ORIGINAL ARTICLE
Real-World Clinical Characteristics, Management, and Outcomes of 44 Paediatric Patients with Hypopigmented Mycosis Fungoides
Zhong-Hui HU, Lu LU, Jin-Di FENG, Hong-Bin SONG, Shi-Yu ZHANG, Lu YANG, Tao WANG and Yue-Hua LIU
Department of Dermatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, State Key Laboratory of Complex Severe and Rare Diseases, National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China
Hypopigmented mycosis fungoides is a rare form of mycosis fungoides that is characterized by achromic lesions, early onset of disease, a predilection for darker skinned populations, and a predominance of CD8+ T cells. Due to the rarity and heterogeneous presentation of hypopigmented mycosis fungoides, there are no criteria that clearly define the clinical characteristics and treatment regimens for this condition. This retrospective study of 44 paediatric patients with hypopigmented mycosis fungoides aimed to summarize their epidemiological and clinical characteristics and assess the effectiveness and safety of different treatment regimens. Clinical manifestations were further classified into 3 morphological groups: hypopigmented lesions, papules overlying hypopigmented lesions, and erythematous plaques overlying hypopigmented lesions. In addition, the results of this study suggest that interferon alpha might be an effective and well-tolerated therapy that could shorten the treatment time to complete response compared with other treatments. Maintenance therapy and long-term follow-up reduced the recurrence rate.
Key words: mycosis fungoides; hypopigmentation; cutaneous T-cell lymphomas; interferon.
SIGNIFICANCE
Hypopigmented mycosis fungoides is an uncommon clinical variant of mycosis fungoides with heterogeneous presentations. The clinical characteristics and treatments of this rare condition have not been clarified. This study retrospectively analysed the clinical characteristics, management, and outcomes of 44 paediatric patients with hypopigmented mycosis fungoides. The clinical manifestations were categorized into 3 morphological groups: hypopigmented lesions, papules overlying hypopigmented lesions, and erythematous plaques overlying hypopigmented lesions. Furthermore, the results of this study suggest that interferon alpha might be an effective treatment that shortens the treatment time to complete response. This study provides additional information about this malignant neoplasm.
Citation: Acta Derm Venereol 2023; 103: adv6226. DOI: https://doi.org/10.2340/actadv.v103.6226.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jun 19, 2023; Published: Aug 22, 2023
Corr: Tao Wang, Yue-Hua Liu, Department of Dermatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, State Key Laboratory of Complex Severe and Rare Diseases, National Clinical Research Center for Dermatologic and Immunologic Diseases, No. 1 Shuaifuyuan, Dongcheng District, Beijing 100730, China. E-mail: wangtaopumch@126.com, yuehualiu63@163.com
The authors have no conflicts of interest to declare.
INTRODUCTION
Mycosis fungoides (MF) is the most common primary cutaneous T cell lymphoma (1). Hypopigmented MF (hMF) is a rare clinical variant of MF that is most prevalent in young individuals and populations with darker skin types (2). Immunohistochemical (IHC) studies have demonstrated melanocyte damage and abnormal melanogenesis and suggested that the appearance of hypopigmented lesions might be attributable to the cytotoxic pathway of melanocyte destruction by CD8+ T cells (2, 3). Because of the rarity and heterogeneous presentation of this condition, there are currently no criteria that clearly define the characteristics of typical hMF. Compared with patients with classical MF, patients with hMF often have a better prognosis (4–7).
This study describes the epidemiological and clinical profiles of patients with hMF onset in childhood at cutaneous lymphoma centre (Beijing, China), and classified the lesions into 3 morphological subgroups. The study also assessed the effectiveness and safety of different treatment regimens.
MATERIALS AND METHODS
This retrospective study analysed the data for all patients with MF onset during childhood from 1 January 2008 to 31 May 2022 at cutaneous lymphoma centre (mainly serves Asian population from China) and collected informed consent for study participation. The study was approved by the institutional ethics committee (The Institutional Review Board of Peking Union Medical College Hospital) (approval number ZS-3454D). The diagnosis of MF, including tumour-node-metastasis-blood (TNMB) classification and response criteria, was based on the guidelines of the World Health Organization and the European Organization for Research and Treatment of Cancer (8).
The inclusion criteria were: (i) persistent and/or progressive patches, papules, and/or plaques of varying sizes that were exclusively or predominantly hypopigmented (9); (ii) conclusive diagnosis of hMF confirmed by pathological examination; (iii) age less than 18 years at onset of disease. Patients who fulfilled at least 1 of the following criteria were excluded from the analysis: (i) incomplete clinical data; (ii) concomitant presence of another type of lymphoma; (iii) non-standard treatment process.
The following data were extracted from the medical records: sex, age at onset, latency period, lesion imaging findings and characteristics, TNMB classification at the time of diagnosis, histopathological and IHC findings, treatment regimen, response to treatment, and follow-up data. Clinical response to therapy was defined as: complete response (CR, 100% clearance of skin lesions), partial response (PR, 50–99% clearance of skin disease compared with baseline), and stable disease (< 25% increase to <50% clearance in skin disease compared with baseline) (10). Recurrence was defined as any disease relapse in a patient with CR.
In addition to the routine IHC required for the diagnosis of hMF, including CD4 and CD8, we also carried out programmed death 1 (PD1) (clone MX033; MXB Biotechnologies, Fuzhou, China), programmed cell death-ligand 1 (PD-L1) (clone E1L3N; MEDx, Suzhou, China) staining, and T-cell receptor (TCR) rearrangement. IHC was performed automatically using the Lecia Bond Max fully automated IHC stainer. The expression of PD1 and PD-L1 within epidermotropic and dermal lymphoid infiltrates was scored as –, negative (<3%); +, rare-scattered (3–10%); or ++, numerous (10–30%). Genomic DNA was extracted using the TIANamp Micro DNA Kit (Tiangen Biotech, Beijing, China) in accordance with the manufacturer’s protocol. Fragment analysis was performed using the IdentiClone TCR Gene Clonality Assay (BIOMED-2, InVivoScribe Technologies, San Diego, CA, USA). All results were independently determined by at least 2 experts.
Statistical analysis
Abnormally distributed continuous variables are expressed as median (interquartile range), and categorical variables are expressed as number (percentage). Non-parametric data with multiple comparisons were analysed by Kruskal–Wallis one-way analysis of variance followed by Holm’s Stepdown Bonferroni procedure for adjusted p-values. Fisher’s exact test was used for the comparison of the proportional composition between 2 categorical variables. Kaplan-Meier survival analysis was used to evaluate the association between treatment regimen and remission rate. Data were coded and analysed with IBM SPSS version 27.0 (IBM Corp., Armonk, NY, USA). p-values <0.05 were considered statistically significant.
RESULTS
Demographics
The cohort comprised 44 patients with hMF with Fitzpatrick phototypes III or IV. Table I summarizes the demographic data, lesion features, TNMB classification, and histopathology findings of the patients with hMF included in the study.
| Characteristic | Values |
| Sex, n (%) | |
| Male | 33 (75.0) |
| Female | 11 (25.0) |
| M:F ratio | 3.0 |
| Age at onset, years, median (IQR) | 8.5 (6, 10.4) |
| Range | 2–17 |
| Age at diagnosis, years, median (IQR) | 11 (9, 14) |
| Range | 4–24 |
| Latency period, months, median (IQR) | 24 (12, 60) |
| Range | 2–192 |
| Morphological group, n (%) | |
| Group Ia | 14 (32.0) |
| Group IIb | 19 (43.0) |
| Group IIIc | 11 (25.0) |
| Desquamation, n (%) | |
| Yes | 17 (39.0) |
| No | 27 (61.0) |
| Atrophy, n (%) | |
| Yes | 2 (5.0) |
| No, n (%) | 42 (95.0) |
| Location | |
| Non-photoexposed | 44 (100.0) |
| Photoexposed | –d |
| TNMB classification, n (%) | |
| T1aN0M0B0-IA | 2 (4.5) |
| T1bN0M0B0-IA | 1 (2.3) |
| T2aN0M0B0-IB | 30 (68.2) |
| T2bN0M0B0-IB | 11 (25.0) |
| Histopathology, n (%) | |
| Epidermotropism | 29 (65.9) |
| Pautrier’s microabscess | 15 (34.1) |
| CD4/CD8 (n = 26), n (%) | |
| Mixed infiltration | 14 (53.8) |
| CD8 pre | 10 (38.5) |
| CD4 pre | 2 (7.7) |
| aGroup I: Localized or generalized hypopigmented macules, patches, or plaques. bGroup II: Papules overlying hypopigmented lesions. cGroup III: Erythematous plaques overlying hypopigmented lesions. dTen patients (22.7%) had lesions in photoexposed areas such as the face or neck, although the lesions were predominantly located on photoprotected areas. pre: predominance; IQR: interquartile range; M: male; F: female. |
|
Lesion characteristics
Based on the lesion features and course of disease, the hMF skin lesion was classified into 3 morphological subgroups.
- Group I: localized or generalized hypopigmented macules, patches, or plaques (Fig. 1a–c).
- Group II: papules overlying hypopigmented lesions (Fig. 1d–f).
- Group III: erythematous plaques overlying hypopigmented lesions (Fig. 1g–i).
In our definition, the papules or erythematous plaques must coexist with the hypopigmented lesions, and hypopigmented lesions must be the primary manifestation of the patient’s lesions. The 3 morphological groups are not necessarily continuous.
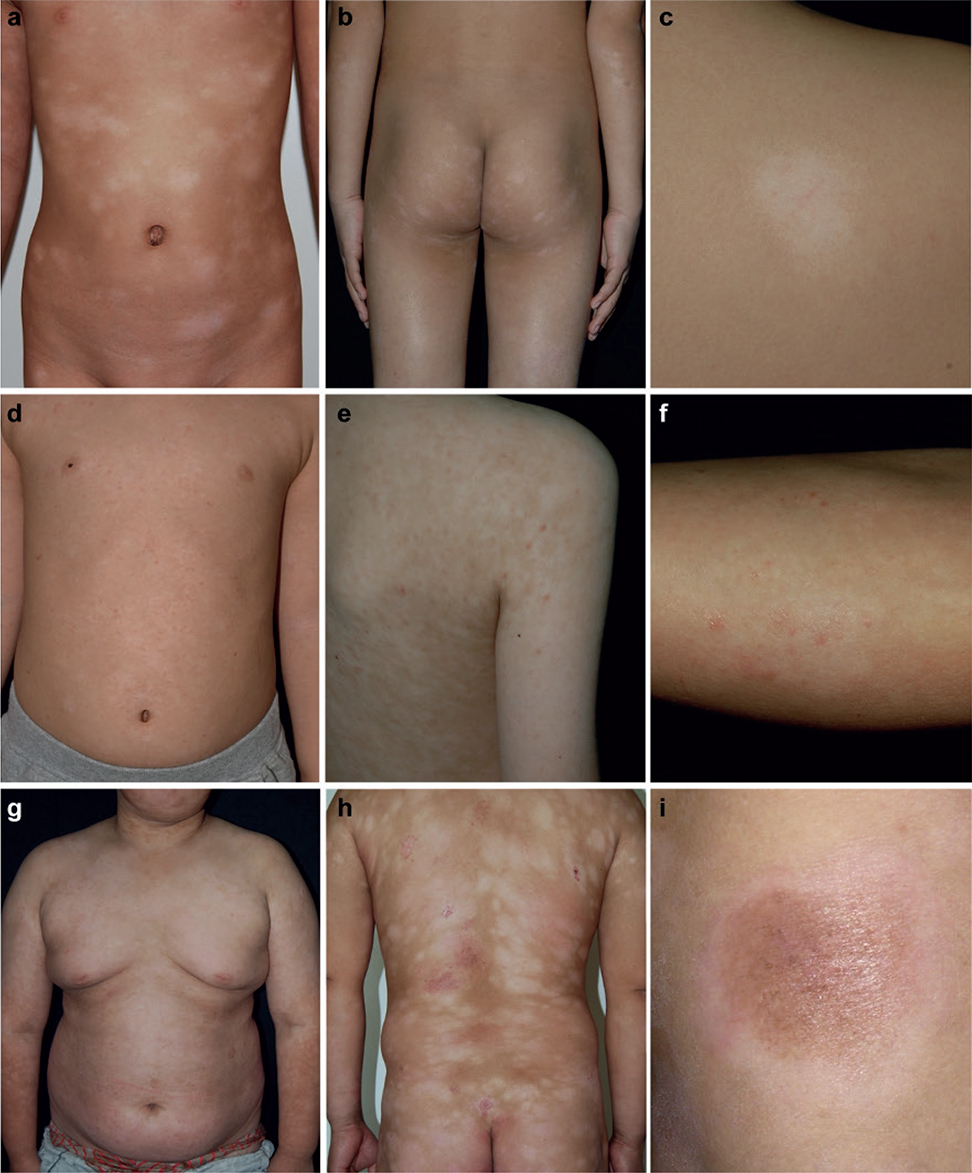
Fig. 1. Clinical spectrum of hypopigmented mycosis fungoides in a range of typical patients from Chinese cohort in our study. (a–c) Group I: Localized or generalized hypopigmented macules, patches, or plaques; (d–f) Group II: Papules overlying hypopigmented lesions; (g, h, i) Group III: Erythematous plaques overlying hypopigmented lesions.
Kruskal–Wallis tests showed no significant difference between the 3 morphological groups in the age at onset (H=1.094, p = 0.579) (Fig. 2a). However, pairwise comparisons showed that patients with Group III lesions had a longer latency period and older age at diagnosis than patients with Group I lesions (adjusted p = 0.038 for latency, adjusted p = 0.016 for age) and Group II lesions (adjusted p = 0.003 for latency, adjusted p = 0.032 for age) (Fig. 2).
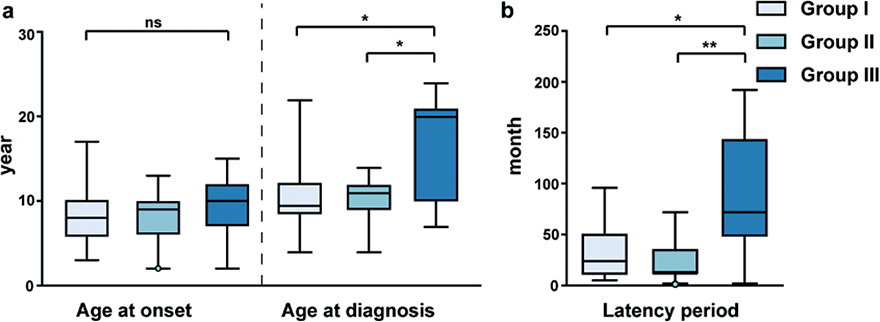
Fig. 2. Box plots of raw data for 3 morphological groups in 44 patients at the age at onset, age at diagnosis, and latency period. (a) Kruskal–Wallis tests showed no statistically significant difference between Group I–III in the age at onset (H=1.094, p = 0.579). Pairwise comparisons of age at diagnosis revealed a statistically significant difference between Group I and Group III (adjusted p = 0.016), and between Group II and Group III (adjusted p = 0.032). (b) Pairwise comparisons of latency period indicated a statistically significant difference between Group I and Group III (adjusted p = 0.038), and between Group II and Group III (adjusted p = 0.003).
Desquamation was present in 17 patients (39%) with cutaneous lesions, with most having fine scales. Some patients had unusual clinical presentations. Patient 26 and patient 43 both had Group I lesion morphology and developed atrophic lesions based on their original hypopigmented patches. Patient 26 also had irregular purpuric lesions on both ankles. The distribution of lesions among all 44 patients was predominantly at non-photoexposed sites, including the trunk, buttocks, and extremities, particularly the proximal extremities. Ten patients (22.7%) had lesions in photoexposed areas such as the face or neck, although the lesions were predominantly located on photoprotected areas.
Histopathology, immunohistochemistry and T-cell receptor rearrangement
Epidermotropism was observed in 29 patients (65.9%), and Pautrier’s microabscess was observed in 15 patients (34.1%). It was observed that the lymphocytes infiltration increased in density and depth from Group I to Group III (Fig. S1). IHC examinations showed a predominance of CD8-positive lymphocytes in 10 patients (38.5%), predominance of CD4 positive lymphocytes in 2 patients (7.7%), and mixed infiltration of CD8- and CD4-positive lymphocytes in 14 patients (53.8%) (n = 26, Table I).
Formalin-fixed, paraffin-embedded skin-punch biopsy specimens from 36 patients were chosen to undergo PD1/PD-L1 staining and TCR rearrangement detection. PD1 expression (+/++) was observed in 6 patients (16.7%), while PD-L1 expression (+/++) was observed in 24 patients (66.7%) (n = 36, Fig. S2, Tables SI, SII). There were no significant differences between the 3 morphological groups in the expressions of PD1 (p = 0.613) and PD-L1 (p = 0.855) (Table SI).
The TCR gene rearrangement results were clonal or positive in 3 patients (9.4%), and polyclonal or negative in 29 patients (90.6%) (n = 32, Table SII). Among the 3 patients with TCR gene rearrangement, monoclonal rearrangements were found in 1 patient for TCRβ, 1 patient for TCRγ, and 2 patients for TCRδ. One patient (3.1%) had 2 or more TCR rearrangements occurring in a single specimen (Table SII).
Tumour-node-metastasis-blood classification
All 44 patients were classified as T1a (4.5%), T1b (2.3%), T2a (68.2%) or T2b (25%) using the TNMB classification criteria, with no internal involvement or metastases (Table I).
Treatment and follow-up
Twenty-four of 44 patients received continuous treatment in our centre. Data on response to initial treatment were available for these 24 patients. The remaining 20 patients were not treated at our institution because they returned to local hospital with the diagnosis of hMF or were lost to follow-up. The main treatment regimens and follow-up data are summarized in Table II.
Among the 24 patients treated in our centre, 22 (91.7%) achieved CR, while 2 (8.3%) achieved PR. Both patients with PR received narrowband ultraviolet B (NBUVB). One of these patients subsequently received a combination of ultraviolet A (UVA) and the other was subsequently treated with UVA and interferon (IFN) alpha. These 2 patients are still being followed up. There was no significant difference between the 4 treatment regimens in the rate of CR (p = 0.645). However, Kaplan–Meier survival analysis found that the median time to CR was shorter in the group that received NBUVB or NBUVB + UVA treatment combined with IFN (5 months, 95 CI% 2.1–7.9 months) than in the group that received NBUVB or NVUVB + UVA treatment without IFN (7 months, 95 CI% 5.6–8.4 months). Furthermore, IFN therapy achieved a superior remission rate over time compared with other treatments (Breslow p = 0.037) (Fig. 3).
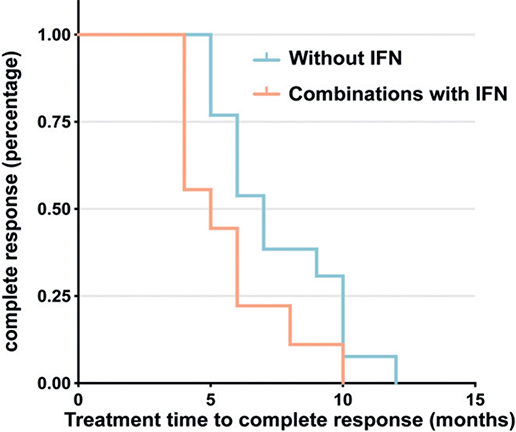
Fig. 3. Kaplan-Meier analysis demonstrated a superior remission rate over time in the group that received narrowband ultraviolet B (NBUVB) or NBUVB + ultraviolet A (UVA) treatment in combination with interferon (IFN) compared with the group that received NBUVB or NBUVB + UVA treatment without IFN (Breslow p = 0.037). The median time to complete response was 5 months (95% confidence interval (95% CI) 2.1–7.9 months) in the group that received NBUVB or NBUVB + UVA treatment plus IFN and 7 months (95% CI 5.6–8.4 months) in the group that received NBUVB or NBUVB + UVA treatment without IFN.
Two patients experienced relapse, giving a global frequency relapse rate of 9.1%. The relapse-free survival times for the 2 patients were 4 months and 24 months. Both patients were able to achieve CR again using the same treatment regimen as the initial regimen.
A total of 24 patients were followed up for a median follow-up of 35 months (range 12–146 months) and had an overall survival of 100%. No patients developed progression or died as a result of hMF or treatment. Among the 20 patients who were not treated in our centre, 13 (65%) achieved CR, 1 (5%) achieved PR, 4 patients (20%) were untreated and achieved SD, and 2 patients (10%) were lost to follow-up.
DISCUSSION
hMF is an unusual subtype of MF that is typically more prevalent in younger individuals with darker skin types and has a better prognosis than other types of MF (2, 11, 12). hMF is histopathologically similar to classical MF, but IHC reveals a predominance of CD8+ T cells. There have been some cohort studies of hMF in recent years (Table III) (4, 5, 9, 13–20). The current study retrospectively summarized the clinical characteristics and treatment regimens of 44 patients diagnosed with hMF in childhood at our centre.
| Study and country | Patients, males/females n | Age at diagnosis, years | Latency period, months | Lesion characteristics | TNMB classification | CD8+ pre % | TCR status | Treatment | Treatment response (n/rate) | Progression to≥IIB | Recurrence rate |
| Current series | 44, 33/11 | 11 (median) | 24 (median) | Hypopigmented only: 14 Papules: 19 Erythematous plaques: 11 |
IA: 3 IB: 41 |
38.5% | 3/32 | NBUVB, UVA, IFN, TCS, TCI, Nitrogen mustard | CR: 22 PR: 2 |
None | 9.1% |
| Shi et al. (13), 2022, China | 32, 21/11 | 16.5 (median) | NS | hypopigmented only | NS | 63.33% | TCS, Nitrogen mustard | Excellent: 30 Not promising: 2 |
NS | NS | |
| Domínguez-Gómez et al. (14), 2021, Mexico | 48, 29/19 | 27.3 (mean) | NS | Hypopigmented only: 39 Erythematous plaques: 9 |
NS | NS | PUVA, NBUVB, TCS, TCI, Prednisone Topical/systemic retinoids |
CR: 47% | NS | 34.78% | |
| *Chen et al. (15), 2021, China | 9, 8/1 | 10.5 (median) | 24 (median) | Hypopigmented only: 4 Scaly erythemas: 5 |
IA: 1 IB: 8 |
66.7% | 2/5 β; β+γ |
NBUVB, TCS | CR: 1 PR: 6 |
None | None |
| Amorim et al. (4), 2018, Brazil | 20, 10/10 | 47.5 (median) | 36 (median) | Hypochromic only | IA: 10 IB: 10 |
NS | PUVA, NBUVB, Radiotherapy Nitrogen mustard, TCS |
CR: 10 PR: 8 NR: 2 |
IIB: 1(death after 4 years) IIIA: 1 |
NS | |
| Rodney et al. (9), 2017, USA | 20, 10/10 | 37.5 (mean) | 63.6 (mean) | Hypopigmented only: 18 Depigmented: 1 Hyperpigmented: 1 |
IA: 8 IB: 12 |
58.3% | PUVA, NBUVB Nitrogen mustard, TCS |
CR: 8 AWD: 6 |
None | 75% | |
| Furlan et al. (16), 2014, Brazil | 20, 6/14 | 32.5 (median) | 102 (median) | Hypopigmented only | IA: 8; IB: 11; IIA: 1 | 80% | NS | NS | NS | NS | |
| Hassab-El-Naby et al. (17), 2013, Egypt | 27, 18/9 | 38.65 (mean) | 39.12 (mean) | Hypopigmented only: 23 Erythematous or pigmented: 4 |
IA: 21 IB: 6 |
51.9% | PUVA, NBUVB, Radiotherapy TCS, TCI |
CR: 13 NR: 1 |
NS | 23.1% | |
| *Castano et al. (5), 2013, USA | 35, 19/16 | 12.6 (mean) | NS | Hypopigmented only: 30 Hyperpigmented: 2 Erythematous: 3 |
NS | 66.7% | 16/35 | PUVA, NBUVB, TCS | CR or PR: 45.7% | Plaque/tumor stage:1 | NS |
| Wongpraparut et al. (18), 2012 Thailand | 9, 3/6 | 42.2 (mean) | 2–120 | Hypopigmented only: 8 Erythematous: 1 |
IA: 8 IB: 1 |
NS | PUVA, NBUVB | CR: 6 PR: 3 |
NS | 66.7% | |
| Kanokrungsee et al. (19), 2012, Thailand | 11, 7/4 | 28 (median, age at onset) | 12 (median) | Hypopigmented only | IA: 5 IB: 6 |
NS | NBUVB | CR: 7 PR: 4 |
None | 50% | |
| Khopkar et al. (20), 2011, India | 15, 10/5 | 32.2 (mean) | 45.96 (mean) | Hypopigmented only: 10 Poikiloderma: 4 Erythematous patches: 1 |
NS | 80% | NS | NS | NS | NS | |
| pre: predominance; NS: not specified; NBUVB: narrowband ultraviolet B; UVA: ultraviolet A; PUVA: psoralen plus ultraviolet A; IFN: interferon; TCS: topical corticosteroids; TCI: topical calcineurin inhibitors; CR: complete response; PR: partial response; NR: no response; AWD: alive with disease; TCR: T-cell receptor. *Studies of childhood/juvenile-onset hypopigmented mycosis fungoides. | |||||||||||
Consistent with some previous studies (14, 17), the male to female ratio of the 44 included patients was 3:1, showing an obvious male predilection. The current study confirmed that hMF affected mainly younger patients, with a median age at onset of 8.5 years and a median age at definitive diagnosis of 11 years. This was also corroborated by previous groups (5, 13–15, 19). Different groups have reported different intervals between disease onset and diagnosis (4, 9, 15–17, 19). In the current study, the interval from disease onset to diagnosis ranged from 2 months to 16 years, with a median of 24 months. In comparison with previous reports (4, 9, 13, 14, 16–20), patients treated for hMF at our cutaneous lymphoma centre were younger and had improved diagnosis and treatment outcomes.
As there are no standardized criteria for defining hMF, the lesion characteristics vary among patients involved in different studies (Table III). Based on our clinical data, we categorized hMF lesions into Group I–III morphologically (Fig. 1). We also observed that fine scales were common secondary lesions, and observed some unusual secondary presentations, such as atrophy and irregular purpura. Further analysis revealed that patients with Group III lesions were older at the time of diagnosis and had a longer latency period than those with Group I and Group II lesions, although there was no difference between Group I–III in the age of onset (Fig. 2). As erythematous lesions usually have a deeper and denser infiltration of atypical lymphoid cells than hypopigmented lesions (15), we postulated that the latency period was correlated with the severity of hMF; that is, the longer the latency period, the more likely the patient was to show more progressive clinical manifestations of Group III lesions. The diversity in the manifestations of hMF has been reported in many studies (4, 13, 16, 19), but no study has performed an in-depth analysis of lesion morphology.
Regarding the distribution of hMF lesions, the primary lesions of all patients were found in non-exposed areas, which was similar to other types of MF. The face or neck was involved in 10 patients (22.7%) in our paediatric cohort, even though these areas were generally not affected. The proportion of patients with lesions on the face and neck was higher in the younger age group (age 0–18 years) than in other age groups with hMF (5, 9, 15). This suggests that children may be more likely to develop hMF lesions on exposed areas such as the face and neck than adults. However, this hypothesis needs to be verified in further studies.
The PD1/PD-L1 pathway is an immune checkpoint pathway that plays a key role in regulating excessive immune responses (21). No studies have evaluated the PD1/PD-L1 expression levels specifically in patients with hMF. In some studies focusing on MF or Sézary syndrome, high expression of PD1 and/or PD-L1 is associated with advanced disease and lower overall survival (22, 23). In the current cohort, the differences in the PD1/PD-L1 expression level between Group I–III and treatment outcome groups did not reach statistical significance.
The assessment of TCR gene rearrangement has become an important adjuvant testing to assist in the diagnosis of hMF (24, 25), and associated with prognostic factors in MF (25–27). In the current cohort, most cases did not show TCR gene rearrangement and the positive rate was lower than classical MF and Sézary syndrome, which may be due to the fact that all our patients were early MF (3 were stage IA and 41 were stage IB) (28). There were no significant differences in main clinical characteristics or treatment outcomes between TCR gene rearrangement positive and negative patients (Table SIII). However, the positive (+/++) rate of PD1/PD-L1 staining and TCR gene rearrangement tended to be higher in Group III than in Group I&II, although the differences were not statistically significant (Table SI).
The treatment of hMF adhered to the treatment guidelines for early MF (29, 30). As shown in Table II, phototherapy was the most common treatment regimen. UVA combined with NBUVB was used to treat deeply infiltrated patches. In general, our treatment regimens yielded encouraging results, with 22 (91.7%) patients achieving CR and 2 (8.3%) patients achieving PR.
For patients with widespread hMF lesions at the time of diagnosis, rapid recent progression, or a strong desire to shorten the remission time, we suggested the addition of IFN to the initial treatment regimen (31–33). In the current study, IFN alpha (Intefen®, 3SBio Inc., Shenyang, China) was administered intramuscularly 3 times a week at a low dose of approximately 1–3 million units each time. The common adverse reactions associated with IFN alpha were flu-like symptoms, such as fever, fatigue, headache and arthralgia; these symptoms disappeared after 1–3 treatments in most patients. The duration of treatment depended on the degree of remission of the skin lesions, and was usually between 3 and 6 months. Although the number of patients in the current study was small, the time taken to reach CR was significantly shorter for the group that received NBUVB or NBUVB + UVA treatment in combination with IFN than the group that received NBUVB or NBUVB + UVA treatment without IFN (median treatment time 5 months vs 7 months; Breslow p = 0.037, Fig. 3). Furthermore, dark-skinned individuals reportedly have a poor response to NBUVB, possibly owing to a photoprotective effect of melanin (34). IFN may be a viable option for such patients.
Patients with hMF required persistent treatment and long-term follow-up. In the current cohort, no patients experienced deterioration or died from the disease or related treatments, indicating that hMF in childhood has an excellent prognosis. The recurrence rate in our cohort was 9.1%, which was lower than that reported in previous studies (range 20–89%) (Table III) (9, 14, 17–19, 35). This might be because even if the lesions were completely cleared, we still recommended the continuation of treatment, usually NBUVB, for another 1–2 years with a gradual reduction in the frequency of treatment sessions. Although hMF has a good prognosis, there is a risk of disease progression or even death in some instances, especially for patients who fail to continue treatment or who remain untreated for a long time (4–7). Therefore, it is imperative to always treat hMF as a malignant neoplasm with lethal potential and frequent relapses.
The limitations of this study were the retrospective design, small sample size, and effects of small amount of punch biopsy tissue and the long preservation time regarding the results of additional IHC and TCR gene rearrangement detection.
In conclusion, this study describes a cohort of paediatric patients with hMF who had clinical and pathological characteristics that were similar, but also differed from, the characteristics reported in previous studies. The hMF skin lesions were classified into 3 morphological subgroups and the results indicated that patients with a longer latency period were more likely to manifest Group III lesions, defined as more progressive than Group I&II. Finally, the results showed that IFN alpha was an effective and well-tolerated therapy for hMF in select patients, and that the addition of IFN shortened the time to CR. These results show that hMF in childhood has an indolent course and a good prognosis. The results also indicate that maintenance therapy and long-term follow-up could reduce the recurrence rate.
ACKNOWLEDGEMENTS
This work was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-B-092) and Beijing Municipal Natural Science Foundation (Z210017).
The study was approved by the institutional ethics committee (approval number ZS-3454D).
REFERENCES
- Dummer R, Vermeer MH, Scarisbrick JJ, Kim YH, Stonesifer C, Tensen CP, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers 2021 7: 61.
- Martínez Villarreal A, Gantchev J, Lagacé F, Barolet A, Sasseville D, Ødum N, et al. Hypopigmented mycosis fungoides: loss of pigmentation reflects antitumor immune response in young patients. Cancers (Basel) 2020; 12.
- Furlan FC, de Paula Pereira BA, da Silva LF, Sanches JA. Loss of melanocytes in hypopigmented mycosis fungoides: a study of 18 patients. J Cutan Pathol 2014; 41: 101–107.
- Amorim GM, Niemeyer-Corbellini JP, Quintella DC, Cuzzi T, Ramos ESM. Hypopigmented mycosis fungoides: a 20-case retrospective series. Int J Dermatol 2018; 57: 306–312.
- Castano E, Glick S, Wolgast L, Naeem R, Sunkara J, Elston D, et al. Hypopigmented mycosis fungoides in childhood and adolescence: a long-term retrospective study. J Cutan Pathol 2013; 40: 924–934.
- Stone ML, Styles AR, Cockerell CJ, Pandya AG. Hypopigmented mycosis fungoides: a report of 7 cases and review of the literature. Cutis 2001; 67: 133–138.
- Ardigó M, Borroni G, Muscardin L, Kerl H, Cerroni L. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol 2003; 49: 264–270.
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019; 133: 1703–1714.
- Rodney IJ, Kindred C, Angra K, Qutub ON, Villanueva AR, Halder RM. Hypopigmented mycosis fungoides: a retrospective clinicohistopathologic study. J Eur Acad Dermatol Venereol 2017; 31: 808–814.
- Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol 2011; 29: 2598–2607.
- Reiter O, Amitay-Laish I, Oren-Shabtai M, Feinmesser M, Ben-Amitai D, Hodak E. Paediatric mycosis fungoides – characteristics, management and outcomes with particular focus on the folliculotropic variant. J Eur Acad Dermatol Venereol 2022; 36: 671–679.
- Hodak E, Amitay-Laish I, Feinmesser M, Davidovici B, David M, Zvulunov A, et al. Juvenile mycosis fungoides: cutaneous T-cell lymphoma with frequent follicular involvement. J Am Acad Dermatol 2014; 70: 993–1001.
- Shi HZ, Jiang YQ, Xu XL, Zhang W, Song H, Wang XP, et al. Hypopigmented mycosis fungoides: a clinicopathological review of 32 patients. Clin Cosmet Investig Dermatol 2022; 15: 1259–1264.
- Domínguez-Gómez MA, Baldassarri-Ortego LF, Morales-Sánchez MA. Hypopigmented mycosis fungoides: a 48-case retrospective series. Australas J Dermatol 2021; 62: e419–e420.
- Chen Y, Xu J, Qiu L, Fu L, Liang Y, Wei L, et al. Hypopigmented mycosis fungoides: a clinical and histopathology analysis in 9 children. Am J Dermatopathol 2021; 43: 259–265.
- Furlan FC, Pereira BA, Sotto MN, Sanches JA. Hypopigmented mycosis fungoides versus mycosis fungoides with concomitant hypopigmented lesions: same disease or different variants of mycosis fungoides? Dermatology 2014; 229: 271–274.
- Hassab-El-Naby HM, El-Khalawany MA. Hypopigmented mycosis fungoides in Egyptian patients. J Cutan Pathol 2013; 40: 397–404.
- Wongpraparut C, Setabutra P. Phototherapy for hypopigmented mycosis fungoides in Asians. Photodermatol Photoimmunol Photomed 2012; 28: 181–186.
- Kanokrungsee S, Rajatanavin N, Rutnin S, Vachiramon V. Efficacy of narrowband ultraviolet B twice weekly for hypopigmented mycosis fungoides in Asians. Clin Exp Dermatol 2012; 37: 149–152.
- Khopkar U, Doshi BR, Dongre AM, Gujral S. A study of clinicopathologic profile of 15 cases of hypopigmented mycosis fungoides. Indian J Dermatol Venereol Leprol 2011; 77: 167–173.
- Xie M, Huang X, Ye X, Qian W. Prognostic and clinicopathological significance of PD-1/PD-L1 expression in the tumor microenvironment and neoplastic cells for lymphoma. Int Immunopharmacol 2019; 77: 105999.
- Di Raimondo C, Rubio-Gonzalez B, Palmer J, Weisenburger DD, Zain J, Wu X, et al. Expression of immune checkpoint molecules programmed death protein 1, programmed death-ligand 1 and inducible T-cell co-stimulator in mycosis fungoides and Sézary syndrome: association with disease stage and clinical outcome. Br J Dermatol 2022; 187: 234–243.
- Nguyen GH, Olson LC, Magro CM. Upregulation of inhibitory signaling receptor programmed death marker-1 (PD-1) in disease evolution from cutaneous lymphoid dyscrasias to mycosis fungoides and Sezary’s syndrome. Ann Diagn Pathol 2017; 28: 54–59.
- Allen PB, McCook-Veal AA, Switchenko JM, Paulino DM, Niyogusaba T, Baird KM, et al. Staging lymph nodes and blood at diagnosis in mycosis fungoides identifies patients at increased risk of progression to advanced stage: a retrospective cohort study. Cancer 2023; 129: 541–550.
- Zimmermann C, Boisson M, Ram-Wolff C, Sadoux A, Louveau B, Vignon-Pennamen MD, et al. Diagnostic performance of high-throughput sequencing of the T-cell receptor beta gene for the diagnosis of cutaneous T-cell lymphoma. Br J Dermatol 2021; 185: 679–680.
- Marks E, Wang Y, Shi Y, Susa J, Jacobson M, Goldstein DY. Specific TCR gene rearrangements in mycosis fungoides: does advanced clinical stage show a preference? J Clin Pathol 2018; 71: 1072–1077.
- Calvani J, de Masson A, de Margerie-Mellon C, de Kerviler É, Ram-Wolff C, Gruber A, et al. Image-guided lymph node core-needle biopsy predicts survival in mycosis fungoides and Sézary syndrome. Br J Dermatol 2021; 185: 419–427.
- Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol 2014; 70: 205.e201–216; quiz 221–202.
- Dummer R, Vermeer MH, Scarisbrick JJ, Kim YH, Stonesifer C, Tensen CP, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers 2021; 7: 61.
- Olsen EA, Whittaker S, Willemze R, Pinter-Brown L, Foss F, Geskin L, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood 2022; 140: 419–437.
- Spaccarelli N, Rook AH. The use of interferons in the treatment of cutaneous T-cell lymphoma. Dermatol Clin 2015; 33: 731–745.
- Olsen EA. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther 2003; 16: 311–321.
- Stadler R, Otte HG, Luger T, Henz BM, Kühl P, Zwingers T, et al. Prospective randomized multicenter clinical trial on the use of interferon-2a plus acitretin versus interferon -2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood 1998; 92: 3578–3581.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol 2013; 88: 954–960.
- Akaraphanth R, Douglass MC, Lim HW. Hypopigmented mycosis fungoides: treatment and a 6(1/2)-year follow-up of 9 patients. J Am Acad Dermatol 2000; 42: 33–39.