SHORT COMMUNICATION
Turner Syndrome, Atypical Naevi and Multiple Melanoma: Coincidence or Causality?
Julia M. K. CLABBERS1,2, Remco VAN DOORN3 and Nicole A. KUKUTSCH3
1Department of Dermatology, Haga Hospital, Els Borst-Eilersplein 275, NL-2545 AA The Hague, 2Department of Dermatology, Maastricht University Medical Centre+, Maastricht and 3Department of Dermatology, Leiden University Medical Centre, Leiden, The Netherlands. E-mail: j.clabbers@hagaziekenhuis.nl
Citation: Acta Derm Venereol 2023; 103: adv5586. DOI: https://doi.org/10.2340/actadv.v103.5586.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Feb 21, 2023; Published: May 5, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
We report here a 42-year-old woman with Turner syndrome, who was under strict surveillance every 3 months at the Department of Dermatology, Leiden University Medical Center, Leiden, the Netherlands (a centre of expertise for melanoma), with > 200 naevi (Fig. 1), approximately 60 atypical naevi and a history of 13 clinically suspicious lesions with the pathological diagnosis of dysplastic naevus. In previous years, she had developed 4 superficial spreading melanomas and 1 melanocytic tumour of uncertain malignant potential (Fig. 2). Three lesions showed histological signs of pre-existing atypical naevi. For the other 2 lesions, pre-existing and considerably smaller naevi were retrieved at the same spot on total-body photographs. Most of the patient’s naevi were already present during adolescence. She had been treated with growth hormones and oestrogen for a brief period after Turner syndrome was diagnosed at the age of 18 years. Her family history was positive for melanoma. High-risk melanoma genes (i.e. CDKN2A, CDK4, BAP1, TERT, POT1, TERF2IP, ACD and MITF) tested negative.
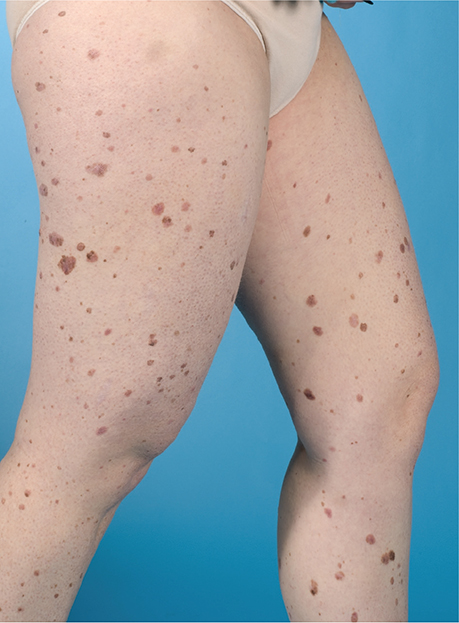
Fig. 1. Overview of the patient’s legs, showing a very high naevus count with multiple clinically atypical naevi.
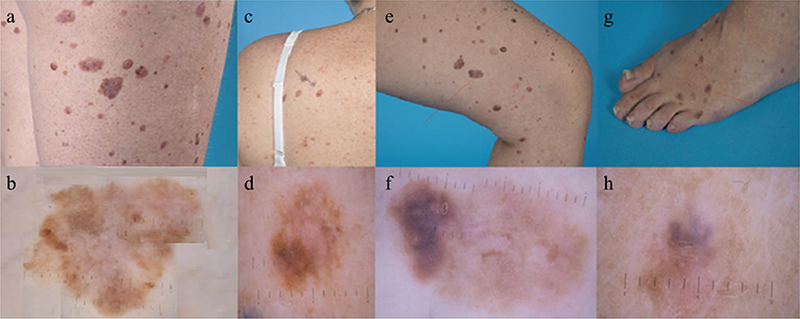
Fig. 2. (a) Clinical photograph of a superficial spreading malignant melanoma on the right thigh (Breslow thickness 0.6 mm, developed from a pre-existent naevus). (b) Dermatoscopic image of the lesion shown in (a). (c) Clinical photograph of a melanocytic tumour of uncertain malignant potential (MELTUMP) on the left shoulder (developed from a pre-existent naevus). (d) Dermatoscopic image of the lesion shown in (c). (e) Clinical photograph of a superficial spreading malignant melanoma on the left thigh (Breslow thickness 0.9 mm). The pathologist did not report any signs of a pre-existing naevus. However, comparison between the current clinical photograph and an overview photograph taken 10 years previously, revealed a considerably smaller and more symmetrical pigmented lesion on the same location in the past. (f) Dermatoscopic image of the lesion shown in (e). (g) Clinical photograph of a superficial spreading malignant melanoma on the left foot (Breslow thickness 1.4 mm). In this lesion, also, the pathologist did not report a pre-existing naevus, but on overview photographs from 10 years previously a considerably smaller pigmented lesion was seen on the same location. (h) Dermatoscopic image of the lesion shown in (g).
The high number of atypical and dysplastic naevi and melanomas in this patient is remarkable. To our knowledge, no similar cases have been reported previously. The aim of this study was to assess the evidence of an association between Turner syndrome, the development of atypical naevi, and the risk of cutaneous melanoma. A further aim was to examine a possible influence of hormone replacement therapy on the risk of developing melanoma.
DISCUSSION
Turner syndrome is associated with a high number of common naevi (1–3). Also, a high naevus count is associated with an increased risk of melanoma (4). There are no studies investigating the presence of atypical naevi in Turner syndrome. Only 1 study described a higher count of naevi sized more than 5 mm (1 of the criteria for an atypical naevus) in Turner syndrome compared with control subjects (2). One patient with mosaic Turner syndrome and atypical naevi has been described, but the mosaicism was not examined in the affected skin (5). The evidence for a higher risk of melanoma in patients with Turner syndrome is weak. Given the known high number of common naevi in Turner syndrome and the association between high naevus count and risk of melanoma, it is remarkable that only 3 previous cases of melanoma in Turner syndrome have been described (choroidal melanoma, peritoneal melanoma metastasis without mention of primary tumour, melanoma not further specified) (1, 6, 7). There are no reports regarding multiple melanoma in Turner syndrome. Three cohort studies on cancer risk in Turner syndrome have reported occurrence of melanoma (8–10). The first cohort, of 597 patients with Turner syndrome, in 1996, did not document any occurrence of melanoma (8). However, 477 of these 597 patients were under 30 years old. This selection of relatively young patients could have negatively influenced the number of melanomas. In 2008, a national cohort study of cancer incidence in 3,425 women with Turner syndrome was conducted in Great Britain (9). This study found 8 cases of melanoma compared with an expected number of 3.6; however, this difference was not statistically significant. Of the 2 reported ocular cancers, 1 was an ocular melanoma, the exact subtype was not mentioned. In 2016, a Swedish national cohort study was published, showing data from a cancer registry of, in total, 1,409 women with Turner syndrome (10). They found a statistically significant higher incidence of melanoma in Turner syndrome (10 cases compared with an expected number of 3.36). Overall these cohort studies are not fully conclusive about the melanoma risk in Turner syndrome, but suggest a higher incidence. In the current patient, multiple melanomas were histologically proven or clinically suspicious to have developed from pre-existing clinically atypical naevi. This is in contrast to sporadic melanoma, as generally approximately 70% of melanomas arise de novo (11).
Even though an increased growth rate of naevi during growth hormone therapy was described in 1 study (12), subsequent studies showed no proof of induction of a higher total naevus count or melanoma by growth hormone therapy (2, 13, 14). A possible relationship with development of more clinically atypical naevi remains unresolved (13, 14). The influence of female sex hormones on melanoma development has also been suggested, but current data found no proof of a higher risk of melanoma by using hormone replacement therapy or oral contraceptives (15).
In conclusion, it is important for paediatricians, endocrinologists, general practitioners, and other doctors treating patients with Turner syndrome, to be aware that these patients might also have atypical naevi, and that there might be an elevated risk of developing melanoma. An international patient registry could help to collect more cases and gather information about their phenotype. We advise close monitoring of naevus count and development of atypical naevi during puberty in patients with Turner syndrome. In general, it is recommended that patients with > 100 naevi or ≥ 5 atypical naevi consult a dermatologist every year for a total body inspection, as well as patient education about sun exposure and skin self-examination.
REFERENCES
- Becker B, Jospe N, Goldsmith LA. Melanocytic nevi in Turner syndrome. Pediatr Dermatol 1994; 11: 120–124.
- Wyatt D. Melanocytic nevi in children treated with growth hormone. Pediatrics 1999; 104: 1045–1050.
- Zvulunov A, Wyatt DT, Laud PW, Esterly NB. Influence of genetic and environmental factors on melanocytic naevi: a lesson from Turner’s syndrome. Br J Dermatol 1998; 138: 993–997.
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer 2005; 41: 28–44.
- Papanikolaou M, Chohan T, Millington GWM. Malignant melanoma, papillary thyroid carcinoma and Erdheim-Chester disease, associated with both BRAF(V600E) and mosaic Turner syndrome. Clin Exp Dermatol 2020; 45: 512–514.
- Buckley CA, Cheng H. Intraocular melanoma, diabetes, and Turner’s syndrome: presentation with proptosis. Br J Ophthalmol 1981; 65: 460–463.
- Gare M, Ilan Y, Sherman Y, Ben-Chetrit E. Malignant melanoma in Turner’s syndrome. Int J Dermatol 1993; 32: 743–744.
- Hasle H, Olsen JH, Nielsen J, Hansen J, Friedrich U, Tommerup N. Occurrence of cancer in women with Turner syndrome. Br J Cancer 1996; 73: 1156–1159.
- Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA. Cancer incidence in women with Turner syndrome in Great Britain: a national cohort study. Lancet Oncol 2008; 9: 239–246.
- Ji J, Zoller B, Sundquist J, Sundquist K. Risk of solid tumors and hematological malignancy in persons with Turner and Klinefelter syndromes: a national cohort study. Int J Cancer 2016; 139: 754–758.
- Pampena R, Kyrgidis A, Lallas A, Moscarella E, Argenziano G, Longo C. A meta-analysis of nevus-associated melanoma: prevalence and practical implications. J Am Acad Dermatol 2017; 77: 938–945.e934.
- Bourguignon JP, Pierard GE, Ernould C, Heinrichs C, Craen M, Rochiccioli P, et al. Effects of human growth hormone therapy on melanocytic naevi. Lancet 1993; 341: 1505–1506.
- Zvulunov A, Wyatt DT, Rabinowitz LG, Esterly NB. Effect of growth hormone therapy on melanocytic nevi in survivors of childhood neoplasia. Arch Dermatol 1997; 133: 795–796.
- Zvulunov A, Wyatt DT, Laud PW, Esterly NB. Lack of effect of growth hormone therapy on the count and density of melanocytic naevi in children. Br J Dermatol 1997; 137: 545–548.
- Gandini S, Iodice S, Koomen E, Di Pietro A, Sera F, Caini S. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer 2011; 47: 2607–2617.