ORIGINAL REPORT
Effects of Early Emollient Use in Children at High Risk of Atopic Dermatitis: A German Pilot Study
Inken HARDER1#, Dora STÖLZL1#, Nicole SANDER1, Jan HARTMANN1, Elke RODRIGUEZ1, Carsten MAZUR1, Sebastian KERZEL2, Michael KABESCH2, Denise KÜSTER3, Jochen SCHMITT3, Regina FÖLSTER-HOLST1, Sascha GERDES1, Hila EMMERT1 and Stephan WEIDINGER1
1Department of Dermatology and Allergy, University Hospital Schleswig-Holstein, Campus Kiel, Kiel, 2Department of Pediatric Pneumology and Allergy, University Children’s Hospital Regensburg (KUNO), Clinic St Hedwig, Regensburg and 3Center of Evidence-based Healthcare, University Hospital and Medical Faculty Carl Gustav Carus, TU Dresden, Dresden, Germany
#Co-first authors.
Several small studies have indicated that daily emollient use from birth might delay, suppress or prevent atopic dermatitis (AD). Two larger trials did not confirm this; however, a recent smaller study indicated a protective effect if daily emollient use is used in the first 2 months of life. Further research is needed to evaluate the effect of emollient use on development of AD. The current study randomly assigned 50 newborns who were at high risk of developing AD (1:1) to receive general infant skin-care advice (control group), or skin-care advice plus emollient with advice to apply emollient at least once daily until 1 year of age (intervention group). Repeated skin examinations, skin physiology measurements and skin microbiome profiling were performed. Of the children in the intervention and control groups, 28% and 24%, respectively, developed AD (adjusted Relative Risk (RR) 1.19, p = 0.65, adjusted risk difference 0.05). Skin pH decreased and transepidermal water loss and stratum corneum hydration increased over time in both groups with no significant differences. In the intervention group skin microbiome alpha diversity increased earlier, and the abundance of Streptococcus and Staphylococcus species were significantly reduced at month 1. Daily early emollient use in children with high risk of AD was safe, but it did not significantly reduce the risk of developing AD or impact skin physiology development.
Key words: atopic dermatitis; early emollient; skin barrier; skin physiology.
SIGNIFICANCE
Atopic dermatitis is an itchy, inflammatory skin disease that affects 10–20% of children and 3% of adults. It often begins in infancy and significantly impairs the quality of life of affected patients and their families. The aim of this study was to determine whether daily use of emollients in neonates at increased risk of developing atopic dermatitis affects the likelihood of onset of the disease.
Citation: Acta Derm Venereol 2023; 103: adv5671. DOI: https://doi.org/10.2340/actadv.v103.5671.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Apr 6, 2023; Published: May 29, 2023
Corr: Stephan Weidinger, Department of Dermatology and Allergy, University Hospital Schleswig-Holstein, Campus Kiel, Arnold-Heller-Str. 3, DE-24105 Kiel, Germany. E-mail: sweidinger@dermatology.uni-kiel.de
Competing interests and funding: SW has received institutional research grants from La Roche Posay, LEO, Pfizer and Sanofi; and has performed consultancies and/or lectures for Abbvie, Almirall, Boehringer, Eli Lilly, Galderma, Kymab, Leo Pharma, Pfizer, Regeneron, and Sanofi. HE received an institutional research grant from LEO Pharma. JS received institutional grants for investigator-initiated research from the German GBA, the BMG, BMBF, EU, Federal State of Saxony, Novartis, Sanofi, ALK, and Pfizer. He also participated in advisory board meetings as a paid consultant for Sanofi, Lilly, and ALK. RF-H has been referee and consultant for the following companies: Astellas, Almirall Hermal, Beiersdorf, Biogen, Johnson&Johnson, La Roche Posay, LEO Pharma, Neubourg GmbH, Novartis, Pierre Fabre, Procter&Gamble, Regeneron, Sanofi. All other authors declare that they have no relevant conflicts of interest.
INTRODUCTION
A topic dermatitis (AD) is a chronic inflammatory skin disease characterized by intense itch and recurrent eczematous lesions, which typically manifests in early infancy and, for most patients, is a lifelong disease (1). Its pathogenesis is based on the interplay of a complex skin barrier dysfunction, a dysbiosis of the skin microbiome with a reduced diversity and a greater abundance of S aureus, and a Th2-dominated local immune dysregulation, which together drive inflammation (2). Both inherited and acquired (e.g. through the cutaneous inflammatory milieu) changes in the protein and lipid composition of the stratum corneum can contribute to the skin barrier deficiency, which is reflected by features such as a decreased hydration, increased water loss and skin pH, and which is postulated to lower inflammatory thresholds to irritants and haptens and to allow increased entry of antigens, allergens and pathogens (1). To date, there is no curative treatment for AD, and thus effective primary prevention strategies would be highly desirable. Currently there is no clear evidence showing that any of the allergy-based interventions examined to date are effective (3, 4). The recognition of the key importance of epidermal dysfunction in AD, and the fact that emollients can improve skin hydration and barrier function (5), have stimulated approaches to using basic barrier-restoring emollients in a preventive fashion. Initial pilot studies indicated that daily application of sunflower seed oil (6) and basic emollients, such as liquid paraffin 50% in white soft paraffin, DoubleBase Gel (Dermal Laboratories, Herts, UK) and Diprobase cream (Bayer, Berks, UK) (7), to ceramide-containing body lotions (4, 7) from birth is a safe and feasible approach and could delay, suppress, or prevent AD (8–10). However, these findings could not be confirmed in 2 larger randomized controlled trials (7, 11), and a recent meta-analysis indicated that early emollient use might delay rather than prevent AD (12). Recently, an approximately 30% reduced relative risk for AD with twice-daily use of an emollient formulation with added ceramides for the first 8 weeks of life was reported. This indicates that the timing and type of emollient may be important, and that additional studies evaluating how different emollients affect skin physiology and AD development are warranted (13).
The aim of this study was to investigate the effect of early use of an emollient containing a prebiotic Vitreoscilla filiformis lysate (14) (Lipikar Baume AP+ balsam (Lipikar), LA ROCHE-POSAY Laboratoire Pharmaceutique, France) on microbiome development and skin physiological parameters. Furthermore, the study investigated the effects of emollient use on the development or delayed onsetand severity of AD.
MATERIALS AND METHODS
Study design and participants
A pragmatic parallel group, assessor-blind, randomized open-label prospective study of emollient use in neonates at the Department of Dermatology, University Hospital Schleswig-Holstein (UKSH) Kiel, Germany, and at the WECARE Study Center of the Hospital St Hedwig, Regensburg, Germany was performed. Term newborns at high risk of developing AD, defined as having ≥1 first-degree relative with physician-diagnosed asthma, AD or allergic rhinitis, were included between days 1 and 21 after birth and assigned randomly (1:1) to an intervention (n = 25) or control (n = 25) group. The study was approved by the ethics committees of the Medical Faculty at the Christian-Albrechts-University in Kiel and the University of Regensburg. The trial was registered at ClinicalTrials.gov (NCT03376243).
Procedures and interventions
During the 2-year study period, data such as skin health and care were collected during 5 face-to-face visits (V1/baseline, V2/month 1, V4/month 6, V5/month 12, and V7/month 24) and 2 telephone interviews (V3/month 3 and V6/month 18). In addition, skin examinations were performed by trained physicians, skin physiological parameters were measured, and biosamples were taken. Follow-up questionnaires will be sent annually to the participating families up to the age of 6 years. If a child developed atopic eczema during the study period, they were excluded from the study and, if requested by the parents, followed up in the outpatients’ care unit.
At baseline, all parents of the participants received a structured information that included best-practice advice on infant skin care in accordance to the German Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) S3 guidelines for prevention (15) and the German Federal Centre of Health Education, Cologne. The parents of children randomized to the intervention group were additionally provided an emollient (Lipikar) and instructed to apply it at least once daily to the child’s entire body surface for the first year of life. An overview of the trial design is shown in Fig. 1.
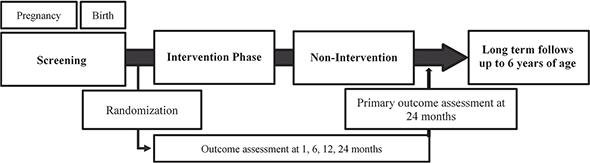
Fig. 1. Overview of trial design. Study participants were randomized to either intervention or control groups and invited to 5 visits at the hospital and 2 telephone interviews.
Outcomes
The primary feasibility outcome was the percentage of families willing to be randomized. The primary clinical outcome was the cumulative incidence of AD as defined by standard diagnostic criteria (16–18) at month 12 and month 24. Secondary outcomes comprised adherence with the intervention, incidence of emollient-related adverse events (AEs), amount of contamination as a result of increased awareness in the control group, development of skin physiology and microbiome diversity over time, and age and severity of AD at onset.
Measurement of skin physiological parameters
All skin physiology measurements were performed with the Multi Display Device MDD 4 (Courage and Khazaka Electronic GmbH, Köln, Germany) on the cheek, the volar forearm and the antecubital fossa (under controlled ambient conditions at room temperature). Transepidermal water loss (TEWL) (g/h/m2) was measured with the Tewameter TM300, stratum corneum (SC) hydration with the Corneometer CM 825 and skin pH with the pH-Meter PH 905 (Courage and Khazaka Electronic GmbH, Köln, Germany) following the manufacturers’ instructions. For the analysis of the SC-hydration the mean of 3 separate measurements was used for analysis.
Sampling of skin microbiota
Skin microbiota were collected from the cheek, the volar forearm and the antecubital fossa by swabbing a 4 cm2 skin area for at least 30 s using sterile Isohelix SK-3S Buccal Swabs (Isohelix, Cell Projects Ltd, Harrietsham Kent, UK) soaked in sterile SCF-1 solution (50 mM Tris buffer, 1 mM EDTA, 0.5% Tween-20 [pH8.0]). Bacterial DNA was extracted using the QIAamp UCP Pathogen mini kit automated on the QIAcube (QIAGEN, Hilden, Germany) following the manufacturer’s instructions.
Processing of bacterial 16S rRNA sequenced data
The V1 and V2 variable regions of the 16S rRNA gene were amplified by PCR with the universal primer pair 27F and 338R. Sequencing was performed with MiSeq Reagent Kit v3 on the Illumina MiSeq (Illumina Inc., San Diego, CA, USA). The ampliseq (Version 2.1.0) pipeline (19) was used with default parameters integrated in the nf-core framework (20). Briefly, the pipeline uses cutadapt for primer trimming, FastQC for quality evaluation, DADA2 to dereplicate, remove bimeras and infer amplicon sequence variants (ASVs) using the SILVA reference (Version 132) (21).
Statistical analysis
Infants were randomized in a 1:1 ratio using random block sizes to either the intervention or control group, with a central, Web-based, computer-generated, randomization service.
This was a pilot study and therefore not powered to establish efficacy of early emollient use vs regular care. The sample size of 50 was chosen pragmatically with reference to expected birth numbers, time and resources available. With an estimated proportion of 40–60% of participating families willing to be randomized, the precision was calculated to provide an estimate within 10% for a 95% confidence interval (95% CI) of the proportion willing to be randomized. With an expected incidence of AD within the first 2 years of life between 14% and 24% and 50 newborns randomized, 25 in each group, the 95% CI was calculated to have a rangeof 18–24%. In the primary analysis, the current study analysed participants as randomized regardless of adherence with allocation and using observed data. In a post hoc sensitivity analysis, actual emollient use was considered and all individuals who reported having used any emollient over the entire body for at least 5 days per week for a period of at least 2 months until visit 3 were assigned to the “emollient” group.
For both scenarios, boxplots of the physiological measurements were created for the healthy children. In each case, different time-points were considered and differences between the emollient and the control group were calculated using the Wilcoxon rank sum test. In addition, linear regression was performed to show the trend of each of the measured values over time. Same analyses were also performed for the comparison of healthy vs diseased children regarding AD. Furthermore, boxplots and the Wilcoxon rank sum test were used to compare Eczema Area and Severity Index (EASI), Objective Severity Scoring of Atopic Dermatitis (oSCORAD), and the time of onset of AD between the different intervention and control groups.
All statistical analyses were performed using R Statistical Software (22) (version 4.0.2 (2020-06-22)). Furthermore, the following packages were used: dplyr (23), tidyverse (24), reshape2 (25), ggplot2 (26) and ggpubr (27).
For microbiome analysis Shannon Index was used as a measurement of alpha diversity, Bray Curtis dissimilarity and Principal Coordinate Analysis (PcoA) for beta diversity, as well as differential abundance analysis using linear models including mode of delivery and sex of the infants as con-founders. For these analyses the R packages MicrobiomeStat (version 1.1) (28) phyloseq (version 1.4) (29) and vegan (version 2.6) (30) were used.
RESULTS
Baseline characteristics
A total of 50 term-born infants were randomized either to the intervention or control group. There were no significant differences in clinical characteristics between the 2 groups (Table SI).
Feasibility
The primary feasibility outcome was the percentage of families willing to participate in the current study with the approach for prevention of AD. A total of 402 families were screened for the study (Fig. SI). Of these, 265 (65.9%) did not fulfil the inclusion criteria. Of the remaining 137 families, 68 (49.6%) agreed to participate the study. Screening was discontinued after inclusion of 50 participants.
Incidence, severity and age of onset of atopic dermatitis
Eleven (44%) participants in the intervention group and 8 (32%) participants in the control group discontinued the study before the 7th visit (month 24); 7 and 6 of them, respectively, because they had developed AD, 1 participant in the intervention group discontinued the study, 3 children in the intervention group and 2 in the control group were lost to follow-up (Fig. SI). Children in the control group who developed AD were younger at onset than children in the intervention group (mean age 6 vs 13 months). There was no significant difference in mean severity at onset between the 2 groups (Table I).
Compliance and contamination rates
To examine adherence to the study protocol, compliance and contamination rates were investigated. Families of the intervention group were advised to apply an emollient (Lipikar) at least once daily to the entire body surface area of the child for the first 12 months. The results showed that 95%, 89.4%, 88.2% and 88.2% of families in the intervention group reported daily use of the emollient since the last visit at month 1, month 3, month 6 and month 12, respectively (mean reported adherence over 12 months in the intervention group: 90.2%). After the intervention phase of 12 months, parents were free to decide whether to continue using the study product. At month 24, 14 (28.6%) of the remaining parents of the intervention group reported that they had continued to apply the emollient daily (Table SII).
The families of the control group were not recommended to use emollients; use of emollients from 5–7 days a week by these families were evaluated as “contamination”. Throughout the study period, we had a “contamination” of 16%, 13%, 23.8% and 10.6% at month 1, month 3, month 6 and month 12 (mean “contamination” in the control group: 15.85%) (Table SIII).
Differences and changes in trans-epidermal water loss, pH and electrical capacitance over time
No significant differences were observed for the TEWL development over time at any of the examined body areas (cheek and volar forearm) between the intervention and the control group (Fig. 2A/B). Linear regression analysis showed an increase in TEWL over time for the volar forearm in both groups (intervention ß1Volar forearm = 0.218 and control ß1Volar forearm = 0.980) (Fig. S2B). On the cheek, TEWL decreased slightly in the intervention group over time (ß1cheek = –0.073) and increased in the control group (ß1cheek = 0.283) (Fig. S2A). Linear regression analysis showed an increase in SC hydration over time for the volar forearm in both groups (control ß1Volar forearm = 0.840 and intervention ß1Volar forearm = 0.781; Fig. S3B). Children in the intervention group had slightly, but not significantly, higher values at all visits (Fig. 3A/B).
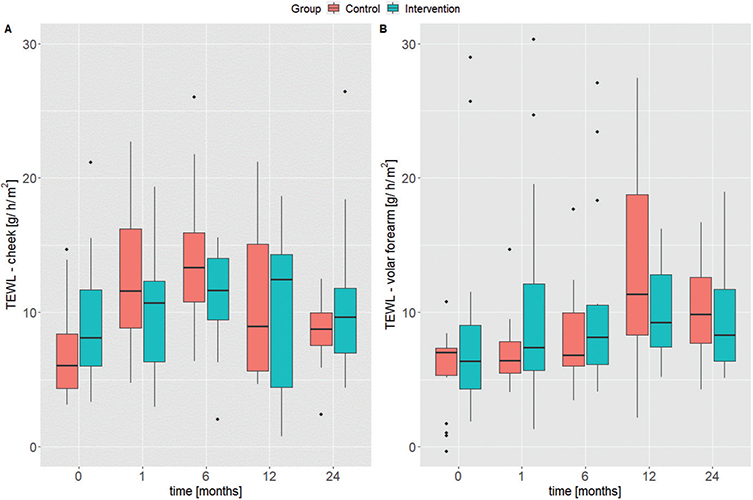
Fig. 2. Box plots for transepidermal water loss (TEWL) measurements (A) at the cheek and (B) the volar forearm. The median is plotted for each visit of the control group (red) and the intervention group (blue). Study time-points are shown in months on the x-axis.
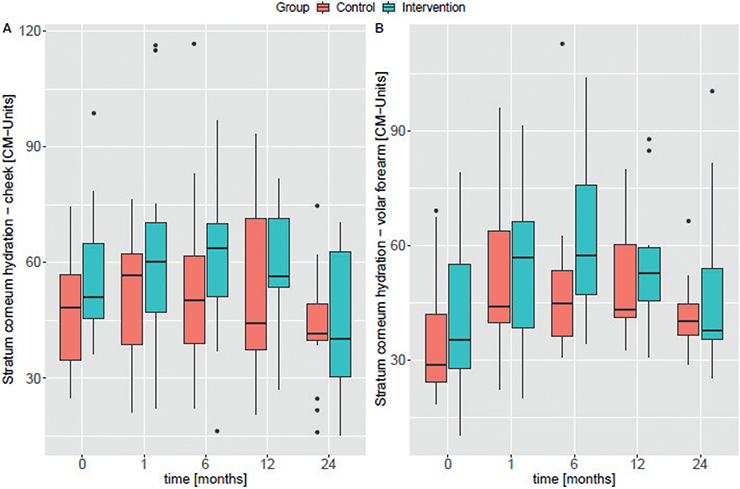
Fig. 3. Box plots for stratum corneum hydration measurements at (A) the cheek and (B) the volar forearm. The median is plotted for each visit of the control group (red) and the intervention group (blue). Study time-points are shown in months on the x-axis. CM: Corneometer units.
Likewise, no significant differences were observed in the skin pH between groups (Fig. 4A/B). In both groups, skin pH decreased slightly over time on the volar forearm (control ß1Volar forearm = –0.016 and intervention ß1Volar forearm = –0.030) (Fig. S4B). The same trend was observed for the cheek of the intervention group (ß1Cheek = –0.018), whereas there was a slight, but not significant, increase over time in the control group (ß1Volar forearm = 0.007) (Fig. S4A).
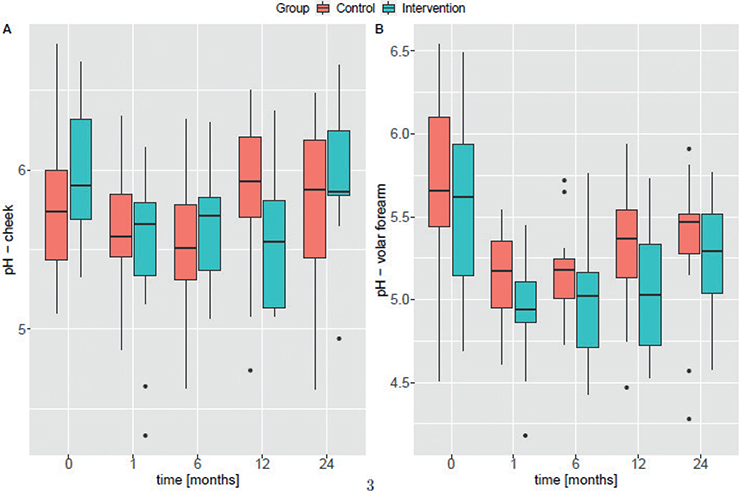
Fig. 4. Box plots for pH measurements at (A) the cheek and (B) the volar forearm. The median is plotted for each visit of the control group (red) and the intervention group (blue). Study time-points are shown in months on the x-axis.
Skin microbiome
Across all time-points alpha and beta diversity did not differ significantly between intervention and control groups (Fig. 5A/B). However, at month 1, a significantly higher alpha diversity (Shannon Index) (Fig. 5C), and significantly decreased abundance of Staphylococcus epidermidis and Streptococcus mitis species and increased Enhydrobacter (unc.) in the intervention group (Fig. 5D) were observed. Until month 24 the proportion of Staphylococcus aureus ASVs was negligible in the both groups (<1%). Considering only those children who were carriers of S. aureus in both groups (control group n = 11, intervention group n = 9) and including the occurrence of AD during the study, no significant difference was found in the group distribution (Fisher exact: p = 0.62). Also, the relative abundance of S. aureus was not significantly different between AD and healthy subjects, either in the intervention or the control group (Wilcoxon: p = 0.26 and p = 0.33, respectively). A full overview of significantly differential ASVs is shown in Table SIV.
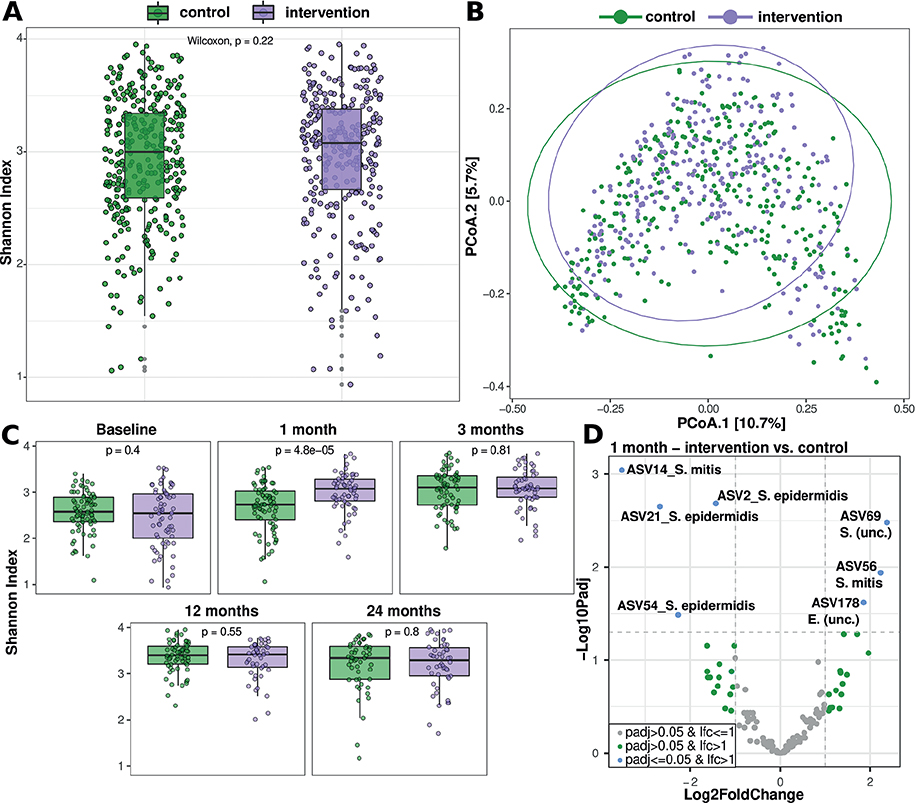
Fig. 5. Microbiome differences between intervention and control groups. (A) Alpha diversity (Shannon index) summarized over all time-points. (B) Beta diversity (PcoA of Bray-Curtis dissimilarity) summarized over all time-points. (C) Alpha diversity (Shannon index) for each time-point, p-value indicates Wilcoxon rank sum test. (D) Volcano plot of linear model based differential abundant amplicon sequence variants (ASVs). Negative Log10 of the Benjamini Hochberg corrected p-value is plotted over the Log2FoldChange (LFC) per ASV, grey dots indicate non-significantly differential ASVs with a LFC of 1 or lower, green dots indicate non-significantly differential ASVs with a LFC >1 and blue dots indicate ASVs with a significantly differential abundance between the groups and a LFC > 1. ASV names are based on the best rank hit by DADA2 classification (using the SILVA database).
Adverse events
No AEs were reported during the duration of the trial.
Sensitivity analysis
For sensitivity analysis, participants who reported an emollient use of at least 5 times per week on the whole body for at least 2 months until visit 3 (month 3) were classified as the intervention group. Based on these definitions, 26 children were assigned to the control group, of whom 6 (23%) developed AD until month 12, and 19 children (42.2%) were assigned to the intervention group, of whom 2 (10.5%) developed AD until month 12. Until month 24, 3 additional children in the intervention group developed AD, resulting in a total of 5 (26%) children developing AD up to month 24. The mean age at onset of AD was higher in children in the intervention group, but not significantly compared with the control group (Table II). Disease severity at onset did not show significant differences between the 2 groups (Fig. S5A/B).
There were no significant differences in TEWL values or SC hydration measurements between the intervention and control groups in the sensitivity analysis at any of the time-points (Fig. S6A/B and Fig. S7A/B). Likewise, there were no significant differences with regard to skin pH, except for the volar forearm at month 6 (p = 0.02), where values were slightly higher in children in the control group (Fig. 6).
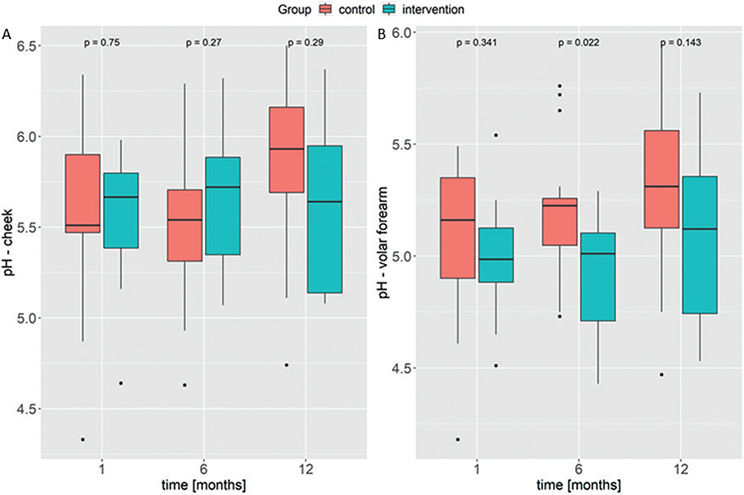
Fig. 6. Box plots for pH measurements on (A) the cheek and (B) the volar forearm in the reclassified groups not including the children who developed atopic dermatitis. The median is plotted for each visit of the control group (red) and the intervention group (blue). Study time-points are shown in months on the x-axis.
In the sensitivity analysis, alpha and beta diversity were also not significantly different between the intervention and control groups across all time-points (Fig. S8A/S8B). Comparing diversity over time between the 2 groups, significantly higher alpha diversity (Shannon index) was observed in the intervention group after 1 month (Fig. S8C) where, in addition, the study found significantly different frequencies of ASVs with a lower frequency of ASVs attributable to the species Staphylococcus epidermidis (ASV2 and ASV21) and Streptococcus mitis (ASV14) in the intervention group, whereas another ASV of the species Streptococcus mitis (ASV56) was increased (Fig. S8D). A complete list of all ASVs that were differentially prevalent in the groups is shown in Table SV.
DISCUSSION
Regular use of emollients in order to enhance skin barrier function is an essential part of any AD management strategy (31). While several smaller studies indicated that daily application of basic emollients from birth might prevent or delay AD (6, 9, 13), larger trials did not confirm this (7, 11). However, it cannot be excluded that the effectiveness of such an intervention depends on the type of emollient and specific subgroups of patients. Likewise, the effects of early emollient use beyond AD, e.g. on the skin physiology and microbiome, have not yet been examined (31).
This current pragmatic, randomized controlled trial of high-risk infants did not find robust evidence that the regular use of a complex emollient formulation with a prebiotic lysate for the first year of life can prevent AD, although there was a trend towards a higher age at onset of AD in children in the intervention group (12).
The current study found no evidence that regular use of emollients from birth affects TEWL or SC hydration. Only the skin pH was found to differ significantly, at 1 time-point (6 months) after regrouping the participants. It is important to note, however, that the current findings relate only to prevention, and not to management, of AD, for which the regular and liberal use of emollients is well established and recommended (31).
In addition to clinical parameters, the current study also found notable early alterations in the skin microbiome with emollient use. An earlier increase in diversity accompanied by a decrease in disease-relevant species was observed within the intervention group for both classifications. The maturation and diversification of the early skin microbiome has previously been shown as a key development during infancy, and an earlier diversification within the intervention group can therefore be regarded as being beneficial (32). Reduced abundances of Streptococcus and Staphylococcus ASVs, as well as increased abundances of rare bacteria, have been previously regarded as the main drivers of this diversification, which the current study confirmed through sensitivity analysis (32).
No emollient-related AEs were reported. Adherence in the intervention group was high (90.2%). The major limitation of this study was the small number of participants and a rather high rate of contamination in the control group, probably due to increased awareness, based on the study title and initial inclusion interview. Strengths of the current study include its randomized controlled trial design, blinded outcome assessment, and the high number of follow-up visits to the clinic, as well as analysis of skin physiology measurements and skin microbiome analysis, which, for the first time, were performed in the context of early emollient use from birth. Long-term follow-up of the study participants until the age of 6 years is ongoing, and, given that there was a trend towards later onset and lower activity of AD at onset in the intervention group, there is still the possibility of beneficial long-term effects of early emollient use, such as a milder course of AD.
ACKNOWLEDGEMENTS
The EARLYemollient Study was financially supported by La Roche-Posay Laboratoire Pharmaceutique, France. The funding source was not involved in the study design, the collection, analysis and interpretation of data, writing of the report, or in the decision to submit the article for publication.
The study was approved by the ethics committees of the Medical Faculty at the Christian-Albrechts-University in Kiel (A101/16, June 20, 2016) and the University of Regensburg (17-882-160, March 12, 2018).
REFERENCES
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360.
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Vol., Nature Rev Dis Primers 2018; 4: 1.
- Abrams EM, Watson W, vander Leek TK, Atkinson A, Primeau MN, Francoeur MJ, et al. Dietary exposures and allergy prevention in high-risk infants. Allergy Asthma Clin Immunol 2022; 18: 36.
- Dissanayake E, Tani Y, Nagai K, Sahara M, Mitsuishi C, Togawa Y, et al. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: a 2 × 2 factorial, randomized, non-treatment controlled trial. Int Arch Allergy Immunol 2019; 180: 202–211.
- Glatz M, Jo JH, Kennedy EA, Polley EC, Segre JA, Simpson EL, et al. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS One 2018; 13: e0192443.
- Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WHI, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014; 134: 818–823.
- Chalmers JR, Haines RH, Bradshaw LE, Montgomery AA, Thomas KS, Brown SJ, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020; 395: 962–972.
- Kvenshagen BK, Carlsen KH, Mowinckel P, Berents TL, Carlsen KCL. Can early skin care normalise dry skin and possibly prevent atopic eczema? A pilot study in young infants. Allergol Immunopathol (Madr) 2014; 42: 539–543.
- Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014; 134: 824–830.e6.
- Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WHI, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014; 134: 818–823.
- Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2020; 395: 951–961.
- Zhong Y, Samuel M, van Bever H, Tham EH. Emollients in infancy to prevent atopic dermatitis: a systematic review and meta-analysis. Allergy 2022; 77: 1685–1699.
- Chaoimh CN, Lad D, Nico C, Puppels GJ, Wong XFCC, Common JE, et al. Early initiation of short-term emollient use for the prevention of atopic dermatitis in high-risk infants – the STOP–AD randomised controlled trial. Allergy 2023; 78: 984–994.
- Gueniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, et al. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled clinical study. Br J Dermatol 2008; 159: 1357–1363.
- Schlüsselwörter Allergie-Evidenz-S3-Leitlinie-Primär-prävention-Überarbeitung.
- Williams HC, Jburney PG, Hay RJ, Archer CB, Shipley MJ, Ahunter JJ, et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994; 131: 383–396.
- Williams HC, Jburney PG, Strachan D, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. II. Observer variation of clinical diagnosis and signs of atopic dermatitis. Br J Dermatol 1994; 131: 397–405.
- Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol 1994; 131: 406–416.
- Straub D, Blackwell N, Langarica-Fuentes A, Peltzer A, Nahnsen S, Kleindienst S. Interpretations of environmental microbial community studies are biased by the selected 16S rRNA (gene) amplicon sequencing pipeline. Front Microbiol 2020; 11: 2652.
- Ewels PA, Peltzer A, Fillinger S, Patel H, Alneberg J, Wilm A, et al. The nf-core framework for community-curated bioinformatics pipelines. Nature Biotechnol 2020; 38: 276–278.
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013; 41: D590–D596.
- R: The R Project for Statistical Computing. [accessed 25.11.2022] Available from: https://www.r-project.org/.
- Function reference • dplyr. Available from: https://dplyr.tidyverse.org/reference/index.html, accessed 25.11.2022
- tidyverse citation info. Available from: https://cran.r-project.org/web/packages/tidyverse/citation.html, accessed 25.11.2022
- reshape2 citation info. [accessed 25.11.2022] Available from: https://cran.r-project.org/web/packages/reshape2/citation.html.
- ggplot2 citation info. [accessed 25.11.2022] Available from: https://cran.r-project.org/web/packages/ggplot2/citation.html.
- CRAN - Package ggpubr. [accessed 25.11.2022] Available from: https://cran.r-project.org/web/packages/ggpubr/index.html.
- Zhou H, He K, Chen J, Zhang X. LinDA: linear models for differential abundance analysis of microbiome compositional data. Genome Biol 2022; 23: 1–23.
- McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013; 8: e61217.
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. 2019. R package version. 2015; 2(10).
- Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020; 34: 2717–2744.
- Capone KA, Dowd SE, Stamatas GN, Nikolovski J. Diversity of the human skin microbiome early in life. J Invest Dermatol 2011; 131: 2026–2032.