SHORT COMMUNICATION
Drug Survival of Anti Interleukin-17 and Interleukin -23 Agents after Adalimumab Failure in Hidradenitis Suppurativa: A Pilot Study
Federica REPETTO#, Gabriele ROCCUZZO#*, Lorenza BURZI, Luca MASTORINO, Paolo DAPAVO, Pietro QUAGLINO and Simone RIBERO
Department of Medical Sciences, Section of Dermatology, University of Turin, Via Cherasco 23, IT-10126, Turin, Italy. *E-mail: gabriele.roccuzzo@unito.it
#These authors contributed equally and share first authorship.
Citation: Acta Derm Venereol 2023; 103: adv5278. DOI: https://doi.org/10.2340/actadv.v103.5278.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Mar 15, 2023; Published: Apr 19, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
The anti-tumour necrosis factor (TNF)-alpha agent adalimumab has represented the main biologic therapy for treatment of moderate to severe hidradenitis suppurativa (HS) since 2015, with achievement rates in clinical response up to 86.7% (1, 2). However, patients with unsatisfactory responses to this therapy represent a therapeutic challenge in clinical practice. As the development of inflammatory nodules, abscesses, and fistulas has been proven to be triggered not only by TNF-alpha, but also by other cytokines, such as interferon (IFN)-γ, interleukin (IL)-1, IL-17, and IL-12/23, selectively targeting these other mediators could represent a promising therapeutic option (3). Recent studies have suggested that anti-IL-17 and anti-IL-23 biologics, originally approved for the treatment of psoriasis, can play a role in targeting the inflammatory cascade responsible for HS, leading to clinical improvement (4–7). However, studies investigating the off-label switch to these agents, following first-line therapy with adalimumab, are lacking. We report here the results of a pilot study carried out at the Dermatology Clinic of the University of Turin, Turin, Italy, aimed at evaluating the safety and efficacy of the switch to either an anti-IL-17 or anti-IL-23 agent after first-line adalimumab in patients with moderate-to-severe HS.
METHODS
The study was conducted in accordance with the Declaration of Helsinki. Inclusion criteria were: age ≥ 18 years, Hurley grade ≥ 2, presence of complete medical records, and switch to an anti-IL-17 or anti-IL-23 agent following primary or secondary failure and/or biologic-related unacceptable side-effects with adalimumab. Ultrasound was performed in cases of doubt in evaluating subclinical lesions (8). Patients were evaluated every 12 weeks with the International Hidradenitis Suppurativa Severity Score System (IHS4) and Hidradenitis Suppurativa Clinical Response (HiSCR) (9). Mann–Whitney, McNemar’s, and χ2 tests were used to analyse continuous and paired nominal data, respectively. p-values < 0.05 were considered statistically significant.
RESULTS
Patients’ characteristics are summarized in Table I. All patients received at least 1 conventional treatment before adalimumab (median number of previous lines of therapy 3, range 1–6), with tetracycline being the most common (69.5% of patients). At adalimumab first prescription (T0), 42.3% of patients were classified as Hurley grade 2 and 57.7% as Hurley grade 3. In terms of latent class analysis (LCA) clinical phenotypes, 7 patients (26.9%) were defined as LC1 (axillary‒mammary‒groin), 6 patients (23.1%) as LC2 (axillary‒gluteal‒groin), and 13 patients (50.0%) as LC3 (axillary‒groin) (10). At the time of switch (Ts), 16 patients were started on the anti-IL-17 agent secukinumab, and 10 patients on an anti-IL-23 agent (risankizumab n = 7, guselkumab n = 3), with mean IHS4 in the 2 groups of 21.1 (range 10–40) and 17.1 (range 8–40) (p = 0.357), respectively. All biologics were given off-label at the standard dosage approved for psoriasis (i.e. 300 mg at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter for secukinumab, 150 mg at week 0, week 4, and every 12 weeks thereafter for risankizumab, and 100 mg at week 0, week 4, and every 8 weeks thereafter for guselkumab). The groups were comparable for all parameters evaluated, with no difference in terms of basal mean IHS4 values (i.e. 22.4 and 22, p = 0.952) or Hurley score (i.e. 11 patients Hurley grade 2 and 15 Hurley grade 3). Drug choice was not influenced by any specific clinical feature, but was determined by chronological factors (i.e. reports in the literature regarding the off-label use of anti-IL-17 agents were published earlier than those on anti-IL-23) and drug availability at our centre. Dermatology Life Quality Index (DLQI) measures were also recorded (11). During follow-up, 8 patients (50%) in the anti-IL-17 and 1 patient (10%) in the anti-IL-23 groups discontinued treatment due to inefficacy (p = 0.037), respectively. Drug survival analysis at 48 weeks is shown in Fig. 1 (p = 0.241). As for clinical responses, a significant difference between the 2 drug classes was found at 6 months in terms of HiSCR (p = 0.0272), with higher rates of achievements in clinical responses in the anti-IL-23 group. At 12 months, mean IHS4 scores of 5.1 (range 0–20) and 14.1 (range 0–30) were recorded in the anti-IL-23 and anti-IL-17 groups, respectively, with a significant improvement compared with baseline achieved only in the IL-23 group (p = 0.0027 vs p = 0.0926). No severe side-effects were observed in either group.
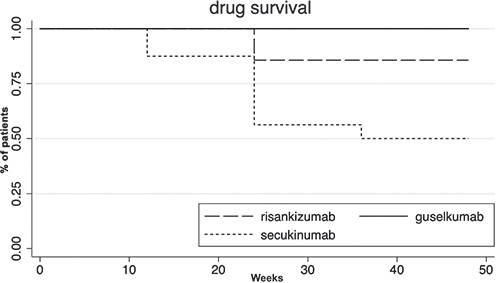
Fig. 1. Drug survival of different anti-interleukin (IL)-17 and anti-IL-23 agents.
DISCUSSION
Some interesting observations can be made based on these results. First, both classes of drug play a role in improving clinical scores in patients with HS refractory to anti-TNF-alpha, but with significant improvement in terms of IHS4 seen only in the anti-IL-23 group. Secondly, anti-IL-17-treated patients had a significantly higher discontinuation rate throughout the year of follow-up due to drug inefficacy compared with those receiving anti-IL-23 agents. Thirdly, anti-IL-23-treated patients showed higher rates of achieving a clinical response at 6 months compared with those treated with anti-IL-17 agents. Finally, both drug classes have a positive impact on patients’ quality of life, as measured by an overall improvement in DLQI measures (p < 0.05). While the small size of this study, along with its retrospective nature, prevents us from drawing definitive conclusions regarding which biologic agent should be prescribed as first choice after adalimumab failure, carefully integrating pre-clinical evidence with real-life data remains essential in pursuing the best therapeutic option for each HS patient.
ACKNOWLEDGEMENTS
The patients in this study gave written informed consent to publication of their case details.
REFERENCES
- Morita A, Takahashi H, Ozawa K, Imafuku S, Takekuni N, Takahashi K, et al. Long-term analysis of adalimumab in Japanese patients with moderate to severe hidradenitis suppurativa: open-label phase 3 results. J Dermatol 2021; 48: 3–13.
- Roccuzzo G, Rozzo G, Burzi L, Repetto F, Dapavo P, Ribero S, Quaglino P. Switching from adalimumab originator to biosimilars in hidradenitis suppurativa: what’s beyond cost-effectiveness? Dermatol Ther 2022; 35: e15803.
- Markota Čagalj A, Marinović B, Bukvić Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci 2022; 23: 3753.
- Repetto F, Burzi L, Ribero S, Quaglino P, Dapavo P. Efficacy and safety of risankizumab in hidradenitis suppurativa: a case series. Acta Derm Venereol 2022; 102: adv00780.
- Melgosa Ramos FJ, García Ruiz R, Mateu Puchades A, Alfageme Roldán F. Guselkumab effectiveness, and posology in patients with moderate to severe hidradenitis suppurativa: a retrospective bicentric experience. Dermatol Ther 2022; 35: e15558.
- Burzi L, Repetto F, Ramondetta A, Rozzo G, Licciardello M, Ribero S, Quaglino P, Dapavo P. Guselkumab in the treatment of severe hidradenitis suppurativa, a promising role? Dermatol Ther 2021; 34: e14930.
- Ribero S, Ramondetta A, Fabbrocini G, Bettoli V, Potenza C, Chiricozzi A, et al. Effectiveness of Secukinumab in the treatment of moderate-severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real-life setting. J Eur Acad Dermatol Venereol 2021; 35: e441–e442.
- Revuz JE, Jemec GB. Diagnosing hidradenitis suppurativa. Dermatol Clin 2016; 34: 1–5.
- Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Giamarellos-Bourboulis EJ, et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017; 177: 1401–1409.
- Cazzaniga S, Pezzolo E, Bettoli V, et al. Characterization of hidradenitis suppurativa phenotypes: a multidimensional latent class analysis of the National Italian Registry IRHIS. J Invest Dermatol 2021; 141: 1236–1242.e1.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.