ORIGINAL REPORT
Determinants of Depressive Symptoms, Quality of Life, Subjective Health Status and Physical Limitation in Patients with Systemic Sclerosis
Stefanie HEYNE1, Eva HAUFE2, Stefan BEISSERT1, Jochen SCHMITT2 and Claudia GÜNTHER1
1Department of Dermatology, University Hospital Dresden and 2Institute of Evidence-Based Healthcare, Technical University Dresden, Dresden, Germany
Systemic sclerosis is a progressive connective tissue disease for which there is limited knowledge about physical limitations, quality of life and depression. The aim of this study was to assess these parameters during the disease process of systemic sclerosis, in a cross-sectional study of 79 patients and a longitudinal study of 33 patients over 10 years. Medical data were collected by physicians’ questionnaires and sociodemographic data, pain, physical limitation, quality of life, subjective health status, risk of depressive symptoms by patients’ questionnaires. Data analysis was descriptive and exploratory. Cross-tabulations, χ2 test and Student’s t-test were used for calculations, Pearson’s correlation to measure dependencies, and logistic regression analyses for categorized parameters. The cross-sectional analysis of 79 patients with systemic sclerosis (81% female, mean ± standard deviation age 61.5 ± 12.6 years) demonstrated a higher rate of patients with risk of depressive symptoms (42.3%) higher physical limitations, lower quality of life, and subjective health status than reference values for the general German population. Moderate to strong correlations between disease-related physical limitation, quality of life, subjective health status, risk of depressive symptoms and pain were detected (correlation according to Pearson –0.459 to –0.638, p < 0.001). Longitudinal analysis revealed a significant increase in disease activity, pain, physical limitation and risk of depressive symptoms (p < 0.001) during the disease process. This study demonstrates that nearly half of patients with systemic sclerosis probably experience depressive symptoms. The rate of patients with risk of depressive symptoms, pain and physical limitations increased during the systemic sclerosis disease process. Health-related quality of life and state of health declined, indicating the need for better interdisciplinary care for patients with systemic sclerosis.
Key words: systemic scleroderma; quality of life; depression; epidemiology.
SIGNIFICANCE
This study highlights the high proportion of patients with systemic sclerosis who have a risk of depressive symptoms (42.3%); this is 5 times greater than reference values for the general German population. Furthermore, patients with systemic sclerosis have higher physical limitations, lower quality of life and subjective health status compared with references for the general German population. These parameters deteriorated increasingly over a 10-year disease process of systemic sclerosis. Correlations between physical limitation, quality of life, subjective health status, high risk of depression and pain were demonstrated. Therefore, early diagnosis of systemic sclerosis, and preventive interdisciplinary treatment of physical and psychological symptoms, are required.
Citation: Acta Derm Venereol 2023; 103: adv6502. DOI https://doi.org/10.2340/actadv.v103.6502.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jul 11, 2023; Published: Sep 6, 2023
Corr: Stefanie Heyne, Department of Dermatology, University Hospital Carl Gustav Carus Dresden, Fetscherstr. 74, DE-01307 Dresden, Germany. E-mail: Stefanie.Heyne@uniklinikum-dresden.de
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Systemic sclerosis (SSc) is a rare connective tissue disease that affects the skin, joints, and internal organs, including the heart, lungs, kidneys and gastrointestinal tract, resulting in pain, dyspnoea and dysphagia, which greatly restrict patients’ physical and mental health status.
SSc is an autoimmune disease that affects women 3–4 times more often than men (1). The disease usually manifests between the 3rd and 5th decade of life, with a prevalence of 100 cases/1 million population worldwide (1, 2). Overall incidence rates range between 8 and 56 new cases/1 million population/year worldwide.
In 1988, Le Roy classified SSc into 2 forms: limited and diffuse (3). Limited cutaneous systemic sclerosis (lcSSc) describes a mostly slowly progressing skin thickening that primarily affects acral areas, such as the hands, forearms, feet, and lower legs. In contrast, diffuse cutaneous systemic sclerosis (dcSSc) spreads rapidly to the proximal areas of the extremities and trunk. In addition to these two main forms of SSc, we also included patients with systemic scerlosis sine scleroderma, mixed connective tissue disease (MCTD), considered SSc like Very early diagnosis of SSc (VEDOSS) or other special forms like seronegative SSc.
The disease criteria were defined by the American College of Rheumatology (ACR) in 1980 and revised in 2013 (4, 5). Antinuclear antibodies (ANA) can be detected in approximately 94% of patients with SSc, autoantibodies directed against topoisomerase I (Scl-70) in 30%, and autoantibodies against centromeres in 36% (6).
Microvascular, fibrotic and immunological changes can lead to vascular symptoms (Raynaud’s phenomenon, digital ulcers, scleroderma), musculoskeletal dysfunction and internal organ failure (gastrointestinal symptoms, kidney function impairment, cardiac/pulmonary dysfunction, impairment of the nervous system or the masticatory apparat).
To date, there is no causative treatment for SSc. The available treatment options only allow control of organ damage (immunosuppressants, tocilizumab, nintedanib) or limited improvement in symptoms (e.g. manual lymph drainage and physiotherapy, ultraviolet A 1 (UVA1) therapy) (1, 7–9).
In addition to disease-related physical limitations, such as joint contractures, ulcerations, pain, muscle weakness, dyspnoea, decreased mouth opening, swallowing, digestive difficulties and pain, patients with SSc may develop a disfiguring appearance, including joint contractures, telangiectasias, reduced finger and toe tips, decreased facial expression, which may impair their psychological well-being. Restrictions in food intake correlated with low subjective state of health in previous analysis (10). It also has been demonstrated that gingivitis, and osteolysis contribute to a decrease in health-related quality of life (HRQoL) (11).
While the prevalence of depressive symptoms in the general German population is 8.5% (12), depressive symptoms in patients with SSc range from 23% to 46% according to previous studies (13–16). This suggests a need for definition of determinants of disability, quality of life, subjective health status, and depressive symptoms.
MATERIALS AND METHODS
Setting and study population
Patients with SSc participating in routine dermatological autoimmune consultations at Dresden University Hospital, Dresden, Germany, were enrolled in the study. Patients were at least 18 years old with a definite diagnosis of prevalent SSc according to the ACR criteria for classification of SSc (5). The study was approved by the ethics committee of Technical University Dresden.
A total of 79 patients participated in a cross-sectional analysis from 2016 to 2018. Of these, 33 patients had been examined in a similar way in 2008, enabling longitudinal analysis to be performed (17).
Study instruments
Patients completed a patient questionnaire about socioeconomic characteristics and demographics.
A subjective assessment of disease severity was made by the patient from mild (severity grade 1), moderate (severity grade 2), severe (severity grade 3) to very severe (severity grade 4), in terms of a Patient Global Assessment (PGA) (18).
Existing pain and resulting limitations of daily activities in the past week were determined by means of 8 questions with 9 response options each (answers 1–3 representing no or mild (category 1), 4–6 moderate (category 2), and 7–9 (category 3) severe pain/restriction).
Weight, height, form of SSc, organ manifestations (skin, vessels, lung, oesophagus, stomach, small intestine, colon, heart, musculoskeletal, nervous system, masticatory system) were documented in a physician questionnaire and verified the SSc diagnosis using the ACR criteria.
Information about disease activity and clinical severity was detected by using the score of Scleroderma Trials and Research Group of European Alliance of Associations for Rheumatology (EUSTAR). Cutaneous involvement was assessed by modified Rodnan Skin Score (mRSS) (19). The EUSTAR score was positive for active disease at a value ≥ 3, and the mRSS score was recorded as abnormal at values > 14.
Antibody status, present or past symptoms due to changes in the vascular system, joints, muscles, gastrointestinal tract, kidneys, heart, lungs, and nervous system were assessed. Current, or previously reported, medication, physical or ultraviolet (UV) therapy were documented.
Physical limitations were determined by means of the Health Assessment Questionnaire (HAQ), which consists of 20 questions and covers 8 domains of daily physical activities, with 2 or 3 questions in each domain: dressing and grooming, getting up, eating, walking, hygiene, reaching, grasping and activity. Possible answers refer to the last 7 days and range from problem-free (scored by 0), slightly difficult (1), very difficult (2) to not possible (3). The highest single score of the respective area is added and divided by the number of all activity areas, i.e. 8. This results in a value between 0 (for no physical limitation) and 3 (for the most severe physical limitation). A clinically significant change is considered to be a change in HAQ score of at least 0.5 units (20)
HRQoL was evaluated by means of EuroQoL questionare with a 3-level version of 5 dimensions (EQ-5D-3L) consisting of 5 questions on mobility, self-care, general activities, pain/physical discomfort and anxiety/low mood, with each domain having 3 possible response levels, ranging from no problems/symptoms to moderate problems/symptoms to severe problems/symptoms. The values of the EQ-5D-3L index are calculated by an internal standard, and range from less than 0 (where 0 describes a state of health equivalent to death; i.e. negative values are considered worse) to 1 (perfect state of health) (21). The mean value of the HRQoL index can vary from country to country; in Germany it is 0.93 (22).
The EQ-5D also includes a visual analogue scale (VAS) for assessment of the current state of health from the patient’s subjective perspective, with 100 indicating the best and 0 the worst possible state of health. The mean values of the respective population (Germany: 77.3) was used as comparative value (22).
Depressive symptoms were assessed by means of the Center for Epidemiologic Studies Depression Scale (CES-D) which contains 20 questions to assess the frequency of depressive symptoms in the past 7 days on a 4-point scale: rarely/or never (less than 1 day, scored by 0), sometimes/occasionally (1–2 days, 1), more often/frequently (3–4 days, 2), mostly/ constantly (5–7 days, 3). Questions 4, 8, 12 and 16 are formulated positively, so that their point value is assigned in reverse. Finally, the points were summed, resulting in a value between 0 and 60. A score ≥ 16 points indicates a high risk for depressive symptoms (23–25).
Statistical analysis
Data analysis was performed pseudonymized using SPSS version 26. It was descriptive and exploratory. Depending on the scale level, frequencies or distribution parameters, such as mean, maximum, minimum, and standard deviation (SD), were used to describe the data obtained.
Associations of sociodemographic parameters, diagnosis types, disease characteristics and symptoms, disease duration, pain, antibody status, categories of EUSTAR score and mRSS with categories of EQ-5D-3L index, EQ-5D-VAS and CES-D score were estimated using cross-tabulations with χ2 test. Correlational analyses were performed to detect associations between several metric scales, i.e. EQ-5D-3L index, EQ-5D-VAS, HAQ score, pain score, and CES-D score. Furthermore, logistic regression analysis with backward elimination was used to detect associations of characteristics of disease with considered risk of depressive symptoms (CES-D ≥ 16) and social impairment (EQ-5D < 0.93, EQ-5D-VAS < 77.3, respectively). For the analyses of longitudinal data χ2 and paired samples t-tests were used.
RESULTS
Cross-sectional analysis
Most of the 79 patients with SSc were female (81%, Table I) with a mean ± SD age of 61.51 ± 12.59 years.
Mean ± SD disease duration was 12.12 ± 10.27 years. The most-detected antibodies were ANA (97.5% of the patients), which correlated with calcification (p = 0.003), vomiting (p = 0.013) and 3-fold increased ceratine kinase (CK) (p = 0.013). Anti-centromere antibodies (35.4% of the patients) were significantly associated with pulmonary fibrosis (p = 0.002), as well as kidney involvement (p = 0.040) and anti-Scl70 antibodies (30.4% of the patients) significantly correlated with pulmonary fibrosis (p < 0.001), with contractures (p = 0.003), calcifications (p = 0.022), ulcers (p = 0.029) and pulmonary hypertension (p = 0.045).
LcSSc was the most common diagnosis (62%). The most frequent organ involvements were skin manifestations (Raynaud’s syndrome), scleroderma, pits, ulcerations, calcifications), restricted mobility, sicca symptoms and, thus, musculocutaneous symptoms, such as ulcers/pits, contractures, tendon rubbing, muscle weakness were currently predominant.
An active disease (EUSTAR score ⁄ 3) was present in 46.8% of the patients. mRSS > 14 was found in 6.3% of the patients and was correlated significantly with disease related physical limitation (p = 0.002), gastrointestinal involvement (p = 0.010) and musculoskeletal involvement (p = 0.004).
Approximately 60% of the patients with SSc experienced severe or very severe pain and a moderate or severe PGA.
More than two-thirds of the patients received physio-therapy; almost half of the patients required vasodilators, and approximately one-third of the patients were treated with angiotensin-converting enzyme inhibitors (ACE inhibitors). Approximately one-quarter of the patients received immunosuppressants, such as azathioprine or methotrexate.
The mean ± SD EQ-5D-3L index was 0.73 ± 0.26, which is below the mean value of the German population (0.93) (22). The EQ-5D-3L depended on the severity of pain (p = 0.020).
Mean ± SD EQ-5D-VAS was 59.4 ± 21.7, which was also lower than the mean of the German population (77.3) (22) and correlated significantly with pain (p < 0.001) and an involvement of the masticatory organ (p = 0.015). The presence of scleroderma tended to correlate with a low EQ-5D-VAS (p = 0.065).
Of the SSc patients, 42.3% were likely to develop depressive symptoms (CES-D ≥ 16). Mean ± SD CES-D was 16.6 ± 9.6. CES-D ≥ 16 correlated significantly with PGA (p = 0.009) as well as pain (p = 0.002) (Fig. 1). Furthermore, pulmonary hypertension (p = 0.006), muscle weakness (p = 0.008) and dyspnoea (p = 0.016) seemed to have a significant effect on the development of depressive symptoms. The percentage of patients with a high risk of depressive symptoms was slightly higher in those treated with immunosuppressants than in those without (p = 0.012). Considering family income, a low risk of depressive symptoms (CES-D < 16) seemed to be associated with a higher family income (p = 0.020) (Table II). It is notable that the mean family income was 1,001–2,000 €/month, which is below the mean family income of the Dresden population (2,200 €) in 2018 (26).
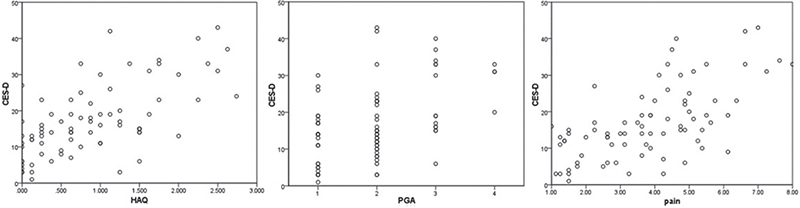
Fig. 1. Significant correlations of Center for Epidemiologic Studies Depression Scale (CES-D) with Health Assessment Questionnaire (HAQ), Patient Global Assessment (PGA), and pain p < 0.001.
| Variable | Patients with CES-D < 16 (N = 45) n (%) | Patients with CES-D ≥ 16 (N = 33) n (%) | p-value* |
| Patient Global Assessment Mild Moderate Severe Very severe |
17 (37.8) 24 (53.3) 4 (8.9) 0 |
8 (24.2) 12 (36.4) 9 (27.3) 4 (12.1) |
0.009 |
| Antibody status Antinuclear antibodies Anti-centromere-antibodies Anti-Scl-70-antibodies |
44 (97.8) 14 (31.1) 17 (37.8) |
32 (97) 13 (39.4) 7 (21.2) |
0.823 0.447 0.117 |
| Diagnosis Limited systemic sclerosis Diffuse systemic sclerosis Systemic sclerosis a sine Scleroderma Mixed connective tissue disease Considered systemic sclerosis Special form |
29 (64.4) 7 (15.6) 1 (2.2) 6 (13.3) 2 (4.4) 0 |
19 (57.6) 6 (18.2) 1 (3) 4 (12.1) 2 (6.1) 1 (3) |
0.882 |
| Organ involvement Raynaud’s phenomenon Scleroderma Calcification Acral ulcerations Pits Pulmonary hypertension Pulmonary fibrosis Oesophagus Stomach/small intestine Colon Kidneys Heart Locomotor system Nervous system Sicca symptoms Masticatory organ |
43 (95.6) 41 (91.1) 6 (13.3) 15 (33.3) 20 (44.4) 3 (6.7) 21 (46.7) 20 (44.4) 1 (2.2) 3 (6.7) 4 (8.9) 10 (22.2) 10 (22.2) 1 (2.2) 13 (28.9) 5 (11.1) |
31 (93.9) 32 (97) 8 (24.2) 13 (39.4) 16 (48.5) 10 (30.3) 11 (33.3) 22 (66.7) 1 (3) 3 (9.1) 3 (9.1) 8 (24.2) 9 (27.3) 0 9 (27.3) 1 (3) |
0.749 0.297 0.215 0.581 0.724 0.006 0.237 0.052 0.823 0.691 0.975 0.834 0.608 0.389 0.875 0.186 |
| Modified Rodnan Skin Score 0–4 5–8 9–14 15–28 |
29 (64.4) 9 (20) 4 (8.9) 3 (6.7) |
22 (66.7) 6 (18.2) 3 (9.1) 2 (6.1) |
0.996 |
| EUSTAR ≥ 3 | 19 (42.2) | 17 (51.5) | 0.416 |
| Current symptoms Ulcers/pits Synovitis Contractures Tendon rubbing 3-fold creatine kinase elevation |
16 (35.6) 4 (8.9) 10 (22.2) 6 (13.3) 0 |
10 (30.3) 4 (12.1) 11(33.3) 4 (12.1) 2 (6.1) |
0.866 0.253 0.315 0.333 0.199 |
| Muscle weakness Muscle atrophy Reflux/dysphagia Diarrhoea/obstipation Vomiting Renal insufficiency Dialysis Dyspnoea NYHA III/IV Palpitations Conduction disorders Polyneuropathy Trigeminal neuralgia |
2 (4.4) 1 (2.2) 11 (24.4) 3 (6.7) 1 (2.2) 7 (15.6) 0 3 (6.7) 2 (4.4) 3 (6.7) 3 (6.7) 1 (2.2) |
8 (24.2) 4 (12.1) 14 (42.4) 8 (24.2) 3 (9.1) 1 (3) 1 (3) 10 (30.3) 5 (15.2) 0 4 (12.1) 1 (3) |
0.008 0.183 0.078 0.074 0.164 0.072 0.240 0.016 0.095 0.166 0.644 0.823 |
| Pain Mild Severe Very severe |
26 (57.8) 14 (31.1) 5 (11.1) |
6 (18.2) 18 (54.5) 9 (27.3) |
0.002 |
| Current therapy Angiotensin-converting enzyme inhibitors Low-dose systemic corticoids Immunosuppressants Vasodilators Ultraviolet therapy Physiotherapy |
15 (33.3) 6 (13.3) 11 (24.4) 22 (48.9) 6 (13.3) 31 (68.9) |
8 (24.2) 7 (21.2) 11 (33.3) 16 (48.5) 2 (6.1) 24 (71.7) |
0.270 0.461 0.012 0.982 0.701 0.553 |
| NYHA: New York Heart Association; EUSTAR: score of Scleroderma Trials and Research Group of European Alliance of Associations for Rheumatology. *χ2 test. Bold p-values represent significant values. | |||
Multivariate logistic regression analysis showed a significant association of CES-D with HAQ (p < 0.001) (Fig. 1).
The Pearson correlation matrix demonstrated mutual significant correlations between EQ-5D-3L index, CES-D, HAQ, pain, and EQ-5D VAS (correlation according to Pearson –0.459 to –0.638, p < 0.001) in the patients with SSc (Fig. 2).
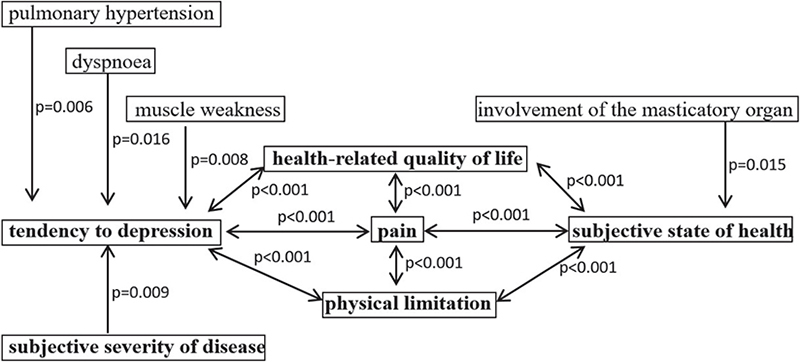
Fig. 2. Significant correlations between Center for Epidemiologic Studies Depression Scale (CES-D), Patient Global Assessment, EQ-5D-3L, EQ-5D-VAS, pain and significant correlating clinical symptoms (pulmonary hypertension, dyspnoea, muscle weakness, and involvement of the masticatory organ).
Longitudinal analysis
A longitudinal analysis of 33 patients from 2008 to 2016 was performed (Table III). In 2016 the majority of patients with SSc were female (84.8%), married (66.7%), had a secondary school degree (39.4%), retired (81.8%), and had a monthly family income of 1,001–2,000 € (37.0%), which is below the mean family income of the Dresden population (2,200 €) in 2018 (26). Considering that the majority of patients were retired, mean family income corresponds to the mean income of retired households in Dresden (27).
| Variable | Patients in 2008 | Patients during the period 2016–2018 | p-value of change* |
| Disease duration, mean ± SD | 18.99 ± 8.05 | ||
| PGA, n (%) Mild Moderate Severe Very severe |
7 (21.2) 21 (63.6) 5 (15.2) 0 |
6 (18.2) 17 (51.5) 9 (27.3) 1 (3.0) |
0.003 |
| PGA, mean ± SD | 1.94 ± 0.61 | 2,15 ± 0.76 | 0.090 |
| PGA decrease, n (%) | 9 (9.1) | ||
| PGA stable/increase, n (%) | 30 (90.9) | ||
| Antibody status, n (%) ANA Anti-centromere-antibodies Anti-Scl-70-antibdies Anti-RNA polymerase 3 antibodies Anti-PmScl antibodies Anti-U1-RNP antibodies |
32 (97) 11 (33.3) 9 (27.3) 2 (6.1) 3 (9.1) 4 (12.1) |
||
| Diagnosis, n (%) Limited SSc Diffuse SSc Systemic sclerosis sine scleroderma MCTD Considered SSc Special form |
19 (57.6) 4 (12.1) 0 7 (21.2) 2 (6.1) 1 (3) |
||
| Organ involvement, n (%) Raynaud’s phenomenon Scleroderma Calcification Acral ulcerations Pits Pulmonary hypertension Pulmonary fibrosis Oesophagus Stomach/small intestine Colon Kidneys Heart Locomotor system Nervous system Sicca symptoms Masticatory organ |
32 (97.9) 33 (100) 10 (30.3) 14 (42.4) 19 (57.6) 9 (27.3) 15 (45.5) 26 (78.8) 1 (3) 4 (12.1) 4 (12.1) 15 (45.5) 12 (36.4) 1 (3) 16 (48.5) 5 (15.2) |
||
| mRSS, n (%) 0–4 5–8 9–14 15–28 |
7 (21.2) 14 (42.4) 3 (9.1) 9 (27.3) |
21 (63.6) 10 (30.3) 1 (3.0) 1(3.0) |
0.484 |
| mRSS > 14, n (%) | 9 (27) | 1 (3) | 0.003 |
| mRSS, mean ± SD | 10.70 ± 8.13 | 4 .27 ± 4.25 | < 0.001 |
| EUSTAR, mean ± SD | 1.07 ± 0.74c | 2.29 ± 1.56 | < 0.001a |
| EUSTAR ≥ 3, n (%) | 0c | 16 (48.5) | – |
| EUSTAR decrease, n (%) | 7 (24.1)c | ||
| EUSTAR stable/increase, n (%) | 22 (75.9)c | ||
| Current symptoms, n (%) Ulcers/pits Synovitis Contractures Tendon rubbing 3-fold CK elevation Muscle weakness Muscle atrophy Reflux/dysphagia Diarrhoea/obstipation Vomiting |
7 (21.2) 1 (3.0) 10 (30.3) 10 (30.3) 0 11 (33.3) 5 (15.2) 13 (39.4) 4 (12.1) 4 (12.1) |
11 (33.3) 5 (15.2) 14 (42.4) 8 (24.2) 0 3 (9.1) 1 (3.0) 21 (63.6) 5 (15.2) 2 (6.1) |
|
| Renal insufficiency Dialysis Dyspnoea NYHA III/IV Palpitations Conduction disorders Polyneuropathy Trigeminal neuralgia |
0 0 3 (9.1) 6 (18.2) 1 (3.1) 17 (51.5) 0 |
1 (3) 0 4 (12.1) 3 (9.1) 2 (6.1) 6 (18.2) 1 (3) |
|
| Pain, n (%) Mild Severe Very severe |
15 (45.4) 13 (39.4) 5 (15.2) |
11 (33.3) 13 (39.4) 9 (27.3) |
< 0.001 |
| Pain, mean ± SD | 3.42 ± 1.61 | 4.41 ± 1.83 | 0.018 |
| Pain decrease, n (%) | 7 (21.2) | ||
| Pain stable/increase, n (%) | 26 (78.8) | ||
| Current therapy, n (%) ACE inhibitors Systemic glucocorticoids Immunosuppressants Vasodilators UV therapy Physiotherapy |
14 (42.4) 8 (24.2) 5 (15.2) 20 (60.6) 1 (3) 25 (75.8) |
||
| HAQ, mean ± SD | 0.69 ± 0.57 | 1.01 ± 0.72 | < 0.001 |
| HAQ change by 0.22–0.49, n (%) | 6 (18.2) | ||
| HAQ change by ≥ 0.5, n (%) | 14 (42.3) | ||
| HAQ decrease, n (%) | 7 (21.2) | ||
| HAQ stable/increase, n (%) | 26 (78.8) | ||
| EQ-5D-3L–Index, mean ± SD | 0.74 ± 0.29 | 0.66 ± 0.31 | 0.057 |
| EQ-5D-3L < 0.93, n (%) | 27 (81.8) | 30 (90.9) | < 0.001 |
| EQ-5D-3L stable/decrease, n (%) | 30 (90.9) | ||
| EQ-5D-3L increase, n (%) | 3 (9.1) | ||
| EQ-5D-VAS, mean ± SD | 62.61 ± 19.16 | 55.97 ± 22.20 | 0.113 |
| EQ-5D-VAS < 77.3 | 23 (69.7) | 27 (81.8) | 0.160 |
| EQ-5D-VAS stable/decrease, n (%) | 23 (69.7) | ||
| EQ-5D-VAS increase, n (%) | 10 (30.3) | ||
| CES-D, mean ± SD | 17.31 ± 9.94d | 18.21 ± 10.59 | 0.523b |
| CES-D ≥ 16, n (%) | 15/32 (46.9)d | 19 of 33 (57.6) | < 0.001 |
| CES-D decrease, n (%) | 14 (43.8) | ||
| CES-D stable/increase, n (%) | 18 (56.2) | ||
| CES-D ≥ 16 in 2008 in correlation with change in BMI | 0.014 | ||
| CES-D change in correlation with change in BMI | 0.098 | ||
| BMI, mean ± SD | 24.2 ± 3.6 | 24.2 ± 4.2 | < 0.001 |
| BMI stable/decrease, n (%) | 19 (57.6) | ||
| BMI increase, n (%) | 14 (42.4) | ||
| a29 comparable patients. b32 comparable patients. Missing: c4 patients, d1 patient. SD standard deviation; PGA: Patient Global Assessment; ANA: antinuclear antibodies; SSc: systemic sclerosis; CES-D: Center for Epidemiologic Studies Depression Scale; HAQ: Health Assessment Questionnaire; EQ-5D-3L: EuroQoL questionare with a 3-level version of 5 dimension; EQ-5D-VAS: EuroQoL questionare with a visual analogue scale; BMI: body mass index; MCTD: mixed connective tissue disease; UV: ultraviolet; CK: creatine kinase; mRSS: modified Rodnan Skin Score; ACE: angiotensin-converting enzyme; NYHA: New York Heart Association; EUSTAR: score of Scleroderma Trials and Research Group of European Alliance of Associations for Rheumatology. *χ2 test/ Student’s t-test Bold p-values represent significant values. | |||
Mean disease duration was 19.0 (± 8.1) years. LcSSc was the most frequent diagnosis (57.6%). Ninety-seven percent of patients tested positive for ANA, 33.3% for anti-centromere antibodies, and 27.3% for anti-Scl70 antibodies.
During the 10-year observation period a worsening of ulcerations/pits, synovitis, contractures, reflux/dysphagia, diarrhoea/obstipation, renal insufficiency, cardiac insufficiency, conduction disorders, trigeminal neuralgia was shown. In contrast, tendon rubbing, muscle weakness, muscle atrophy, vomiting, palpitations and polyneuropathy slightly improved (Fig. 3).
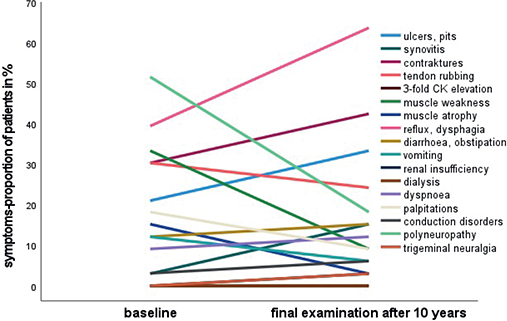
Fig. 3. Changes in clinical symptoms over time. CK: creatine kinase.
The number of patients with mRSS > 14 decreased significantly from 2008 (9 patients) to 2016–2018 (1 patient) (p = 0.003), as did mean values (2008: 10.7 (± 8.1); 2016–2018: 4.3 (± 4.3); (p < 0.001)). It should be noted that assessing mRSS is investigator dependent and thus might have been influenced by the change in investigators from 2008 to 2016.
Mean EUSTAR score increased significantly (p < 0.001) over time. While in 2008 no patients had a EUSTAR score ≥ 3, 48.5% of patients had a EUSTAR score ≥ 3 in 2016 to 2018, 75.9% of patients had a stable or increased EUSTAR score. In addition to this objective increase in disease activity, there was also a trend towards an increase (p = 0.090) in mean values of subjective PGA over this time period. Ninety percent of patients reported a stable or worsened PGA. Mean pain score increased significantly (p = 0.036) over time, with a stable or increased pain score in 78.8% of patients. There was also a shift in the proportion of patients with mild and moderate PGA and pain to severe and very severe PGA and pain.
Regarding physical limitation, patients with SSc showed a significantly increased mean HAQ (p < 0.001) from 2008 to 2016–2018, with a change of 0.22–0.49 in 18.2% of patients and a change ≥ 0.50 in 42.4% of patients. Overall, 78.8% of the patients showed a stable or increased HAQ, representing worsening of physical limitation over time with the disease process.
Disease-related quality of life (mean EQ-5D-3L: p = 0.057) and subjective state of health (mean EQ-5D-VAS: p = 0.113) tended to decrease. Compared with reference values for the mean score of the German population (0.93) (22), 81.8% of patients with SSc in 2008 and 90.9% of patients with SSc in 2016–2018 had a lower EQ-5D-3L index with significant change over time (p < 0.001). Most (90.9%) of the patients had a stable or worsened EQ-5D-3L index over time. Consequently, disease-related quality of life decreased over the course of the disease. Of patients with SSc, 69.7% in 2008 and 81.8% in 2016 to 2018 had an EQ-5D-VAS below the a mean score of the general German population (77.3) (22) (p = 0.160); 69.7% had a stable or lower EQ-5D-VAS over time; 46.9% in 2008 and 57.6% in 2016–2018 had a CES-D ≥ 16 and thus a high risk of depressive symptoms, with significant change over time (p < 0.001); 21.9% of patients developed a high risk of depressive symptoms, and 12.5% recovered from that. There was a significant association between change in BMI and conspicuous CES-D in 2008 (p = 0.014).
Mean BMI increased significantly (p < 0.001): 57.6% of patients had a stable or lower BMI, and 42.4% had a higher BMI over time.
DISCUSSION
This study demonstrates that nearly half of the investigated 79 patients with SSc were likely to develop depressive symptoms. This is approximately 5 times higher than the prevalence of depressive symptoms in the general German population, which was determined among 868 Germans in a previous study using the CES-D (1) or Patient Health Questionnaire 8 (PHQ-8) (28).
Importantly, the prevalence of depressive symptoms in patients with SSc exceeded the number determined in patients with atopic dermatitis, psoriasis or rheumatological disorders, such as lupus or rheumatoid arthritis (29–31). Depressive symptoms could occur as a consequence of the physical and psychological changes during SSc disease and have been reported worldwide. A Canadian study showed a mean CES-D score of 12.9 (± 9.7) with a CES-D ≥ 16 in 32.1% of patients with SSc (3). In Italy, a similar study was conducted using the Beck Depression Inventory (BDI). Depressive symptoms were detected above a threshold of 10. The mean BDI score was 10.5 (± 8.3), with 46.2% having a score > 10 and thus depressive symptoms (32). In a French study, the Hospital Anxiety Depression Scale (HADS) (suspected depressive symptoms ≥ 8) identified clinical depressive symptoms and anxiety disorder in 40.4% of patients with lcSSc and 58.8% of patients with dcSSc (4). A Japanese study of 50 patients with SSc showed depressive symptoms in 46% (2).
The current study revealed that a high risk of depressive symptoms (CES-D ≥ 16) correlated with subjective disease severity and pain (Fig. 1). Pain is a known influencing factor of physical limitation and depressive symptoms in patients with SSc (34–36) and is also an important indicator of sexual dysfunction in women with SSc (37) that can lead to depression. Furthermore, pulmonary hypertension with associated dyspnoea and muscle weakness influenced the high risk of depressive symptoms. This is supported by the significant correlation of a high risk of depressive symptoms with health-related physical limitation (Fig. 1) that has been reported previously (5).
The subjective state of health (EQ-5D-VAS) and disease-related quality of life (EQ-5D-3L) of this SSc cohort were below the mean values for the general German population (22) and correlated both with pain, as also shown by a previous study (37), and with associations between quality of life and physical limitations (38). The significant correlation of a low subjective state of health with the involvement of the masticatory organ suggests that patients with SSc were strongly impaired by restrictions during food intake (mouth opening, chewing, swallowing) (15). It has been demonstrated that sicca symptom, gingivitis and osteolysis contribute to a decrease in HRQoL (29).
The significant correlations between EQ-5D-3L index, CES-D, HAQ, pain and EQ-5D-VAS, together with the above-described associations with clinical symptoms (Fig. 2), demonstrate the close interactions between physical and psychological factors in this disease.
Longitudinal analysis of HRQoL, subjective state of health, high risk of depressive symptoms, physical limitations, pain and disease symptoms revealed an increase in disease severity, indicated by EUSTAR score ≥ 3 and PGA, during the 10 years of observation. This was associated with an increasing mean pain score, which might be caused by chronification of pain during long-term SSc in association with physical limitation and risk of depressive symptoms (33). Indeed, analysis of mean disease-related physical limitations (HAQ) demonstrated a significant increase with a change of 0.22–0.49 in 18.2% of patients and a change ≥ 0.50 in 42.4% of patients.
In agreement with previous observations, the mean HRQoL (EQ-5D-3L index) tended to decrease over time (39, 40). In addition, mean subjective health status also declined during the 10-year observation. As a consequence, the risk of depressive symptoms increased significantly.
Study limitations
In this study, patients with SSc were compared with available data for the general German population, to enable comparison with a larger group of individuals. A direct control group was not investigated.
In 2008, a total of 78 patients were examined, of whom 33 participated again in 2016–2018. Most patients were lost-to-follow-up due to death, relocation or declining to participate in the follow-up study.
It is possible that the depression rate, of 5 times higher than the reference of Stein et al. (1), is overestimated, because they analysed a population 10 years younger in comparison with the current cohort. In addition, the number of female patients was higher in the current population. Both parameters may affect the incidence of depressive symptoms, and therefore limit the value of comparison.
Conclusion
Significant correlations between physical limitation due to the disease, disease-related quality of life, subjective state of health, high risk of depressive symptoms and pain warrant interdisciplinary cooperation in the treatment of patients with SSc, especially as pulmonary hypertension associated with dyspnoea as well as muscle weakness directly impacted the rate of depressive symptoms, and involvement of the masticatory correlated with the subjective state of health (Fig. 2).
Pain, physical limitation and prevalence of patients with high risk of depressive symptoms increased, and disease-related quality of life and subjective state of health declined with the course of the disease, indicating that current levels of care and support might be insufficient to prevent this development. To facilitate the early recognition of this problem, measures for the detection of depressive symptoms should be used by the treating physician and subsequently linked to additional psychological care. Early recognition of the disease-associated symptoms and preventive interdisciplinary treatment should be enforced in patients with SSc in order to limit depressive symptoms and declining quality of life.
ACKNOWLEDGEMENTS
The study was reviewed and approved by the ethics committee of the Technical University of Dresden (EK53032008).
REFERENCES
- Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. J Eur Acad Dermatol Venereol 2017; 31: 1401–1424.
- Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 2008; 37: 223–235.
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988; 15: 202–205.
- Masi AT. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980; 23: 581–590.
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013; 72: 1747–1755.
- Mierau R, Moinzadeh P, Riemekasten G, Melchers I, Meurer M, Reichenberger F, et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German Network for Systemic Scleroderma: correlation with characteristic clinical features. Arthritis Res Ther 2011; 13: R172.
- Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al.; EUSTAR Coauthors. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017; 76: 1327–1339.
- Bongi SM, Del Rosso A, Passalacqua M, Miccio S, Cerinic MM. Manual lymph drainage improving upper extremity edema and hand function in patients with systemic sclerosis in edematous phase. Arthritis Care Res (Hoboken) 2011; 63: 1134–1141.
- Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al.; SENSCIS Trial investigators. nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528.
- Smirani R, Truchetet ME, Poursac N, Naveau A, Schaeverbeke T, Devillard R. Impact of systemic sclerosis oral manifestations on patients’ health-related quality of life: a systematic review. J Oral Pathol Med 2018; 4: 808–815.
- Nietert PJ, Mitchell HC, Bolster MB, Curran MY, Tilley BC, Silver RM. Correlates of depression, including overall and gastrointestinal functional status, among patients with systemic sclerosis. J Rheumatol 2005; 32: 51–57.
- Stein J, Luppa M, Mahnke J, Weyerer S, Schomerus G, Riedel-Heller SG. Depressionsscreening am Telefon mittels der Allgemeinen Depressionsskala (ADS) [Screening for depression by telephone using the German version of the Center for Epidemiological Studies Depression Scale (CES-D)]. Psychiatr Prax 2014; 41: 135–141.
- Matsuura E, Ohta A, Kanegae F, Haruda Y, Ushiyama O, Koarada S, et al. Frequency and analysis of factors closely associated with the development of depressive symptoms in patients with scleroderma. J Rheumatol 2003; 30: 1782–1787.
- Kwakkenbos L, Arthurs E, van den Hoogen FH, Hudson M, van Lankveld WG, Baron M et al; Canadian Scleroderma Research Group. Cross-language measurement equivalence of the Center for Epidemiologic Studies Depression (CES-D) scale in systemic sclerosis: a comparison of Canadian and Dutch patients. PLoS One 2013; 8: e53923.
- Nguyen C, Ranque B, Baubet T, Bérezné A, Mestre-Stanislas C, Rannou F, et al.; Groupe Français de Recherche sur la Sclérodermie. Clinical, functional and health-related quality of life correlates of clinically significant symptoms of anxiety and depression in patients with systemic sclerosis: a cross-sectional survey. PLoS One 2014; 9: e90484.
- March C, Huscher D, Preis E, Makowka A, Hoeppner J, Buttgereit F, et al. Prevalence, risk factors and assessment of depressive symptoms in patients with systemic sclerosis. Arch Rheumatol 2019; 34: 253–261.
- Müller H, Rehberger P, Günther C, Schmitt J. Determinants of disability, quality of life and depression in dermatological patients with systemic scleroderma. Br J Dermatol 2012; 166: 343–353.
- Nikiphorou E, Radner H, Chatzidionysiou K, Desthieux C, Zabalan C, van Eijk-Hustings, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther 2016; 18: 251.
- Khanna D, Furst DE, Clements PJ, Allanore Y, Baron M, Czirjak L, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2017; 2: 11–18.
- Langer HE. 2004. HAQ (Health Assessment Questionnaire) – rheuma-online. HAQ (Health Assessment Questionnaire) [accessed 2022 Dec 28]. Available from: https://www.rheuma-online.de/a-z/h/haq-health-assessment-questionnaire/
- EuroQol Research Foundation. 2018. EQ-5D User Guides – EQ-5D. [accessed 2022 Dec 28]. Available from: https://euroqol.org/publications/user-guides/
- Szende A, Janssen B, Cabases J, editors. Self-Reported population health: an international perspective based on EQ-5D. Dordrecht (NL): Springer; 2014. PMID: 29787044.
- Thombs BD, Hudson M, Schieir O, Taillefer SS, Baron M; Canadian Scleroderma Research Group. Reliability and validity of the center for epidemiologic studies depression scale in patients with systemic sclerosis. Arthritis Rheum 2008; 59: 438–443.
- Kwakkenbos L, Arthurs E, van den Hoogen FH, Hudson M, van Lankveld WG, Baron M, van den Ende CH, Thombs BD; Canadian Scleroderma Research Group. Cross-language measurement equivalence of the Center for Epidemiologic Studies Depression (CES-D) scale in systemic sclerosis: a comparison of Canadian and Dutch patients. PLoS One 2013; 8: e53923.
- Riediger M, Linden M, Wilms H-U. 1998. Die deutsche Version der CES-D als Instrument der gerontologischen Forschung. Zeitschrift für Klinische Psychologie, Psychiatrie und Psychotherapie, 46 (4): 344–364 [accessed 2022 Dec 28]. Available from: http://library.mpib-berlin.mpg.de/ft/mr/MR_Deutsche_1998.pdf.
- Municipal Statistical Office Dresden. 2018. Municipal Citizens’ Survey Dresden 2018 [accessed 2022 Dec 28]. Available from: https://www.dresden.de/media/pdf/onlineshop/statistikstelle/KBU_2018_Hauptaussagen.pdf.
- Öffentlichkeitsarbeit, R. K. und. (o. J.). Rentner–Sozialberichterstattung–Sachsen.de. [accessed 2022 Oct 24]. Available from: https://www.sozialbericht.sachsen.de/rentner-4149.html.
- Hapke U, Cohrdes C, Nübel J. Depressive symptoms in a European comparison – Results from the European Health Interview Survey (EHIS) 2. J Health Monit 2019; 4: 57–65.
- Park EH, Strand V, Oh YJ, Song YW, Lee EB. Health-related quality of life in systemic sclerosis compared with other rheumatic diseases: a cross-sectional study. Arthritis Res Ther 2019; 21: 61.
- Patel KR, Immaneni S, Singam V, Rastogi S, Silverberg JI. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis. J Am Acad Dermatol 2019; 80: 402–410.
- Richards HL, Fortune DG, Weidmann A, Sweeney SK, Griffiths CE. Detection of psychological distress in patients with psoriasis: low consensus between dermatologist and patient. Br J Dermatol 2004; 151: 1227–1233.
- Tedeschini E, Pingani L, Simoni E, Ferrari D, Giubbarelli C, Giuggioli D, et al. Correlation of articular involvement, skin disfigurement and unemployment with depressive symptoms in patients with systemic sclerosis: a hospital sample. Int J Rheum Dis 2014; 17: 186–194.
- Mouthon L, Mestre-Stanislas C, Bérezné A, Rannou F, Guilpain P, Revel M, et al. Impact of digital ulcers on disability and health-related quality of life in systemic sclerosis. Ann Rheum Dis 2010; 69: 214–217.
- Evers C, Jordan S, Maurer B, Becker MO, Mihai C, Dobrota R, et al. Pain chronification and the important role of non-disease-specific symptoms in patients with systemic sclerosis. Arthritis Res Ther 2021; 23: 34.
- Knafo R, Haythornthwaite JA, Heinberg L, Wigley FM, Thombs BD. The association of body image dissatisfaction and pain with reduced sexual function in women with systemic sclerosis. Rheumatology (Oxford) 2011; 50: 1125–1130.
- Ostojic P, Jankovic K, Djurovic N, Stojic B, Knezevic-Apostolski S, Bartolovic D. Common causes of pain in systemic sclerosis: frequency, severity, and relationship to disease status, depression, and quality of life. Pain Manag Nurs 2019; 20: 331–336.
- Racine M, Hudson M, Baron M, Nielson WR; Canadian Scleroderma Research Group. The impact of pain and itch on functioning and health-related quality of life in systemic sclerosis: an exploratory study. J Pain Symptom Manage 2016; 52: 43–53.
- Sierakowska M, Doroszkiewicz H, Sierakowska J, Olesińska M, Grabowska-Jodkowska A, Brzosko M, et al. Factors associated with quality of life in systemic sclerosis: a cross-sectional study. Qual Life Res 2019; 28: 3347–3354.
- van Leeuwen NM, Ciaffi J, Liem SIE, Huizinga TWJ, de Vries-Bouwstra JK. Health-related quality of life in patients with systemic sclerosis: evolution over time and main determinants. Rheumatology (Oxford) 2021; 60: 3646–3655.
- Morrisroe K, Hudson M, Baron M, de Vries-Bouwstra J, Carreira PE, Wuttge DM, et al. International Systemic Sclerosis Inception Cohort (INSYNC) collaboration. Determinants of health-related quality of life in a multinational systemic sclerosis inception cohort. Clin Exp Rheumatol 2018; 113: 53–60.