ORIGINAL REPORT
Prolonged Sick Leave Before and After Diagnosis of Generalized Pustular Psoriasis: A Swedish Population-based Register Study
Sofia LÖFVENDAHL1,2 , Jenny M. NORLIN1
, Jenny M. NORLIN1 , Oskar ERICSON1
, Oskar ERICSON1 , Malin HANNO3
, Malin HANNO3 and Marcus SCHMITT-EGENOLF4
and Marcus SCHMITT-EGENOLF4
1The Swedish Institute for Health Economics (IHE), Lund, 2Division of Occupational and Environmental Medicine, Department of Laboratory Medicine, Lund University, Lund, 3Boehringer Ingelheim, Stockholm and 4Department of Public Health and Clinical Medicine, Dermatology, Umeå University, Umeå, Sweden
The aim of this study was to analyse sick leave in generalized pustular psoriasis, the most severe form of pustular psoriasis. Prolonged sick leave of >14 days was analysed for 502 patients with generalized pustular psoriasis compared with controls with psoriasis vulgaris and matched controls from the general population. Using data from the Swedish National Patient Register, and the Longitudinal integrated database for health insurance and labour market studies, the study estimated the mean number of sick leave days in the year of first diagnosis of generalized pustular psoriasis (index year) and for 2 years before and after the index year. Patients with generalized pustular psoriasis were on sick leave to a larger extent than both control populations for all study years. The number of sick leave days peaked in the index year and then reduced. Compared with the control populations, sick leave in generalized pustular psoriasis was already higher prior to diagnosis, indicating delayed diagnosis and/or a comorbidity burden.
Key words: generalized pustular psoriasis; sick leave; healthcare register; population-based; epidemiology.
SIGNIFICANCE
Generalized pustular psoriasis is a rare, but serious, type of psoriasis. This study used Swedish register data to investigate sick leave among patients with generalized pustular psoriasis. The results show that these patients had more sick leave than both the general population and patients with the more common type of psoriasis, psoriasis vulgaris. Sick leave peaked in the year the patients received their first diagnosis of generalized pustular psoriasis, indicating a higher disease burden due to generalized pustular psoriasis. In the 2 subsequent years, sick leave declined.
Citation: Acta Derm Venereol 2023; 103: adv6497. DOI https://doi.org/10.2340/actadv.v103.6497.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jun 22, 2023; Published: Sep 13, 2023
Corr: Marcus Schmitt-Egenolf, Department of Public Health and Clinical Medicine, Dermatology, Umeå University, Umeå, Sweden. E-mail: marcus.schmitt-egenolf@umu.se
Competing interests and funding: MSE is responsible for dermatology in the project management for the national guidelines for psoriasis at the Swedish Board of Health and Welfare. JMN and SL have been involved in the health economic analyses of the national guidelines for psoriasis at the Swedish Board of Health and Welfare. MSE, JMN, OE and SL have no further conflict of interest to declare. MH is an employee of Boehringer Ingelheim AB, Sweden.
INTRODUCTION
Generalized pustular psoriasis (GPP) is a severe form of pustular psoriasis (1–3). The occurrence of acute flares is typical. The flares, which can be life-threatening, regularly require inpatient care (1). The patient is often severely ill with general symptoms and complications from systemic inflammation.
Epidemiological studies have reported a wide range of GPP prevalence estimates, ranging from 0.18 to 18/100,000 (4–8). Reasons for this variation may be that GPP is an uncommon disease with no consensus in diagnostic criteria. Approximately half of patients with GPP have concomitant psoriasis vulgaris (PV) (2).
Due to the chronicity and risk for comorbidities, GPP has implications both for the patients and for society in terms of direct and indirect costs, due to healthcare use and production loss. Recent studies indicate that direct medical costs (healthcare use and drugs) are higher in GPP compared with both the general population and people with PV (10–12).
Even indirect costs due to production loss from sick leave (SL) may have an impact on the economic burden of GPP. However, there are no studies investigating the presence of SL in patients with GPP. The aim of this study was to analyse SL exceeding 14 consecutive days in patients with incident GPP compared: (i) with people with PV without GPP; and (ii) with the general population.
MATERIALS AND METHODS
Data sources
The National Patient Register (NPR) is an individual-level data register covering inpatient and secondary outpatient care in Sweden. The NPR contains primary and secondary diagnostic codes (International Classification of Diseases, 10th revision; ICD-10) and admission/discharge dates from private and public caregivers. The NPR does not cover primary care, nor healthcare provided by caregivers other than physicians. The NPR has been described elsewhere (13).
The Total Population Register (TPR) is the civil registration of vital events (births, deaths) of all Swedish inhabitants, administrated by Statistics Sweden (14). In the TPR, all citizens are identified by their unique personal identification number (PIN). By law, all healthcare provided must be registered with the person’s PIN, which is automatically assigned to all residents.
The Longitudinal integrated database for health insurance and labour market studies (LISA) is held by Statistics Sweden and contains data on all individuals 15 years of age and older. LISA integrates data from the labour market, educational and social sectors, and is updated annually. This study retrieved information about SL from LISA for the study populations.
Generalized pustular psoriasis cohort
The GPP cohort was identified from a psoriasis cohort identified with a primary or secondary diagnosis of psoriasis in the NPR 2004–2015 (7). The inclusion criteria for the GPP cohort were: (i) at least 1 visit to a physician at any department with a GPP primary or secondary diagnosis (ICD-10 code L40.1) registered 2006–2015; and (ii) 20 years of age or older at the date of first registration of a GPP diagnosis (index date).
Persons in the GPP cohort were not to be registered with GPP in the 2 years prior to the index date. The 2-year decision was based on data availability and an effort to include comorbidities prior to the index date. It was allowed to have a PV diagnosis prior to the first GPP diagnosis, although risking a selection bias due to longer “disease duration”. However, logistic regression did not show any association between SL in the GPP cohort and PV prior to the index date (data not shown).
Psoriasis vulgaris controls
A PV population (PVP) was created from the psoriasis cohort. To be defined as a patient with PV, the requirement was at least 1 registered ICD-10 code of L40.0 or L40.9 as primary or secondary diagnosis 2006–2015. For each patient with GPP, 3 patients with PV were matched on year of birth, sex, and index date (index dates for the PVP reflected the date of PV diagnosis that occurred during the same year as the date of GPP diagnosis for the corresponding individual in the GPP cohort). The PVP were required to have no history of healthcare consistent with PV; GPP or palmoplantar pustulosis.
General population controls
A general population control group (GP) was created from the TPR by matching 5 controls on year of birth, sex and residential area for each included person with GPP by linkage to the NPR. The referents were required to have no history of registered healthcare use consistent with psoriasis (ICD-10 code L40) in the NPR 2004–2017.
Setting
Residents (16–64 years) in Sweden can be granted economic security in case of sickness, injury, or disability. For the first day of sickness, the qualifying day, no benefit is paid. From the 2nd day to the 14th day, sick pay is obtained by the employer. From day 15, economic compensation is provided by the Swedish Social Insurance Agency (Försäkringskassan). The benefit is payable as full-time (100%) or part-time (25%, 50%, or 75%), owing to the reduction in work capacity. For people with a permanent or long-term work incapacity ≥1 year, sickness compensation can be obtained for either a limited or an indefinite period. Data from Försäkringskassan is forwarded to LISA at Statistics Sweden.
Outcomes and follow-up
Data on SL were collected from LISA for 5 calendar years: 2 years before index year (years –2 and –1), index year and 2 years after index (years +1 and +2). Data were also retrieved on employment status in the index year from LISA. In the SL analysis, people were included regardless of employment status. The reason for this is that sickness allowance is also granted for some people not categorized as employed.
Outcomes were: (i) number of persons with SL for each year; (ii) occurrence of SL and length of SL in the index year and during follow-up accounting for the covariates education, comorbidity 730 days prior to index date and SL in the 2 calendar years prior to index year.
SL was expressed as annual net days of SL (part-time days adjusted to full days). Since periods of SL shorter than 15 days are not registered in the LISA, 14 days were added to the net number of SL days for individuals with SL>0 days SL. Analyses were performed per calendar year (due to data structure in LISA) and not between exact dates. People who died during follow-up were excluded for the entire death year due to lack of data in LISA for that year. Data on SL includes weekends and holidays, i.e. the maximum number of annual SL days was 365.
Charlson Comorbidity Index (CCI) was employed to evaluate comorbidity burden (17). Comorbidities were captured from primary and secondary ICD-10 codes for inpatient and outpatient care in the NPR 2 years prior to the index date. In accordance with other studies (18, 19), CCI was categorized into groups according to CCI scores (0 = normal, 1 = moderate, 2 = high and ≥3 = very high).
Subgroup analysis
In a subgroup analysis of SL, the GPP cohort was limited to patients with at least 1 additional registration of a diagnostic code of GPP (L40.1) in the NPR within 1 year from the index date.
Statistical analysis
Descriptive statistics summarized the demographic variables and patterns of SL. The difference in annual net days of SL was compared separately between the GPP cohort and control populations. Student’s t-test and χ2 test were used, as appropriate, to examine groups differences. Violin plots were presented to visualize distribution of SL data.
This study explored the association between GPP and SL compared with controls using regression analysis. As data were skewed towards the right with an excess of zeros and overdispersion (variance > mean) zero-inflated negative binomial (ZINB) regression was used. The ZINB model tests the difference in adjusted relative counts (accounting for covariates) of SL days between the GPP cohort and the control populations accounting for the likelihood of incurring any SL. The model calculates estimates in 2 steps: (i) the likelihood that someone will incur a zero value of SL; and (ii) actual days of SL, weighted by the likelihood of being a zero SL person. For easier interpretation the outcome of the first step of the ZINB was switched (inversion of odds ratio), i.e. to incur SL (instead of not incur). To better understand the model mean predicted number of SL days was calculated for the GPP cohort and the controls (STATA postestimation command). p-values < 0.05 were considered statistically significant. STATA statistical software, version Stata/IC 14.2 (StataCorp LLC, College Station, TX, USA) was used.
Ethical approval
The study was approved by the Regional Ethical Review Board at Umeå University (Dnr 2010-194-31M, 2011-286-32M, 2016-126-32M).
RESULTS
Baseline characteristics
Between 2006 and 2015, this study identified 502 patients with GPP, 1,505 patients with PV and 2,344 GP controls who fulfilled the inclusion criteria (Table I), (Appendix S1; flowchart S1). The mean age (SD) was 50 years (12) and 60% were women across populations at the index date. Among the GPP cohort, 356 (71%) received their first GPP diagnosis in a dermatology/internal medicine department, 89 (18%) had their first diagnosis of GPP in inpatient care, 163 (32%) received 1 additional diagnosis of GPP within 365 days from the index date, and 149 (30%) had a diagnosis of PV in the 2 years prior to the index date. In the GPP cohort, there was a higher proportion of people with SL and higher CCI scores prior to the index year compared with control populations. The GPP cohort also differed in that they had a lower proportion of people with employment in the index year and a higher proportion of deaths during follow-up.
| GPP cohort | Control populations | ||||
| PV population | p-valuea | General population | p-valueb | ||
| N (alive at the beginning of year 0) | 502 | 1,505 | 2,344 | ||
| Women, n (%) | 301 (60) | 906 (60) | 1,419 (61) | ||
| Age, years, mean (SD) | 49.9 (12.0) | 49.9 (12.0) | 50.1 (11.9) | ||
| Median (p25; p75) | 53 (41; 59) | 53 (41; 59) | 54 (42; 60) | ||
| Age groups, n (%) | |||||
| 20–30 years | 49 (10) | 146 (10) | 219 (9) | ||
| 31–40 years | 70 (14) | 222 (15) | 318 (14) | ||
| 41–50 years | 84 (17) | 250 (17) | 393 (17) | ||
| 51–60 years | 198 (39) | 586 (39) | 932 (40) | ||
| 61–65 years | 101 (20) | 301 (20) | 482 (21) | ||
| Employed in November in index year | 318 (63) | 1,115 (74) | < 0.001 | 1,787 (76) | < 0.001 |
| First GPP diagnosis in department: | NA | NA | |||
| Dermatology/internal medicinec | 356 (71) | ||||
| Infection | 26 (5) | ||||
| Other departments | 67 (13) | ||||
| Missing value | 53 (11) | ||||
| First GPP diagnosis in inpatient care | 89 (18) | NA | NA | ||
| Two diagnoses of GPPd | 163 (32) | NA | NA | ||
| Diagnosis of PVe, n (%) | 149 (30) | 1,505 (100) | NA | ||
| CCIe, n (%) | < 0.001 | < 0.001 | |||
| Low, 0 | 388 (77) | 1,294 (86) | 2,149 (92) | ||
| Moderate, 1 | 71 (14) | 128 (9) | 78 (3) | ||
| High, 2 | 20 (4) | 53 (4) | 87 (4) | ||
| Very high, >2 | 23 (5) | 30 (2) | 30 (1) | ||
| History of sick leavef | 141 (28) | 363 (24) | 0.044 | 420 (18) | < 0.001 |
| Mortality, n (%) | |||||
| Year 0 | 10 (2.0) | 4 (0.3) | 11 (0.5) | ||
| Year 1 | 2 (0.4) | 3 (0.2) | 21 (0.9) | ||
| Year 2 | 6 (1.2) | 8 (0.5) | 13 (0.6) | ||
| Mortality in 2 years after diagnosis, n (%) | 18 (3.5) | 15 (1.0) | < 0.001 | 45 (1.9) | < 0.001 |
| Persons in overall analysis, n | |||||
| Year –2 | 502 | 1,505 | 2,344 | ||
| Year –1 | 502 | 1,505 | 2,344 | ||
| Index year | 492 | 1,501 | 2,333 | ||
| Year +1 | 486 | 1,498 | 2,312 | ||
| Year +2 | 484 | 1,490 | 2,299 | ||
| Persons with data for the entire study period, n | 484 | 1,490 | 2,299 | ||
| aComparing generalized pustular psoriasis (GPP) vs psoriasis vulgaris (PV) population. bComparing GPP vs general population. cIn Sweden, dermatology may be incorporated in an internal medicine department. dWithin 365 days from the index date. eIn the 2 years (730 days) prior to the index date. fSick leave in the 2 years (calendar years) prior to the index year | |||||
| Diagnosis of psoriasis vulgaris = ICD-10 code of L40.0 or L40.9 as primary or secondary diagnosis. | |||||
| SD: standard deviation; NA: not applicable; CCI: Charlson Comorbidity Index. | |||||
Number of persons with any sick leave
Compared with PVP, the proportion with SL was significantly larger for patients with GPP in the index year and in years –1 and +1 (Fig. 1). Compared with the GP, the proportion with SL days was significantly larger for the GPP cohort across all years. The proportion with SL in the GPP cohort increased significantly from 20% to 30% over the 2 years preceding the index date (Fig. 1). In the years following the index year, the proportion on SL decreased gradually to the same level as before the diagnosis of GPP. Among PVP and the GP, the proportion on SL was stable over time; 16% and 11%, respectively.
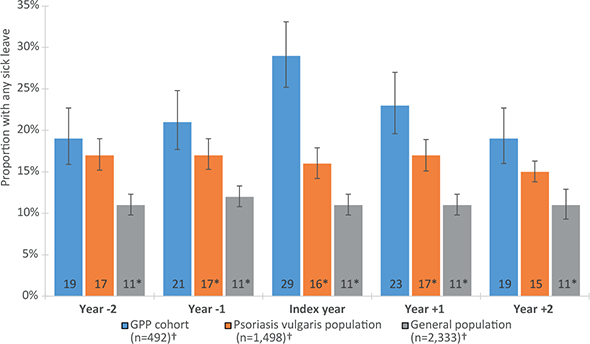
Fig. 1. Proportions of persons with sick leave >14 days in the 5-year study period in the generalized pustular psoriasis (GPP) cohort and the control populations. Error bars indicate 95% confidence intervals (95% CI). *Indicates a statistically significant (p-value<0.05) difference in proportion of persons with sick leave compared with the GPP cohort (Χ2 test). †Population size in index year.
Association between GPP and sick leave
Table II shows the results of the ZINB regression adjusted for covariates, regarding presence of any SL (ZI part) and length of SL (NB part) in the GPP cohort compared with controls. All covariates were adjusted for in each model. Descriptive data and violin plots of crude SL, see Appendix S1; S2–S5. For covariates estimates, see Appendix S1; S6 and S7. Compared with PVP, the odds of having any SL were significantly increased for patients with GPP for the index year and year +1. Compared with the GP, the odds of incurring any SL were significantly increased for the GPP cohort across all years. The expected number of SL days (the NB part) was only significantly increased for the GPP cohort compared with the GP in the index year (rate ratio 1.21, 95% confidence interval (95% CI) 1.02–1.45, p = 0.033). Among the covariates, SL in the 2 years prior to the index year was significantly associated both with odds of having any SL and length of SL across all 3 years and across populations (Appendix S1; S6 and S7).
| GPP vs psoriasis vulgaris population | GPP vs general population | |||||||
| Zero inflated part | Negative binomial part | Zero inflated part | Negative binomial part | |||||
| ORa (95% CI) | p-value | RRb (95% CI) | p-value | ORa (95% CI) | p-value | RRb (95% CI) | p-value | |
| Index year | ||||||||
| Reference population | 1 | 1 | 1 | 1 | ||||
| GPP cohort | 2.17 (1.69–2.85) | < 0.001 | 1.19 (1.00–1.41) | 0.050 | 2.78 (2.13–3.57) | < 0.001 | 1.21 (1.02–1.45) | 0.033 |
| Year +1 | ||||||||
| Reference population | 1 | 1 | ||||||
| GPP cohort | 1.37 (1.04–1.81) | 0.021 | 1.10 (0.90–1.35) | 0.361 | 2.00 (1.52–2.63) | < 0.001 | 1.14 (0.92–1.14) | 0.219 |
| Year +2 | ||||||||
| Reference. population | 1 | 1 | 1 | 1 | ||||
| GPP cohort | 1.19 (0.90–1.59) | 0.215 | 1.01 (0.80–1.27) | 0.942 | 1.51 (1.14–2.00) | 0.004 | 1.21 (0.96–1.51) | 0.108 |
| GPP compared with patients with psoriasis vulgaris and the general population, respectively. | ||||||||
| aLogistic regression part from the ZINB model: analysis sick leave, calculated as inverted odds ratios to denote the odds of being in the non-zero group. bNegative binomial regression part from the ZINB model: analysis of the length of sick leave, i.e. number of absence days among persons with any sick leave. | ||||||||
| Both parts controlled for the covariates education in the index year, comorbidity in the 2 years prior to the index date and sick leave in the 2 years prior to the index year. | ||||||||
| OR: odds ratio; RR: rate ratio; 95% CI: 95% confidence interval. Significant values given in bold. | ||||||||
The predicted number of SL days was significantly increased in the GPP cohort compared with the PVP in the index year and in year +1 (mean difference in days: (16.6; 95% CI: 9.8–23.4; p < 0.001) and (7.1; 95% CI: 0.4–13.8; p = 0.037) respectively) (Fig. 2). The predicted number of SL days was significantly increased in the GPP cohort compared with the GP across all years with pronounced difference in the index year (mean difference; 16.6; 95% CI: 10.8–22.5; p < 0.001) (Fig. 3). The model of predicted number of SL days between the GPP cohort and the control populations can be interpreted as a comparison of 2 hypothetical populations. Each case is treated as a GPP case (even if it is not) while all other independent variable values are kept as is, and the predicted number of SL days is computed. The same procedure is performed treating all cases as PV cases (alternatively GP cases). Thereafter, the mean of all the individual marginal effects is computed for the GPP vs PV and GP, respectively. This explains why the predicted number of SL days for the GPP cohort differs between Fig. 2 and Fig. 3.
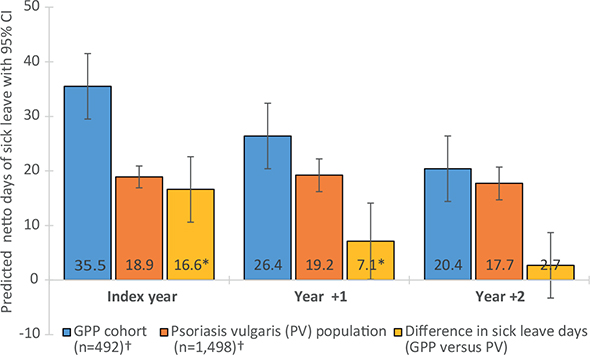
Fig. 2. Predicted days of sick leave (SL) in the generalized pustular psoriasis (GPP) cohort compared with the psoriasis vulgaris population. Error bars indicate 95% confidence (95% CI) intervals. *Indicates a statistically significant (p-value<0.05) difference in predicted days of sick leave compared with the GPP cohort. †Population size in index year.
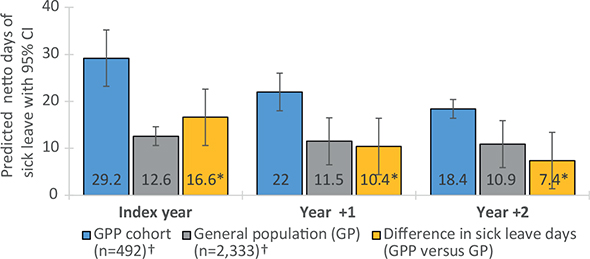
Fig. 3. Predicted days of sick leave (SL) in the generalized pustular psoriasis (GPP) cohort compared with the general population. Error bars indicate 95% confidence intervals. *Indicates a statistically significant (p-value<0.05) difference in predicted days of sick leave compared with the GPP cohort. †Population size in index year.
Subgroup analysis
Among the 502 patients in the GPP cohort, 163 (32%) received at least 1 additional diagnosis of GPP within 1 year from the index date. In this group of patients 132 (80%) received their first GPP diagnosis within a dermatology/internal medicine/infection department. The corresponding figure for the additional diagnosis was also 80%.
Compared with patients with only 1 diagnosis of GPP, this subgroup consisted of a lower proportion of women (55% vs 62%), a higher proportion of patients with a history of PV (36% vs 26%) and a first GPP diagnosis in inpatient care (25% vs 14%).
The group with 2 diagnoses had an overall higher proportion with any SL, ranging from 20% in year –1 and +1 to 37% in the index year. For both groups, mean SL days peaked at the index year, but the group with 2 diagnoses had more days of SL overall (Fig. 4). The difference in mean number of SL days was largest in the index year (56 vs 30 days). For descriptive statistics, see Appendix S1; S8.
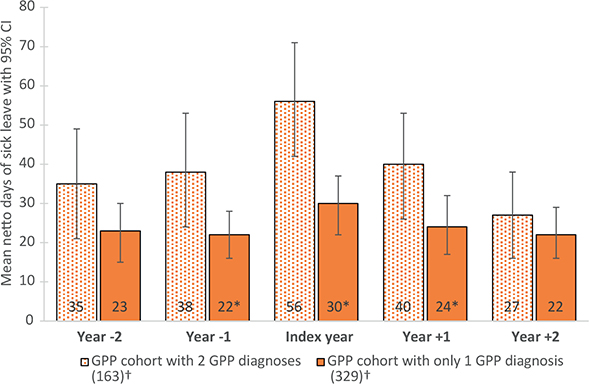
Fig. 4. Mean days of sick leave for persons with at least 1 additional diagnosis of generalized pustular psoriasis (GPP) within 1 year from the index date compared with persons with only 1 diagnosis of GPP. Error bars indicate 95% confidence intervals (95% CI). *Indicates a statistically significant (p-value<0.05) difference in mean days of sick leave between the 2 groups (Student’s t-test). †Population size in index year.
DISCUSSION
This study found an increase in prolonged SL of more than 14 days in a GPP cohort compared with both the general population and patients with psoriasis vulgaris, after controlling for education, CCI and history of SL. This difference was driven by a minority of patients in the GPP cohort with a relatively large number of SL days, e.g. in the index year, one-third of the GPP cohort had SL, with a crude mean number of 131 SL days (median 78.5).
The predicted number of days with SL was higher in the GPP cohort compared with the controls across all 3 years except for year +2 compared with the PVP. The difference in predicted SL days was most pronounced in the index year; a difference of 16.6 SL days between the GPP cohort and the control populations.
In the overall GPP cohort, the crude mean number of SL days in the index year was 39, which represented an increase of 12 days compared with the 2 years prior to the index date (Appendix S1; S2). Two years after the index year, SL had decreased to levels below those observed 2 years prior to the index date, indicating a disease improvement over the study period. This pattern was retained when analyses were limited to those with full data for the entire observation period, i.e. excluding persons lost to death (data not shown).
Notably, compared with the controls, the mean number of SL days was already increased in the GPP cohort in the 2 years before the index year (Table I). One explanation for this may be that symptoms of GPP are present long before the confirmation of diagnosis. The delay in getting a GPP diagnosis was recognized in a US study in which 66 patients with GPP were interviewed about the impact of the disease on their life (15). Nearly 40% of the patients reported that it took several years to receive an accurate diagnosis. Due to its rarity, many healthcare professionals may not have sufficient experience of GPP, leading to incorrect treatment or a delay in referral to a specialist (16).
An additional explanation for the high SL prior to diag-nosis of GPP may be GPP comorbidity. Studies have shown that GPP is associated with several comorbidities (12, 17–22). The current study data suggest that the potential comorbidity burden in the GPP cohort prior to the index date (Table I) may reflect part of the excess SL compared with the controls.
A strength of this study is the employment of large population-based registers, enabling a relatively large sample size. Furthermore, use of administrative register data on SL is less prone to bias compared with self-reported information. The population-based design minimizes selection bias and fluctuations over time, and differences across subgroups stress the importance of matched populations.
Limitations were a lack of information on the specific reasons for SL. Furthermore, the study did not capture the full extent of work loss in the study populations. Firstly, people who die during follow-up are excluded for the whole year of death due to lack of SL data in LISA for that year. Secondly, SL periods of ≤14 days are not registered and people with no registered days in the study data may therefore have multiple short periods of SL.
The validity of the GPP diagnosis has not been examined. GPP is rare and clinical awareness is limited; hence deciding the diagnosis may be challenging (9, 23, 24). There are differential diagnoses to consider in the clinical context (3). Also, an international consensus for the diagnosis of GPP is lacking (23, 25) and there is no standard case definition of GPP in Sweden. The current study case definitions were based on coded diagnoses of GPP and not on classification criteria or validation through medical record review, and thus potentially subject to misclassification.
This study tried to mitigate possible misclassification by a subgroup analysis that included patients with at least 1 additional diagnostic code of GPP in the NPR within 1 year of the first diagnosis (for GPP case definitions, see Löfvendahl et al. (7)). Two registered GPP diagnoses may strengthen the probability of a true GPP diagnosis, but it is also likely to indicate more severe GPP. This reasoning was, to some extent, confirmed by the current data, showing that patients with an additional diagnosis of GPP had a higher number of mean SL days in the index year and in the years immediately before and after the index date.
The results of this study indicate that SL is overrepresented among Swedish patients with GPP compared with both the GP and PV controls, and that the difference is driven by a minority of GPP patients with long periods of SL. Compared with PVP, the difference in SL was not consistently pronounced in the 2 years after the index year. A higher level of SL among patients with GPP compared with controls, as already observable in the years prior to GPP diagnosis, indicates a delayed diagnosis and/or the presence of comorbidities.
ACKNOWLEDGEMENTS
The authors thank Gunnar Brådvik, data analyst, for valuable data management, and Karin Wahlberg, medical writer, for writing and editorial support, both at The Swedish Institute for Health Economics (IHE). We also thank Harald Gyllensvärd at Boehringer Ingelheim AB, Sweden for valuable comments on the manuscript.
This research is the result of a research platform, which has received financial support from AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen Cilag, Leo Pharma and Novartis. This study’s design and analysis was funded by Boehringer Ingelheim. None of the authors has any conflict of interest in connection with the article. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. The authors had full independence regarding study design, data collection, analysis, result interpretation and decision to publish.
REFERENCES
- Kharawala S, Golembesky AK, Bohn RL, Esser D. The clinical, humanistic, and economic burden of generalized pustular psoriasis: a structured review. Expert Rev Clin Immunol 2020; 16: 239–252.
- Twelves S, Mostafa A, Dand N, Burri E, Farkas K, Wilson R, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol 2019; 143: 1021–1026.
- Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol 2018; 32: 1645–1651.
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol 2006; 16: 669–673.
- Fabbri P. Psoriasis. From clinical diagnosis to new therapies. Florence, Italy: SEE-Firenze; 2004. (ISBN:978L000002112).
- Lee JY, Kang S, Park JS, Jo SJ. Prevalence of psoriasis in Korea: a population based epidemiological study using the Korean National Health Insurance Database. Ann Dermatol 2017; 29: 761–767.
- Löfvendahl S, Norlin JM, Schmitt-Egenolf M. Prevalence and incidence of generalised pustular psoriasis in Sweden - a population-based register study. Br J Dermatol 2022; 186: 970–976.
- Ohkawara A, Yasuda H, Kobayashi H, Inaba Y, Ogawa H, Hashimoto I, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol 1996; 76: 68–71.
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol 2022; 23: 5–12.
- Hanna ML, Singer D, Valdecantos WC. Economic burden of generalized pustular psoriasis and palmoplantar pustulosis in the United States. Curr Med Res Opin 2021; 37: 735–742.
- Löfvendahl S, Norlin JM, Schmitt-Egenolf M. Economic burden of generalized pustular psoriasis in Sweden: a population-based register study. Psoriasis 2022; 12: 89–98.
- Morita A, Kotowsky N, Gao R, Shimizu R, Okubo Y. Patient characteristics and burden of disease in Japanese patients with generalized pustular psoriasis: results from the Medical Data Vision claims database. J Dermatol 2021; 48: 1463–1473.
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450.
- Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaëlsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31: 125–136.
- Reisner DV, Johnsson FD, Kotowsky N, Brunette S, Valdecantos W, Eyerich K. Impact of generalized pustular psoriasis from the perspective of people living with the condition: results of an online survey. Am J Clin Dermatol 2022; 23: 65–71.
- Strober B, Leman J, Mockenhaupt M, Nakano de Melo J, Nassar A, Prajapati VH, et al. unmet educational needs and clinical practice gaps in the management of generalized pustular psoriasis: global perspectives from the front line. Dermatol Ther (Heidelb) 2022; 12: 381–393.
- Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol 2014; 53: 676–684.
- Jin H, Cho HH, Kim WJ, Mun JH, Song M, Kim HS, et al. Clinical features and course of generalized pustular psoriasis in Korea. J Dermatol 2015; 42: 674–678.
- Löfvendahl S, Norlin JM, Schmitt-Egenolf M. Comorbidities in patients with generalized pustular psoriasis – a nationwide population-based register study. J Am Acad Dermatol 2023; 88: 736–738.
- Okubo Y, Kotowsky N, Gao R, Saito K, Morita A. Clinical characteristics and health-care resource utilization in patients with generalized pustular psoriasis using real-world evidence from the Japanese Medical Data Center database. J Dermatol 2021; 48: 1675–1687.
- Umezawa Y, Ozawa A, Kawasima T, Shimizu H, Terui T, Tagami H, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res 2003; 295: S43–54.
- Zheng J, Chen W, Gao Y, Chen F, Yu N, Ding Y, et al. Clinical analysis of generalized pustular psoriasis in Chinese patients: A retrospective study of 110 patients. J Dermatol 2021; 48: 1336–1342.
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol 2022; 23: 31–38.
- Ly K, Beck KM, Smith MP, Thibodeaux Q, Bhutani T. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl) 2019; 9: 37–42.
- Navarini AA, Burden AD, Capon F, Mrowietz U, Puig L, Koks S, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 1792–1799.