSCC of the right foot (Fig. 2a and b). Inguinal lymph node metastasis was suspected on ultrasonography. At that time, the patient declined surgery.
IHC of cSCC from the right forearm showed strong IDO expression in stromal immune cells but remained negative in tumour cells (Fig. 3 and Appendix S1; Table SI). PD-1 and TIM-3 were strongly expressed in the stroma, whereas LAG-3 expression was low in the stromal cells. PD-L1 expression in tumour and stromal cells was moderate and graded lower than the cSCC of patient 1. The poorly differentiated cSCC of the right foot demonstrated a distinct pattern of immune markers: in stromal cells, IDO, PD-1, PD-L1, and TIM-3 expression was very low, IDO was not expressed by tumour cells, and LAG-3 expression was similar to that of the cSCC on the forearm (Fig. 3 and Appendix S1; Table SII).
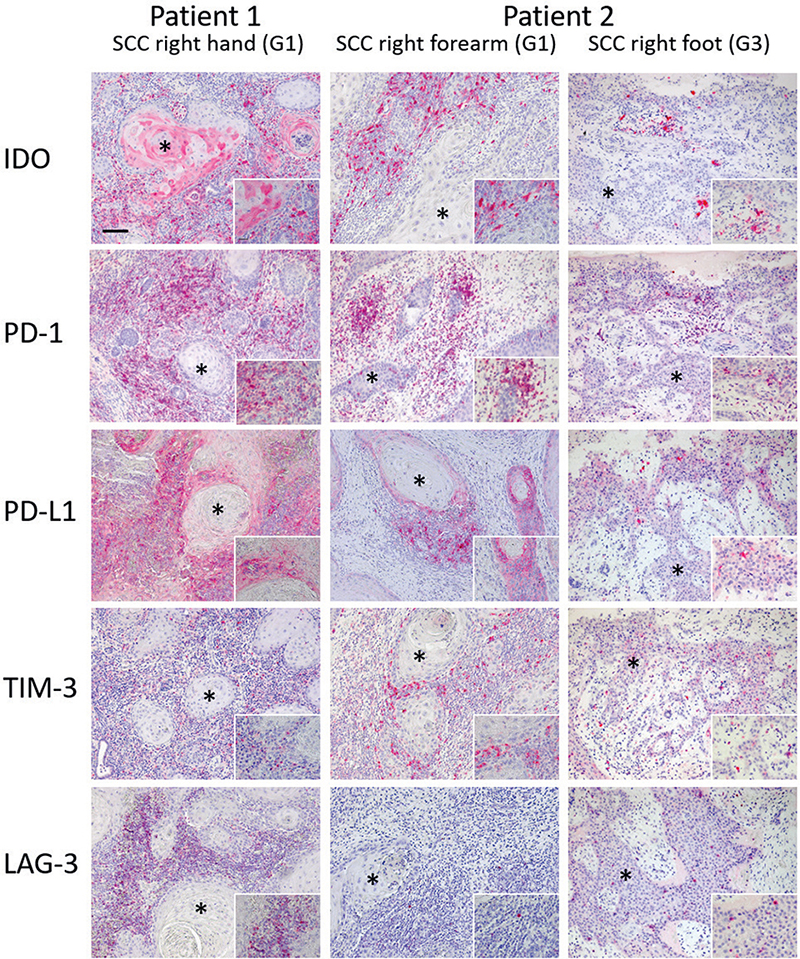
Fig. 3. Immunohistochemical (IHC) staining of cutaneous squamous cell carcinomas (cSCC). Patient 1: IHC staining of the cSCC on the patient’s right hand showed a strong indoleamine 2,3-dioxygenase (IDO) expression in stromal immune cells and tumour cells. Intense staining for programmed cell death protein 1 (PD-1) and lymphocyte-activation gene 3 (LAG-3) and moderate staining for T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) was present in stromal cells. Tumour cells and immune cells in the stroma showed a strong PD-L1-expression. Patient 2: IHC staining of the tumour from the right forearm showed a strong IDO expression in stromal immune cells but in almost no tumour cells. Strong PD-1 and TIM-3 expression was present in the stroma. LAG-3 expression was low in the stromal cells. Programmed cell death protein ligand 1 (PD-L1) expression on tumour and stromal cells is moderate and lower than those in the cSCC of patient 1. Finally, the cSCC of the right foot showed much lower IDO, PD-1, PD-L1 and TIM-3 expression on stromal cells. IDO expressing tumour cells were not present. LAG-3 expression was similar to the cSCC of the right forearm. *Tumour islets of the cSCC. G1: well differentiated; G3: poorly differentiated. Scale bar=100 µm; Insert scale bar=25 µm.
These 2 cSCC lesions in patient 2 demonstrated different profiles of somatic mutations (Table I). Importantly, the cSCC on the foot had a relatively low mutational rate of 3.41/Mbp, few TP53, FAT1, KMT2B, and NOTCH1 driving mutations, and a dominant APOBEC signature (Table I).
The multidisciplinary tumour board recommended treatment with cemiplimab (350 mg every 3 weeks). However, after administering cemiplimab for 3 months, the cSCC on the foot increased in size, while that on the forearm remained stable (Fig. 2a and b). The treatment was discontinued, and the patient agreed to undergo amputation of his right lower leg and resection of the right inguinal lymph nodes (Fig. 2c). Histopathology confirmed tumour infiltration of the inguinal lymph nodes; however, the resection was incomplete. Because of residual metastatic lymph nodes, cetuximab and celecoxib were initiated (12). Three months later, the postoperative wound in the right groin did not heal, and a large ulcerative cSCC developed and grew rapidly on the upper left leg. The therapy was stopped, and the patient died 2 months later from complications related to the non-healing wound.
DISCUSSION
The clinical course in patient 1 showed that PD-1 inhibition with cemiplimab may be a well-tolerated and effective therapy for disease control of cSCC in patients with RDEB. Intralesional IL-2 serves as an additional local treatment option for newly developed cSCC. The high rate of somatic mutations and the immune microenvironment of cSCC with high expression of IDO and PD-L1 in tumour cells and LAG-3 expression on immune cells may predict a beneficial response to immunotherapy. PD-L1 and LAG-3 are markers for T cell exhaustion and are targetable via monoclonal antibodies, as is already being performed for malignant melanoma (18). IDO confers immunosuppressive functions in the tumour microenvironment (TME) and specific blockage via peptide vaccines is being tested in a clinical study on the treatment of melanoma (NCT03047928). In contrast, the poorly differentiated cSCC on the foot of patient 2 did not respond to immunotherapy. In this tumour, a low rate of somatic mutations was associated with low stromal expression of immune markers IDO, PD-1, PD-L1, and TIM-3, compared with cSCC on the patient’s forearm. Thus, a high mutational burden and high expression of targetable immune markers in the TME may lead to a favourable treatment response to immunotherapy in this group of patients.
These 2 cases underscore the challenges of cSCC treatment for RDEB. Multiple tumours with different molecular and immune profiles occur concomitantly or sequentially, and surgical excision is not always possible because of the anatomical and tissue constraints imposed by the disease itself. Cemiplimab, an approved drug for advanced cSCC, had a beneficial effect on the target cSCC in 1 of the patients but not in the other. The treatment was well tolerated without any side-effects, with a standard flat dose. It is improbable that cemiplimab was involved in the development of the oesophageal carcinoma in patient 1, but it was not able to prevent it. PD-1 inhibitors are approved for the treatment of advanced oesophageal carcinoma dependent on the PD-L1 status of the tumour (19). This was not assessed, but we can assume that the PD-L1 expression was low or negative in the oesophageal carcinoma in the current patient.
Immune escape mechanisms under treatment with checkpoint inhibitors can cause secondary treatment failure/resistance, leading to the development of new cancer types (20). Therefore in the 2 patients with RDEB in this study it is not possible to differentiate between SCC development due to selection pressure/secondary resistance and spontaneous development due to the underlying disease, RDEB. There are few case reports on systemic immunotherapy with PD-1 inhibitors for cSCC in RDEB (Table II). Somatic mutations and the immune microenvironment should be characterized to predict therapeutic response and guide therapeutic decisions, particularly in aggressive undifferentiated tumours.
The major limitations of this study are the small number of patients and the evaluation of only the target lesions in patients with multiple tumours.
ACKNOWLEDGEMENTS
IRB approval status: EK-Freiburg 45/18 and 5/20.
This study was financially supported by Sanofi.
LT is a Fellow of the Else-Kröner-Fresenius Foundation. MB was supported by the German Federal Ministry of Education and Research (BMBF) for MIRACUM within the Medical Informatics Funding Scheme (FKZ 01ZZ1606B), CoNfirm (FKZ 01ZX1708F), and Deutsche Forschungsgemeinschaft CRC850, project Z1.
DRS was supported by the Clinician Scientist Program Excellent Clinician Scientists in Freiburg-Education for Leadership (EXCEL at the Medical Center, University of Freiburg, Faculty of Medicine, University of Freiburg, Germany) and funded by the Else-Kröner-Fresenius Foundation (funding number: EXCEL2016_Kolleg.15).
REFERENCES
- Fine J-D, Johnson LB, Weiner M, Li K-P, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: The National EB Registry experience, 1986–2006. J Am Acad Dermatol 2009; 60: 203–211.
- Montaudié H, Chiaverini C, Sbidian E, Charlesworth A, Lacour J-P. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis 2016; 11: 117.
- Mallipeddi R. Epidermolysis bullosa and cancer. Clin Exp Dermatol 2002; 27: 616–623.
- Cianfarani F, Zambruno G, Castiglia D, Odorisio T. Pathomechanisms of altered wound healing in recessive dystrophic epidermolysis bullosa. Am J Pathol 2017; 187: 1445–1453.
- Mellerio JE, Robertson SJ, Bernardis C, Diem A, Fine JD, George R, et al. Management of cutaneous squamous cell carcinoma in patients with epidermolysis bullosa: best clinical practice guidelines. Br J Dermatol 2016; 174: 56–67.
- Robertson SJ, Orrin E, Lakhan MK, O’Sullivan G, Felton J, Robson A, et al. Cutaneous squamous cell carcinoma in epidermolysis bullosa: a 28-year retrospective study. Acta Derm Venereol 2021; 101: adv00523.
- Harrs C, van den Akker PC, Baardman R, Duipmans JC, Horváth B, van Kester MS, et al. The aggressive behaviour of squamous cell carcinoma in epidermolysis bullosa: analysis of clinical outcomes and tumour characteristics in the Dutch EB Registry. Br J Dermatol 2022; 187: 824–826.
- Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 2008; 9: 628.
- Hoste E, Arwert EN, Lal R, South AP, Salas-Alanis JC, Murrell DF, et al. Innate sensing of microbial products promotes wound-induced skin cancer. Nat Commun 2015; 6: 5932.
- Cho RJ, Alexandrov LB, den Breems NY, Atanasova VS, Farshchian M, Purdom E, Nguyen TN, et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci Transl Med 2018; 10: eaas9668.
- Condorelli AG, Dellambra E, Logli E, Zambruno G, Castiglia D. Epidermolysis bullosa-associated squamous cell carcinoma: from pathogenesis to therapeutic perspectives. Int J Mol Sci 2019; 20: 5707.
- Reimer A, Lu S, He Y, Bruckner-Tuderman L, Technau-Hafsi K, Meiss F, et al. Combined anti-inflammatory and low-dose antiproliferative therapy for squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. J Eur Acad Dermatol Venereol 2020; 34: e1–3.
- Kim M, Li M, Intong LR, Tran K, Melbourne W, Marucci D, et al. Use of cetuximab as an adjuvant agent to radiotherapy and surgery in recessive dystrophic epidermolysis bullosa with squamous cell carcinoma. Br J Dermatol 2013; 169: 208–210.
- Medek K, Koelblinger P, Koller J, Diem A, Ude-Schoder K, Bauer JW, Laimer M. Wundheilungsstörungen während der antitumorösen Therapie mit Cetuximab bei schwerer generalisierter dystropher Epidermolysis bullosa. J Dtsch Dermatol Ges J Ger Soc Dermatol 2019; 17: 448–450.
- Piccerillo A, El Hachem M, De Vito R, De Luca EV, Peris K. Pembrolizumab for treatment of a patient with multiple cutaneous squamous cell carcinomas and recessive dystrophic epidermolysis bullosa. JAMA Dermatol 2020; 156: 708–710.
- Duong T, Wong D, Barrett A, Price H. Successful use of immunotherapy to treat advanced cutaneous squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. BMJ Case Rep 2021; 14: e238966.
- Schwieger-Briel A, Trefzer L, Schumann H, Reimer-Taschenbrecker A, Rafei-Shamsabadi D, Meiss F, Has C. Esophageal carcinoma in severe recessive dystrophic epidermolysis bullosa – an underestimated complication? J Eur Acad Dermatol Venereol 2022; 36: e293–e295.
- Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022; 386: 24–34.
- Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 2022; 33: 992–1004.
- Li Z, Sun G, Sun G, Cheng Y, Wu L, Wang Q, et al. Various uses of PD1/PD-L1 inhibitor in oncology: opportunities and challenges. Front Oncol 2021; 11: 771335.
- Khaddour K, Gorell ES, Dehdashti F, Tang JY, Ansstas G. Induced remission of metastatic squamous cell carcinoma with an immune checkpoint inhibitor in a patient with recessive dystrophic epidermolysis bullosa. Case Rep Oncol 2020; 13: 911–915.
- Robertson SJ, Orrin E, Lakhan MK, O’Sullivan G, Felton J, Robson A, et al. Cutaneous squamous cell carcinoma in epidermolysis bullosa: a 28-year retrospective study. Acta Derm Venereol 2021; 101: adv00523.
- Vasilev P, Kalev D, Karamanliev M, Dimitrov D, Troyanova P, Yordanova I. Cemiplimab treatment of squamous cell carcinoma in a patient with severe recessive dystrophic epidermolysis bullosa. J Dtsch Dermatol Ges 2023; 21: 295–297.