QUIZ SECTION
Erythematous Plaque with Crusting on the Lower Leg: A Quiz
Kei HAYASHI1, Shin IINUMA1,2* and Akemi ISHIDA-YAMAMOTO2
1Department of Dermatology, Japanese Red Cross Kitami Hospital, North 6, East 2, Kitami, 090-8666, Japan, and 2Department of Dermatology, Asahikawa Medical University, Asahikawa, Japan. E-mail: iinuma@asahikawa-med.ac.jp
Citation: Acta Derm Venereol 2023; 103: adv00877. DOI: https://doi.org/10.2340/actadv.v103.6236.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Published: Feb 28, 2023
INTRODUCTION
A 92-year-old woman presented with a 3-year history of a growing plaque on her left lower leg. Physical examination revealed a well-circumscribed, irregularly shaped, erythematous, and brownish plaque with crusting, measuring 5×4 cm, on her left lower leg (Fig. 1). The lesion was completely excised. Histopathological examination showed atypical keratinocytes with multinucleation and focal dyskeratosis throughout the thickened epidermis (Fig. 2A). In the adjacent areas, thin anastomosed strands of cuboidal cells protruded from the epidermis to the upper dermis (Fig. 2B). These strands of cells were arranged in a lattice pattern and were embedded in the fibrovascular stroma. Small ductal structures were observed within the interconnected strands of cells without nuclear atypia.
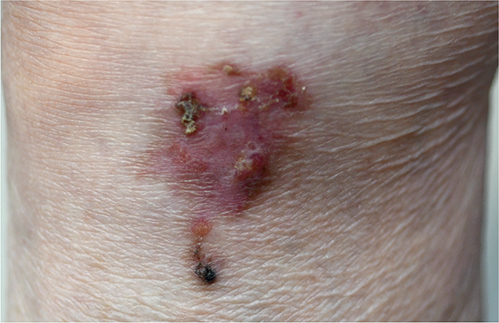
Fig. 1. Clinical presentation. A skin lesion on the left lower leg: well-circumscribed, 5×4 cm irregular shape, erythematous brownish plaque with crusts.
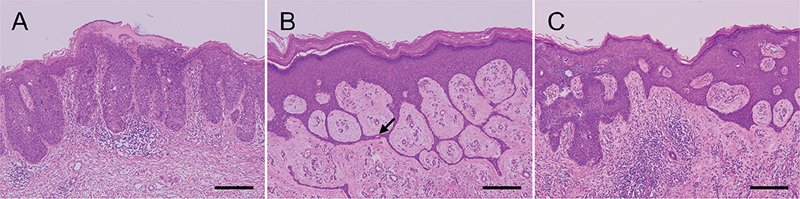
Fig. 2. Histopathological finding. (A) Skin biopsy showing atypical keratinocytes with multinucleation and focal dyskeratosis throughout the thickened epidermis. (B) Thin anastomosed strands of cuboidal cells arising from the epidermis to the upper dermis. The strands of cells exhibited a lattice pattern and were embedded in the fibrovascular stroma. The arrow indicates a sweat duct-like structure. (C) Thick interconnected strands composed of atypical keratinocytes in the epidermis (haematoxylin-eosin, scale bars: 200 µm.)
What is your diagnosis? See next page for answer.
ANSWERS TO QUIZ
Erythematous Plaque with Crusting on the Lower Leg: A Commentary
Diagnosis: Bowen’s disease (squamous cell carcinoma in situ) accompanied by reactive eccrine syringofibroadenoma
Histopathological examination showed atypical keratinocytes with multinucleation and focal dyskeratosis throughout the thickened epidermis, which was indicative of Bowen’s disease (BD) (Fig. 2A). In the areas adjacent to the BD, thin anastomosed strands of cuboidal cells protruded from the epidermis to the upper dermis (Fig. 2B). These strands of cells were arranged in a lattice pattern and were embedded in the fibrovascular stroma. Small ductal structures were observed within the interconnected strands of cells without nuclear atypia, showing typical features of eccrine syringofibroadenoma (ESFA). Thick interconnected strands composed of atypical keratinocytes were observed in multiple regions of the epidermis, exhibiting features of both BD and ESFA (Fig. 2C). No skin lesion recurrence was observed 1 year after tumour excision.
Eccrine syringofibroadenoma is a rare benign skin lesion with eccrine ductal differentiation that presents with varying clinical features (1). ESFA is histologically characterized by anastomosed cords and strands of homogenous cuboidal cells embedded in the fibrovascular stroma. ESFA is classified into 5 subtypes: (i) solitary ESFA, (ii) multiple ESFA without associated cutaneous findings (eccrine syringofibroadenomatosis), (iii) multiple ESFA associated with hidrotic ectodermal dysplasia, (iv) non-familial unilateral linear ESFA, and (v) reactive ESFA. Reactive ESFA is a type of eccrine duct remodelling or repair associated with various inflammatory dermatoses and skin tumours (2). Several case reports have described reactive ESFA associated with carcinomas, including BD, squamous cell carcinoma, porocarcinoma, and basal cell carcinoma (2, 3). While most non-reactive ESFA cases are identified as benign skin adnexal tumours, its malignant transformation into squamous cell carcinoma or porocarcinoma has been documented previously (4). Reactive ESFA is histologically indistinguishable from the other types of ESFA, even with immunohistochemical analysis (5). Therefore, it is difficult to determine whether ESFA is a reactive change secondary to carcinoma or a precancerous lesion that will undergo malignant transformation. In the current case, the BD lesion did not exhibit ductal differentiation. Therefore, ESFA was more likely to be a reactive process caused by the development of BD, rather than a precursor lesion of BD. The treatment strategy for ESFA involves complete surgical excision, as malignancy or malignant transformation is difficult to exclude. In summary, we report here a case of BD accompanied by reactive ESFA. Dermatologists should be aware that ESFA can occur during reactive processes associated with several skin tumours, including BD. Although rare, recognition of this histological change is essential in order to ensure an appropriate diagnosis.
REFERENCES
- Chukwuma O, Walker A, Motaparthi K, Montanez-Wiscovich M. Recalcitrant erosive plaques on the palms and soles: a rare manifestation of eccrine syringofibroadenoma. JAAD Case Rep 2020; 6: 590–592.
- Lee JS, Park H, Yoon HS, Cho S. Eccrine syringofibroadenoma associated with Bowen’s disease: a case report and review of the literature. Ann Dermatol 2020; 32: 57–63.
- Ishida M, Okabe H. Case of Bowen’s disease accompanying syringofibroadenoma. Rinsho Byori 2011; 59: 470–474.
- Pagliuca F, Moscarella E, Argenziano G Ronchi A, Franco R. Longstanding eccrine syringofibroadenoma with evidence of carcinomatous transformation. Am J Dermatopathol 2020; 42: 780–782.
- Tey HL. Characterizing the nature of eccrine syringofibroadenoma: illustration with a case showing spontaneous involution. Clin Exp Dermatol 2009; 34: e66–68.