ORIGINAL REPORT
Common Histological Features Suggesting Enchondral Ossification Pathways in Calciphylaxis of Various Origins: A Study of Human Subcutaneous Tissue Biopsies
Simon ABERGER1, Barbara FINDENIG1,2, Jane BEIL2, Nicole AICHINGER2, Josef KOLLER3, Cees VERMEER4, Leon SCHURGERS4, Elke THEUWISSEN4, Elena MORÉ1, Michael FRANZEN1, Cornelia KRONBERGER2 and Hermann SALMHOFER1
1Department of Internal Medicine I, 2Department of Pathology and 3Department of Dermatology, Paracelsus Medical University, Salzburg, Austria and 4Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands
Calciphylaxis is a rare, yet underdiagnosed condition causing high mortality in patients with severe renal and cardiovascular disease. Since knowledge of the pathophysiology of calciphylaxis is limited, a differential analysis of histological alterations in patient subgroups with various comorbidities might expose different disease phenotypes and allow deeper insights into the pathophysiology of the condition. Histological markers of osteogenesis and calcification were investigated in a group of 18 patients with clinically and histologically verified calciphylaxis, using immunohistochemical staining. Analysis of staining intensity and distribution of marker proteins in histological structures was performed to evaluate distinct patterns between subgroups with different clinical comorbidities in comparison with a control group. In all cases, immunohistochemical staining for bone matrix proteins, bone-morphogenic proteins and matrix-Gla proteins co-localized with subcutaneous vascular and interstitial calcifications. Significant expression of bone-morphogenic protein-7 and active matrix-Gla protein was observed. Mortality was associated with renal comorbidities and increased expression of bone-morphogenic protein-7. However, no distinct histological patterns were found between subgroups with renal disease, warfarin intake or coexisting micro- and macro-angiopathies. The upregulation of osteogenic markers (including bone-morphogenic protein-7) plays a major role in the development of calciphylaxis. Clinical outcome correlates with kidney function and phosphate handling, suggesting different pathophysiological mechanisms. However, biopsy at late-stage disease shows a common histological phenotype, involving enchondral ossification.
Key words: bone morphogenic proteins; calcific uraemic arteriolopathy; enchondral ossification; rare diseases.
SIGNIFICANCE
Calciphylaxis is a rare, calcifying, occlusive vessel disease with different aetiopathological origins and high mortality. Previous histological studies have shown upregulation of bone-morphogenic proteins, bone matrix proteins, and dysbalance of calcification inhibitors. This study confirmed the upregulation of osteogenic markers, including the first evidence of upregulation of bone-morphogenic-protein 7, in calciphylaxis. Renal function, sequential comorbidities, phosphate handling and high expression of bone-morphogenic protein-7 were identified as predictors of clinical outcome. However, the histological appearance did not differ between patients with different comorbidities. Therefore, disease evolution may have different pathophysiological drivers with a common pathophysiological endpoint. This underlines the need for individualization of multimodal treatment in calciphylaxis.
Citation: Acta Derm Venereol 2023; 103: adv5755. DOI: https://doi.org/10.2340/actadv.v103.5755.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 30, 2023; Published: Jul 10, 2023
Corr: Hermann Salmhofer, Department of Internal Medicine I, Paracelsus Medical University, University Hospital Salzburg, Müllner Hauptstrasse 48, AT-5020 Salzburg, Austria. E-mail: h.salmhofer@salk.at
Competing interests and funding: The authors have no conflict of interest to declare.
INTRODUCTION
Calciphylaxis is a rare calcifying skin disease involving local as well as systemic dysregulation of calcium and phosphate homeostasis. Pathological calcium depositions are found within the vascular wall of subcutaneous arterioles and surrounding interstitium, leading to soft tissue ulcerations by vessel occlusion and thrombosis (1). Diagnostic delay arises from undifferentiated symptoms, including pain, myopathy and skin mottling in early disease stages (2).
The incidence of calciphylaxis in patients on dialysis was reported at below 0.01% over a 4-year period, as derived from the EVOLVE trial data by Floege et al. (3), and is even rarer in patients with preserved renal function. Cardiovascular comorbidities, such as obesity, diabetes, occlusive coronary or peripheral artery disease, as well as dysregulation of calcium-phosphate homeostasis, have been described as risk factors for disease development in retrospective cohort studies on chronic kidney disease (CKD) and dialysis registries (4, 5). Notably, in patients without renal impairment, these comorbidities demonstrate a particular aetiopathological association. In a report of 3 cases of non-uraemic calciphylaxis, primary hyperparathyroidism, chronic inflammatory diseases, high calcium-phosphate product and use of vitamin-K antagonists (VKA) were noticed (6). A risk relationship between VKA use and calciphylaxis was established by case-control studies of haemodialysis patients (7, 8), and Floege et al. (3) reported the use of VKA in approximately 45% of patients with calciphylaxis in the EVOLVE trial.
Concerning the pathophysiology of calciphylaxis, previous research has yielded 2 possible mechanisms, involving either precipitation of serum calcium phosphate or de novo local production of bone-like matrix. Regarding increased precipitation of calcium and phosphate, the role of fetuin-A and matrix-Gla proteins (MGP) have been studied as systemic and local inhibitors of calcification, respectively. Fetuin-A is found in the blood, and acts as a calcium scavenger by forming complex multimers, so-called calciprotein particles (9), whereas MGP mainly elicit inhibitory effects on tissue calcification in response to vitamin-K dependent post-translational modification (as reviewed in (10, 11)). However, it is unclear whether patients treated with VKA exhibit distinct histological or molecular features compared with untreated patients.
In recent decades, scientific interest has refocused on the role of active bone formation in vascular calcification, which was recognized as early as in the first half of the 20th century (12). In vitro investigations offered evidence for transdifferentiation of vascular smooth muscle cells (VSMC) into osteoblast-like cells via activation of Runx2 (also known as core-binding factor α-1) triggered by high phosphate exposure, inflammatory stress and loss of calcification inhibitors (13, 14). The expression of bone matrix proteins and ossification markers, such as osteopontin and members of the bone-morphogenic protein (BMP) family, was shown in human skin biopsies of patients with calciphylaxis (15, 16). BMPs activate downstream Smad-effector proteins and Runx2, facilitating the phenotypic plasticity of VSMCs (17). Furthermore, spectrometric analysis of human biopsy material identified carbonate apatite as the main component of calcifications in several calcifying skin disorders (18), and bone scintigraphy studies detected tracer uptake in calciphylaxis lesions (19). Therefore, involvement of active bone metabolism in the development of calciphylaxis has been implicated by experimental as well as clinical studies. However, it is not known whether histomorphological differences or a temporal sequence of effector protein expression is associated with different comorbidities.
In summary, our current understanding of the pathophysiological mechanisms involved in the development of calciphylaxis and other calcifying skin disorders is limited. The aim of the current study was to analyse the expression of molecular markers of bone matrix formation and osteoblast differentiation in a retrospective collection of patient tissue samples. Further analysis of a thoroughly documented medical history with regard to the various origins of calciphylaxis was carried out. The results contribute to a better understanding of the pathophysiology of calciphylaxis, possibly involving enchondral ossification.
MATERIALS AND METHODS
Study cohort
Archival tissue biopsies were identified in a search of the local database, at the Department of Pathology, University Hospital Salzburg, Austria. Human cutaneous biopsies of 18 patients with histologically and clinically proven calciphylaxis were collected for immunohistochemical (IHC) staining. Furthermore, 22 subcutaneous biopsies were obtained from patients without calciphylaxis, who were undergoing hip surgery, to serve as a control group.
The use of archival biopsy material for retrospective analysis, as well as the collection of subcutaneous tissue material during hip surgery, was approved by the Institutional Review and Ethics Board, County of Salzburg before performing the experiments (Study ID 415-EP315).
Detailed medical history, and data on renal function and comorbidities were present in all 18 cases of calciphylaxis.
Histology and staining techniques
After biopsy, tissues were routinely prepared, fixed in 4% neutral buffered formalin, embedded in paraffin and cut to 3-µm thickness. Standard haematoxylin-eosin and Von Kossa staining were performed. Immunoperoxidase staining for antibodies against various bone and calcification markers was performed on an automated DAKO autostainer platform (Agilent Technologies, Santa Clara, CA, USA) with standard staining protocols for all markers. A list of all markers, company information and catalogue numbers are given in Appendix S1.
Microscopic scoring and measurements
Light microscopic assessment was performed by using a standardized protocol and documentation form to ensure equal classification of all slides (provided in the Appendix S1). The procedure was repeated by an independent, experienced pathologist blinded to the case/control status. According to the standardized protocol, histological slides were subdivided into regions of interest (calcifications, endothelium, vascular layers, interstitium, macrophages, adipose and connective tissue). Staining intensity was semiquantitatively expressed on a scale of 0 to 3 (0: “no staining/negative” to 3: “very strong staining”) for each region.
Statistical analysis
For basic statistical analysis and data processing Microsoft Excel 2016 (Microsoft, Redmond, WA, USA) and Graphpad Prism 9 (Graphpad, Sandiego, CA, USA) were used. Data were expressed as mean ± standard deviation (SD). For p-value analysis between 2 groups a Mann–Whitney U test was performed and between multiple groups a Kruskal–Wallis test with Dunn’s post-hoc test was performed. Kaplan–Meier curves with log-rank test and univariate Cox regression model were used for mortality analysis. Statistical significance was accepted for p < 0.05.
RESULTS
Study cohort
As shown in Table I, there was a 1:1 male to female ratio with a median age of 63 years and 1-year survival rate of 50%. In total, 10/18 of patients were on dialysis, 4/18 were in predialysis care, including 2 patients with a renal posttransplant status, and 4/18 had preserved renal function. Among those with preserved renal function, 4/4 were on VKA, 3/4 had diabetes as well as occlusive peripheral artery disease (PAD), and there was 1 case of primary hyperparathyroidism (Table II).
In contrast, among patients on dialysis or severely impaired kidney function, the proportion with diabetes, PAD and VKA treatment did not exceed 50% (Table II). Arterial hypertension (AHT) and secondary hyperparathyroidism (HPT) were the most frequent comorbidities.
Immunohistochemistry
The comparison of mean staining intensities of each histological marker between the calciphylaxis and control groups showed statistically significant increases in bone-morphogenic protein 7 (BMP-7), active matrix-Gla protein (Gla-MGP), inactive matrix-Gla protein (Glu-MGP), Indian-hedgehog (IHH), as shown in Fig. 1. Staining for BMP-7 and Gla-MGP both co-localized with calcified regions, the vascular wall, surrounding interstitial space and macrophages (Fig. 2).
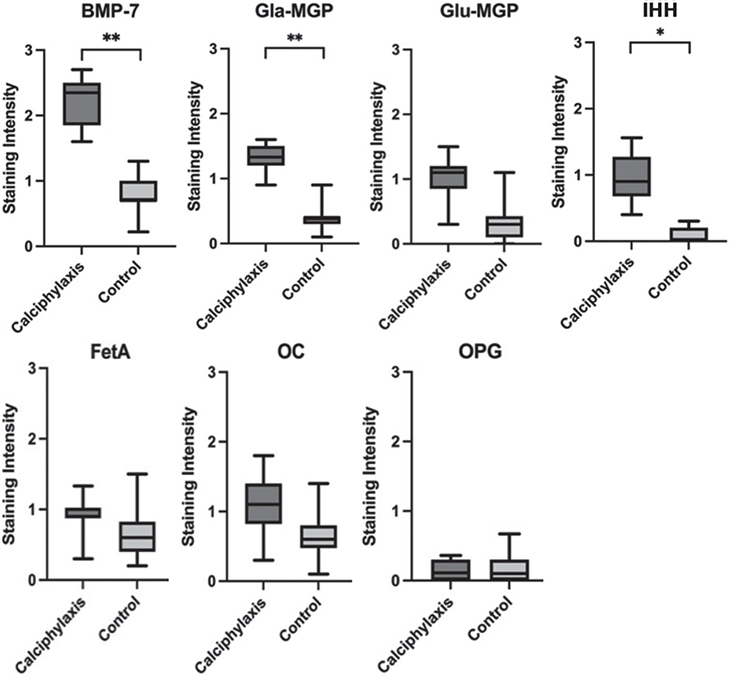
Fig. 1. Data comparison of staining intensities between the calciphylaxis and control group for bone-morphogenic protein-7 (BMP-7), active matrix-Gla protein (Gla-MGP), inactive matrix-Gla protein (Glu-MGP), Indian-hedgehog (IHH), fetuin-A, osteocalcin (OC) and osteoprotegerin (OPG) with box plots. Mann–Whitney U test; statistical significance (*p < 0.05) and (**p < 0.01).
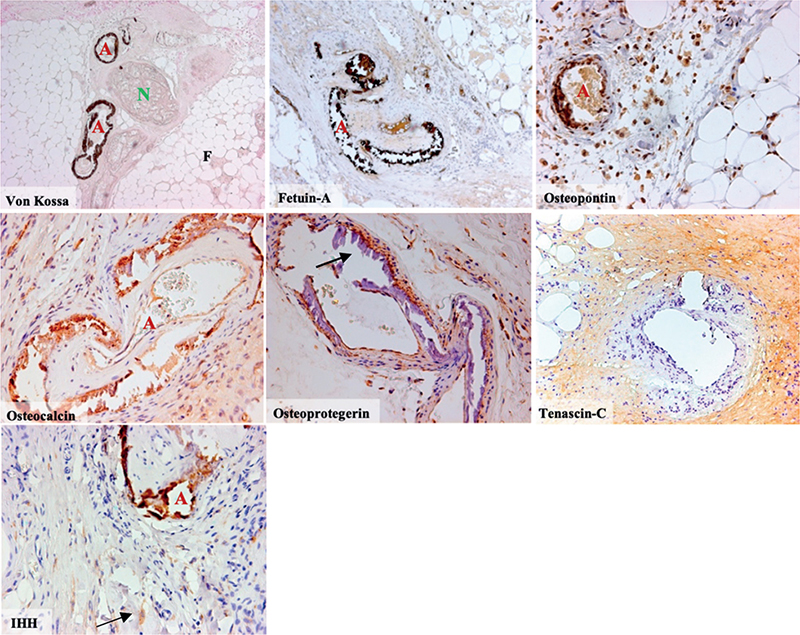
Fig. 2. Von Kossa and immunohistochemical (IHC) staining of different osteogenic markers in biopsies from patients with calciphylaxis, (10x magnification for VonKossa, fetuin-a, tenascin-c and 40x magnification for osteopontin, osteoprotegerin, osteocalcin and indian-hedgehog [IHH]). Von Kossa staining depicting typical calcification of subcutaneous arterioles marked “A”, a cutaneous nerve marked “N” and surrounding adipose tissue marked “F”. IHC staining of fetuin-A, osteopontin and osteocalcin revealing strong co-localizations with calcified arterioles marked “A”. IHC staining of osteoprotegerin did not co-localize with vascular calcifications marked with arrow, weak expression was detected within the vascular wall. IHC staining of tenascin C was positive in the surrounding tissue only. IHC staining of IHH co-localizes with calcifications marked “A” and reveals IHH-positive cells (arrow) in the subcutaneous connective tissue of patients with calciphylaxis.
When comparing local differences in staining intensities, a strong co-localization of fetuin-A, IHH, osteopontin and osteocalcin to calcified regions was found (Fig. 3). For osteoprotegerin much weaker staining was identified within calcifications. BMP-7, Gla-MGP and Glu-MGP strongly co-localized with calcifications. BMP-7 was also found in the surrounding tissues (Fig. 2).
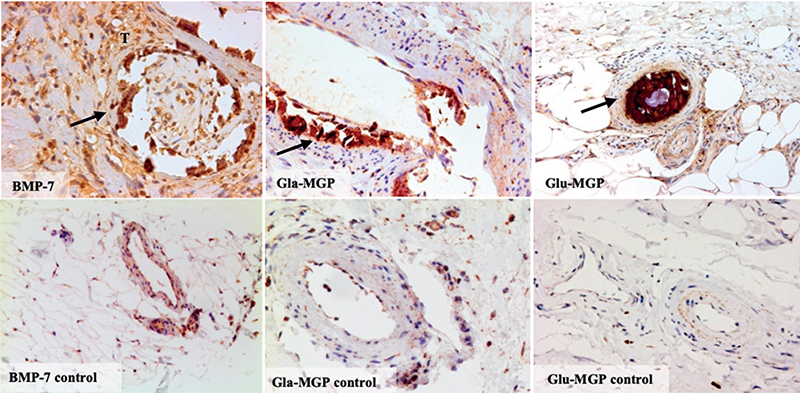
Fig. 3. Differential staining intensities for bone-morphogenic protein-7 (BMP-7), active matrix-Gla protein (Gla-MGP) and inactive matrix-Gla protein (Glu-MGP) in biopsies from patients with calciphylaxis compared with controls. Arrows mark intravascular calcifications. BMP-7-positive cells can be seen in the surrounding tissue marked “T”. Intraluminal thrombosis can be seen in the vascular cross section of BMP-7 and Glu-MGP stains. 40x magnification.
Staining malfunction occurred for BMP-2, BMP-4, Col2α1, Runx2, Sox9 and β-catenin (data not shown). Therefore, these markers were not included in the final analysis.
Mortality analysis and renal function
A statistically significant survival benefit in patients with preserved renal function was evident on Kaplan–Meier curve analysis (Fig. 4). Comparison of staining intensity and renal function showed a trend towards higher BMP-7 expression among patients with reduced renal function, which translated into a risk-correlation between BMP-7 expression and mortality (Fig. 4, Table III).
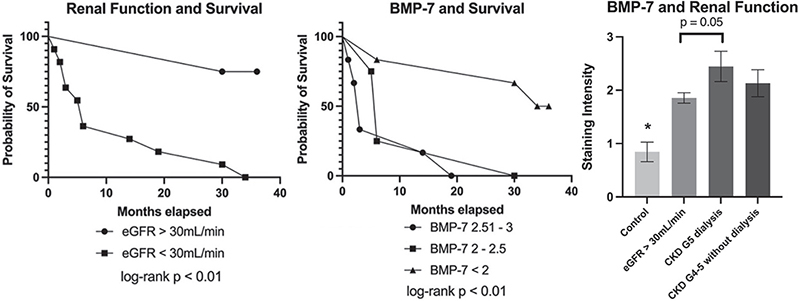
Fig. 4. Kaplan–Meier curves show significantly better 3-year survival in patients with calciphylaxis and eGFR >30 ml/min compared to eGFR <30 ml/min as well as in patients with BMP-7 expression < 2 compared to BMP-7 expression >2 (log-rank test p < 0.01). A trend towards higher expression of bone-morphogenic protein-7 (BMP-7) in patients with calciphylaxis and reduced renal function is depicted as a bar graph with mean±standard deviation (SD) staining intensities. Kruskal–Wallis test; (*) indicates statistical significance (p < 0.05) compared with all other groups.
Subgroup analysis
For further comparison, the calciphylaxis group was subdivided according to dialysis status, the presence of diabetes, occlusive peripheral artery disease, hyperparathyroidism, and anticoagulation with VKA. However, subgroup analysis did not reveal differences in staining intensity or marker distribution (Fig. 5). Therefore, no distinct histological patterns could be identified for any risk factor.
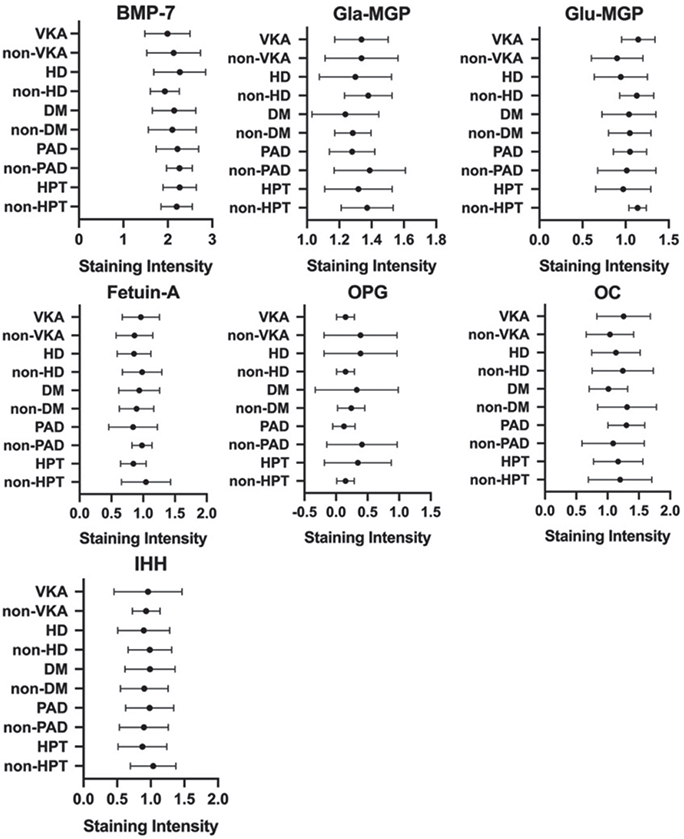
Fig. 5. Subgroup comparisons of markers and risk factors in patients with calciphylaxis. Mean staining intensities (± standard deviation (SD)) for bone-morphogenic protein-7 (BMP-7), active matrix-Gla protein (Gla-MGP), inactive matrix-Gla protein (Glu-MGP), fetuin-A, osteocalcin (OC), Indian-Hedgehog (IHH) and osteoprotegerin (OPG) were depicted vs clinical disease subgroups. No significant differences were detected between groups compared using Mann-Whitney U test (all p > 0.05). VKA: vitamin-K antagonist; HD: haemodialysis; DM: diabetes mellitus; PAD: peripheral artery disease; HPT: hyperparathyroidism. Note: for illustrative purpose the scale of the x-axis has been adjusted for each marker (staining intensity ranges from 0 to 3 for all markers).
DISCUSSION
The pathophysiology of calciphylaxis has been studied extensively, but is not fully understood. The current study characterized histopathological changes in a collection of human tissue samples with proven calciphylaxis and thoroughly documented medical history. Median age at the time of diagnosis was 63 years, with approximately 80% of patients having severe kidney disease (CKD G4–5). Thus, 70% of these patients were on dialysis, 35% on VKA, 35% had diabetes, 35% had atherosclerotic diseases, and 85% had a manifestation of secondary HPT. On the other hand, in patients with preserved kidney function (CKD G1–3) 100% were on VKA, 75% were diabetic, 75% had atherosclerotic disease, and there was 1 case of primary hyperparathyroidism. Mean survival time after the diagnosis was approximately 1.7 years in the current cohort, with 50% of patients surviving less than 1 year upon manifestation of calciphylaxis.
These findings are in agreement with previous larger scale cohort analysis describing the predominant impact of uraemia and phosphate retention on the development of calciphylaxis and its high mortality (3, 4). Preserved kidney function was linked to markedly increased survival time in the current study, and might therefore be a prognostic factor. Nevertheless, early recognition and multidisciplinary treatment can decrease mortality even in patients with severe comorbidities (20).
Concerning the pathophysiology of calciphylaxis, previous histological and molecular studies revealed a potential role of local de novo production of bone-like matrix caused by transdifferentiation of VSMC into osteoblast-like cells via Runx2 activation (21). Upregulated gene expression of BMP-2 and BMP-4 act as main inducers of an osteogenic milieu including deposition of bone matrix proteins and ECM remodelling (22, 23). In the current study, staining for BMP-2, BMP-4 and Runx2 was not reproducible, due to malfunction of the antibodies. However as a new finding, the current study revealed strong expression of BMP7 within calcifications and vascular layers. Current knowledge of the role of BMP-7 in vascular calcification is limited. In experimental studies using a murine model of severe CKD and vascular calcification, infusion of synthetic BMP-7 has been shown to elicit inhibitory effects on calcification (23) and to protect against atherosclerosis (24). Furthermore, BMP-7 plays an important role in kidney development and has been shown to inhibit renal fibrosis in kidney disease (25, 26). BMP-7 elicits its effects by counteracting signalling pathways of osteogenic transdifferentiation of VSMC and transforming growth factor beta (TGF-β)-pathway (27). To our knowledge, this is the first study to show the presence of BMP-7 in calcified areas of subcutaneous biopsies from patients with calciphylaxis. Given its pleiotropic biological activities, functional analysis of BMP-7 in the context of vascular calcification will be important in future follow-up studies.
In line with these findings, we identified co-expression of calcification inhibitors, Gla-MGP and fetuin-A, in calcified lesions. Gla-MGP was also present within the vascular wall and surrounding tissues. However, the current study did not detect osteoprotegerin expression in calcifications and found only low staining activity in the surrounding tissue. Osteoprotegerin has been linked to protective properties against VKA-induced vascular calcification (28), but high serum levels correlate with overall increased calcification risk (29). Previous studies provided evidence of synergistic effects of vitamin K dependent matrix-Gla protein, fetuin-A and osteoprotegerin as calcification inhibitors in calciphylaxis (30) and CKD (31, 32). This suggests that osteoprotegerin might be suitable as a biomarker of calcification, but that local expression does not play a critical role in the calcification process.
Furthermore, the current study showed a strong co-localization of bone-matrix proteins, osteocalcin and osteopontin, within calcified areas, in agreement with previous data on upregulated expression of these markers (22, 33). The finding of bone-matrix proteins further underlines the role of active bone formation in the process of vascular calcification. In addition, the current study identified increased expression of IHH in calcified regions. To date, information about IHH-signalling and atherosclerosis is scarce, mainly describing inhibitory effects (34, 35) and a potential role in osteoarthritic calcification (36) as well as osteoblastic differentiation (37). Given the critical role of IHH in bone formation, this finding warrants further analysis of this signalling pathway in the context of calciphylaxis and vascular calcification.
In addition to our protein expression studies, the current study evaluated putative histological differences between patients with preserved and impaired kidney function, warfarin intake, and coexisting cardiovascular comorbidities in a translational approach. Survival was significantly better in patients with preserved renal function, and mortality risk among histological markers correlated significantly with the expression of BMP-7 (hazard-ratio 6.77, p = 0.03, 95% confidence interval). Subgroup analysis revealed a trend towards increased expression of BMP-7 in patients with severe CKD and haemodialysis. Although BMP-7 has so far been associated with protective properties against vascular calcification and renal fibrosis, there was a positive correlation between local BMP-7 expression and mortality in the current study. This suggests possible pathophysiological differences depending on renal function and phosphate handling, and raises the question regarding whether BMP-7 may be suitable as a biomarker. Otherwise, disease aetiology did not seem to induce different histological phenotypes regarding the distribution of osteogenic markers.
Study limitations
Histological slides were assessed semiquantitatively, possibly resulting in reduced accuracy and statistical power. To minimize this effect, the current study used a standardized assessment file and 2 independent experts blinded to the case/control status to verify the current results. A few slides had to be created from 2 different tissue blocks from the same patient, which might decrease comparability. The relatively small cohort of 18 patients limits the statistical power of the current data. Furthermore, the control group was not matched regarding clinical comorbidities.
Conclusion
In summary, this study demonstrates the presence of bone-morphogenic proteins, bone-matrix proteins and regulatory proteins of the matrix-Gla family within calcifications and the vascular wall of calciphylaxis lesions, underlining the pivotal role of extraosseous bone formation in the pathogenesis of subcutaneous calcifications. Using a translational approach, the study identified differences in renal function, phosphate handling and expression of osteogenic markers as influencers of clinical outcome. Mortality was highest in patients with impaired renal function, sequential renal comorbidities and high expression of BMP-7. The presence of BMP-7 in human subcutaneous biopsies of patients with calciphylaxis is a novel finding. Pathophysiological functions of BMP-7 and its potential as a biomarker in the context of vascular calcification and CKD should be addressed in future follow-up studies.
Skin biopsy in patients with clinically overt calciphylaxis captures advanced disease manifestations. This late-stage histomorphological picture did not seem to be influenced by kidney function, VKA intake, or coexisting peripheral artery disease. Therefore, disease evolution may have different pathophysiological drivers, but a common pathophysiological endpoint can be assumed in this late stage of the condition. Future studies including animal models are needed to better understand the temporal progression of histological changes in calciphylaxis and to identify markers for the individualization of treatment.
ACKNOWLEDGEMENTS
The support of Michael Antosch and Ulrich Dorn, orthopaedic surgeons, Paracelsus Medical University Salzburg, Austria, is gratefully acknowledged.
This project was funded by a research grant from the Paracelsus Medical University (Salzburg, Austria) to H. Salmhofer and C. Kronberger. Further financial support was provided by a research grant from Amgen Corporation (Vienna, Austria) and the Reiner-Brettenthaler-fellowship of the Salzburg Physicians Board (Salzburg, Austria), both granted to H. Salmhofer.
REFERENCES
- Colboc H, Moguelet P, Bazin D, Carvalho P, Dillies AS, Chaby G, et al. Localization, morphologic features, and chemical composition of calciphylaxis-related skin deposits in patients with calcific uremic arteriolopathy. JAMA Dermatol 2019; 155: 789–796.
- Ghosh T, Winchester DS, Davis MDP, El-Azhary R, Comfere NI. Early clinical presentations and progression of calciphylaxis. Int J Dermatol 2017; 56: 856–861.
- Floege J, Kubo Y, Floege A, Chertow GM, Parfrey PS. The effect of cinacalcet on calcific uremic arteriolopathy events in patients receiving hemodialysis: the EVOLVE Trial. Clin J Am Soc Nephrol 2015; 10: 800–807.
- Gaisne R, Péré M, Menoyo V, Hourmant M, Larmet-Burgeot D. Calciphylaxis epidemiology, risk factors, treatment and survival among French chronic kidney disease patients: a case-control study. BMC Nephrol 2020; 21: 63.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56: 569–579.
- Maroz N, Mohandes S, Field H, Kabakov Z, Simman R. Calciphylaxis in patients with preserved kidney function. J Am Coll Clin Wound Spec 2014; 6: 24–28.
- Galloway PA, El-Damanawi R, Bardsley V, Pritchard NR, Fry AC, Ojha SK, et al. Vitamin K antagonists predispose to calciphylaxis in patients with end-stage renal disease. Nephron 2015; 129: 197–201.
- Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y. A case-control study of calciphylaxis in japanese end-stage renal disease patients. Nephrol Dial Transplant 2012; 27: 1580–1584.
- Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, et al. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 2012; 23: 1744–1752.
- Jirak P, Stechemesser L, Moré E, Franzen M, Topf A, Mirna M, et al. Clinical implications of fetuin-A. Adv Clin Chem 2019; 89: 79–130.
- Kutikhin AG, Feenstra L, Kostyunin AE, Yuzhalin AE, Hillebrands J-L, Krenning G. Calciprotein particles: balancing mineral homeostasis and vascular pathology. Arterioscler Thromb Vasc Biol 2021; 41: 1607–1624.
- Bunting CH. The Formation of true bone with cellular (red) marrow in a sclerotic aorta. J Exp Med 1906; 8: 365–376.
- Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 2001; 89: 1147–1154.
- Engelse MA, Neele JM, Bronckers AL, Pannekoek H, de Vries CJ. Vascular calcification: expression patterns of the osteoblast-specific gene core binding factor alpha-1 and the protective factor matrix gla protein in human atherogenesis. Cardiovasc Res 2001; 52: 281–289.
- Griethe W, Schmitt R, Jurgensen JS, Bachmann S, Eckardt KU, Schindler R. Bone morphogenic protein-4 expression in vascular lesions of calciphylaxis. J Nephrol 2003; 16: 728–732.
- Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 2013; 93: 365–373.
- Nigwekar SU, Jiramongkolchai P, Wunderer F, Bloch E, Ichinose R, Nazarian RM, et al. Increased bone morphogenetic protein signaling in the cutaneous vasculature of patients with calciphylaxis. Am J Nephrol 2017; 46: 429–438.
- Franzen M, Moré E, Cadamuro J, Koller J, Salmhofer W, Wohlmuth-Wieser I, et al. Mineral depositions of calcifying skin disorders are predominantly composed of carbonate apatite. Acta Derm Venereol 2017; 97: 1178–1181.
- Paul S, Rabito CA, Vedak P, Nigwekar SU, Kroshinsky D. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol 2017; 153: 101–103.
- Rrapi R, Chand S, Gabel C, Ko L, Moore KJ, Steele D, et al. Early diagnosis and intervention of calciphylaxis leading to rapid resolution. JAAD Case Rep 2021; 13: 65–70.
- Jane B, Findenig B, Berr F, Dietze O, Hauser-Kronberger C, Salmhofer H. Calciphylaxis – calcified vsmcs show similarities with chondrocytes in the process of endochondral ossification. Wiener klinische Wochenschrift 2010; 122: A4–A4
- Kramann R, Brandenburg VM, Schurgers LJ, Ketteler M, Westphal S, Leisten I, et al. Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant 2013; 28: 856–868.
- Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res 2005; 97: 105–114.
- Davies M, Lund R, Hruska K. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Nephrol 2003; 14: 1559–1567.
- Liu L, Wang Y, Yan R, Liang L, Zhou X, Liu H, et al. BMP-7 inhibits renal fibrosis in diabetic nephropathy via miR-21 downregulation. Life Sci 2019; 238: 116957.
- Park CH, Yoo TH. TGF-β inhibitors for therapeutic management of kidney fibrosis. Pharmaceuticals (Basel) 2022; 15: 1485.
- Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol 2000; 184: 37–45.
- Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 2001; 21: 1610–1616.
- Makarović S, Makarović Z, Steiner R, Mihaljević I, Milas-Ahić J. Osteoprotegerin and vascular calcification: clinical and prognostic relevance. Coll Antropol 2015; 39: 461–468.
- Brandenburg V, Al-Fakhri N, Nemeth K, Goettsch C, Schurgers LJ, Vermeer C, et al. Calcification inhibitors in vascular calciphylaxis associated with normal renal Function. Thromb Haemost 2012; 108: 1241–1243.
- Moe SM, Reslerova M, Ketteler M, O’Neill K, Duan D, Koczman J, et al. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 2005; 67: 2295–2304.
- Wei FF, Trenson S, Thijs L, Huang QF, Zhang ZY, Yang WY, et al. Desphospho-uncarboxylated matrix gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol Dial Transplant 2018; 33: 1122–1128.
- Rashdan NA, Sim AM, Cui L, Phadwal K, Roberts FL, Carter R, et al. Osteocalcin regulates arterial calcification via altered Wnt signaling and glucose metabolism. J Bone Miner Res 2020; 35: 357–367.
- Dunaeva M, Waltenberger J. Hh signaling in regeneration of the ischemic heart. Cell Mol Life Sci 2017; 74: 3481–3490.
- Dashti M, Peppelenbosch MP, Rezaee F. Hedgehog signalling as an antagonist of ageing and its associated diseases. Bioessays 2012; 34: 849–856.
- Shuang F, Zhou Y, Hou SX, Zhu JL, Liu Y, Zhang CL, et al. Indian hedgehog signaling pathway members are associated with magnetic resonance imaging manifestations and pathological scores in lumbar facet joint osteoarthritis. Sci Rep 2015; 5: 10290.
- Deng A, Zhang H, Hu M, Liu S, Gao Q, Wang Y, et al. Knockdown of Indian hedgehog protein induces an inhibition of cell growth and differentiation in osteoblast MC3T3‑E1 cells. Mol Med Rep 2017; 16: 7987–7992.