SHORT COMMUNICATION
Preferences of Patients with Atopic Dermatitis Regarding Patient Self-administered Tools Used in Clinical Practice
Johanna M. MANDELIN1, Anna EKMAN2, Suvi T. RUOHONEN2 and Laura KORHONEN3
1Department of Dermatology, Helsinki University Central Hospital, Meilahdentie 2, FIN-00250 Helsinki, 2Sanofi Oy, Espoo, and 3Department of Dermatology, Tampere University Hospital, Tampere, Finland. E-mail: johanna.mandelin@hus.fi
Citation: Acta Derm Venereol 2023; 103: adv5261. DOI https://doi.org/10.2340/actadv.v103.5261.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 21, 2023; Published: Apr 6, 2023
Competing interests and funding: JMM is an advisor, consultant, speaker or investigator for AbbVie, Eli Lilly, LEO Pharma, Novartis, Pfizer, Sanofi, Sidekick and Takeda. AE and STR are currently employed by Sanofi. LK is an advisor, consultant, or speaker for AbbVie, Eli Lilly, LEO Pharma, Orion, Novartis, Pfizer, Sanofi, and Sidekick.
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease affecting approximately 20% of children and 10% of adults in high-income countries. AD typically presents with an itchy rash, unpredictable flares and changes in severity over time and decreases patients’ quality of life (QoL) (1).
In clinical practice, the severity of AD is often assessed through clinical examination and AD history. Patient-reported outcome (PRO) measures, apart from the non-disease specific Dermatology Life Quality Index (DLQI), are rarely used. The new treatment paradigm in AD has created a need for AD-specific measurement tools that are easily applicable in clinical settings. The Harmonising Outcome Measures for Eczema (HOME) initiative recommends the use of Patient-Oriented Eczema Measure (POEM) and/or Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD) for measuring patient-reported AD symptoms in clinical practice (2). For measuring disease control, the recommended tools are either Atopic Dermatitis Control Tool (ADCT) or Recap of Atopic Eczema (3). ADCT is a 6-question patient self-administered tool designed and validated to assess AD disease control (4). ADCT has been reported to correlate well with POEM, itch numerical rating scale (NRS) and Eczema Area and Severity Index (EASI) scores (4, 5). In addition to eczema symptoms, ADCT also includes questions about the impact of AD on daily activities, emotions, and sleep, thus covering a wider range of factors than most PROs (5).
This study assessed AD symptoms and disease control with ADCT, POEM and DLQI questionnaires in Finnish patients with AD. The study aim was to evaluate patients’ preferences between these tools and to determine whether patients’ own overall estimations of AD severity are in agreement with the PRO results.
MATERIALS AND METHODS
The patient questionnaire was developed by the authors and distributed through the Finnish Allergy, Skin and Asthma Federation. Respondents were AD patients with an itchy dermatitis during the last 12 months. Answers were collected from 22 October until 7 December 2021. The questionnaire included background questions, patients’ own estimation of disease severity and control, and the POEM, ADCT and DLQI tools. Patients were also asked about their preference between ADCT and POEM in evaluating their own disease situation and when discussing their AD during appointments with healthcare professionals (HCPs).
RESULTS
The survey was answered by 439 respondents (89% female, 11% male):13% were under 30 years old, 35% 30–49 years old, 34% 50–65 years old and 17% over 65 years old (1% did not want to disclose their age). A majority (80%) had immediate type allergies, 62% allergic rhinitis, 49% asthma and 19% ocular allergic disease. Also, anxiety (15%), cardiovascular diseases (13%) and depression (13%) were relatively common.
The respondents self-evaluated the current severity of their AD and disease control during the last week. AD was reported to be under control by 56% of respondents. Severity was reported to be mild by 46%, moderate by 44% and severe by 5%. Treatments used during the last year included moisturizers (98%), topical corticosteroids (94%), tacrolimus ointment (30%) and pimecrolimus cream (15%). In addition, 18% had used oral cortico-steroids and 7% had received ultraviolet (UV) therapy. Other treatments (traditional systemics, biologics, JAK inhibitors) were rarely used.
According to POEM scores, half (52%) of the patients had moderate AD and 23% had severe or very severe AD. The patients who estimated their disease as mild (n = 200) had a mean POEM score of 9.3, corresponding to moderate disease. ADCT scores implied that AD was uncontrolled in 67% of respondents (7 or more points on a scale of 0–24, mean 9.4). The mean ADCT score of patients estimating their disease to be under control was 6.8. Based on DLQI results, AD had an at least moderate effect on QoL in 59% of the respondents, and a very large or extremely large effect in 27%.
The correlation between ADCT and POEM scores, and between ADCT and DLQI scores was high: Pearson’s coefficients were r = 0.748 and r = 0.754, respectively. The correlation between POEM and DLQI scores was weaker (r = 0.59).
Patients’ estimations about the relevancy of different tools were collected. POEM and ADCT were rated equally meaningful, while DLQI was perceived less meaningful (Fig. 1A). In the whole study population, 47% would choose POEM for self-evaluation and 46% for routine consultations with HCPs, whereas ADCT was preferred by 26% and 28%, respectively (Fig. 1B). Approximately 25% of the respondents could not choose between the tools, and 2% said they would use neither. However, the preferred tool for HCP consultations in severe AD patients was POEM in 32% and ADCT in 28%, whereas 40% were undecided (Fig. 1B). Thus, in this subgroup of patients, both tools were rated equally useful.
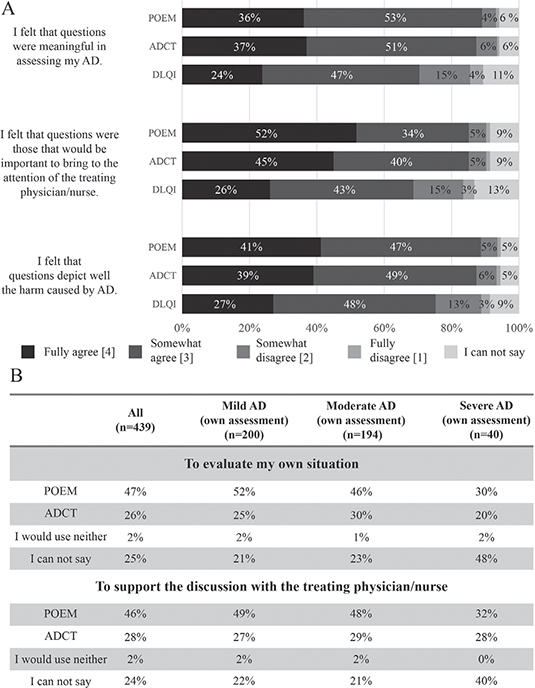
Fig. 1. (A) Survey responders (n = 439) evaluated different patient-reported tools (Patient-Oriented Eczema Measure (POEM), Atopic Dermatitis Control Tool (ADCT), Dermatology Life Quality Index (DLQI)) about the relevance of questions in assessing their atopic dermatitis (AD), importance of bringing these topics to their treating physician’s/nurse’s attention, and suitability of the questions in measuring the burden of the disease. For each statement, the responders were given the following options to choose from: 4 = Fully agree, 3 = Somewhat agree, 2 = Somewhat disagree, 1 = Fully disagree and 0 = I can not say. (B) Percentage of responders with different AD severities to choose POEM, ADCT or neither in evaluating their own situation or for routine consultations with their treating physician or nurse. Five patients could not assess their disease severity, and were excluded from disease state subgroup analyses.
DISCUSSION
A total of 439 Finns responded to the questionnaire concerning symptoms, disease control and AD severity. A majority (89%) of the respondents were female, which mirrors the sex distribution of the patient federation’s members (86% female, 14% male). 46% of the respondents evaluated their AD as mild and 56% estimated their AD to be currently under control.
When comparing patient-reported disease severity with the outcomes of the validated AD measurement tools, the results differed: 53% of the respondents estimated their disease to be moderate or severe, whereas POEM suggested it in 75%. The ADCT tool implied that AD was uncontrolled in 67% vs 44% by self-estimation. Patient underestimation compared with treating physicians’ assessments has previously been reported in a US-based adult patient population (6) and in a Singapore-based paediatric population (7). This underestimation might result from adjusting to a long-term disease often affecting the patient since infancy. Thus, one advantage of PROs is that they enable the hidden burden of the disease to become more evident.
ADCT and POEM performed similarly when patients were asked to rate the relevancy of the tools in describing the overall disease burden and the usefulness of the questionnaires in discussions with HCPs. Nearly all (98%) respondents were willing to use either POEM or ADCT to assess the disease severity and disease control, implying that patients see benefit in disease self-assessment.
When specifically asked about the preference between ADCT and POEM tools, POEM was preferred by the total study population. It was somewhat surprising that patients preferred a skin symptom-focused questionnaire over a more holistic assessment. It is possible that patients are more accustomed to describing and discussing skin symptoms rather than everyday inconveniences and emotions with their HCPs and, thus, also consider those more relevant. There are also structural differences between the questionnaires (8) as POEM does not address the intensity or severity of the symptoms but merely their frequencies. In fact, it was noted in the current study that patients with severe AD preferred ADCT and POEM equally, possibly reflecting the greater impact of severe disease on QoL, and the need to address this with the medical personnel.
In this study, ADCT scores correlated well with those of POEM and DLQI, whereas correlation between POEM and DLQI was weaker. This is in line with previous studies (4, 5) and could be explained by POEM and DLQI measuring different aspects of the disease (severity and QoL, respectively) and ADCT combining the components of both.
In conclusion, treating physicians should encourage patients through holistic discussions about AD symptoms, disease burden and the impact of the disease on emotions and daily activities, when striving for optimal management of AD.
ACKNOWLEDGEMENTS
The authors thank Ville Haikola from Sales Questor for the work with the questionnaire form. This study was funded by Sanofi.
REFERENCES
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360.
- Leshem YA, Chalmers JR, Apfelbacher C, Furue M, Gerbens LAA, Prinsen CAC, et al. Measuring atopic eczema symptoms in clinical practice: the first consensus statement from the Harmonizing Outcome Measures for Eczema in clinical practice initiative. J Am Acad Dermatol 2020; 82: 1181–1186.
- Leshem YA, Chalmers JR, Apfelbacher C, Katoh N, Gerbens LAA, Schmitt J, et al. Measuring atopic eczema control and itch intensity in clinical practice: a consensus statement from the Harmonising Outcome Measures for Eczema in Clinical Practice (HOME-CP) Initiative. JAMA Dermatol 2022; 158: 1429–1435.
- Pariser DM, Simpson EL, Gadkari A, Bieber T, Margolis DJ, Brown M, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr Med Res Opin 2020; 36: 367–376.
- Kido-Nakahara M, Yokote G, Yoshida M, Furue M, Nakahara T. Atopic dermatitis Control Tool (ADCT): a useful tool for self-evaluation in patients with atopic dermatitis. J Dermatol 2021; 48: 1951–1952.
- Wei W, Anderson P, Gadkari A, Blackburn S, Moon R, Piercy J, et al., Discordance between physician- and patient-reported disease severity in adults with atopic dermatitis: a US cross-sectional survey. Am J Clin Dermatol 2017; 18: 825–835.
- Xu X, Olsson M, Bajpai R, Koh Jean Aan M, Weng Yew Y, Wong S, et al. Concordance between physician-rated and caregiver-perceived disease severity in children with atopic dermatitis: a cross-sectional study. Acta Derm Venereol 2020; 100: adv00308.
- Maintz L, Bieber T, Bissonnette R, Jack C. Measuring atopic dermatitis disease severity: the potential for electronic tools to benefit clinical care. J Allergy Clin Immunol Pract 2021; 9: 1473–1486.e2.