ORIGINAL REPORT
Factors Associated with Eczema Clinical Trial Awareness, Interest, and Participation in Adults
Erin E. GRINICH1#, Isabelle J. THIBAU2#, Emile LATOUR3, Kyla N. PRICE4, Allison R. LOISELLE2, Eric SIMPSON1 and Wendy SMITH BEGOLKA2
1Department of Dermatology, 2National Eczema Association, 3Biostatistics Shared Resource, Knight Cancer Institute, Oregon Health & Science University and 4College of Medicine, University of Illinois, Chicago, IL, USA
#These authors contributed equally to this work.
Despite the need for improved eczema therapies and a rapid increase in available eczema clinical trials, participation remains low. The aim of this study was to identify factors associated with clinical trial awareness, interest, and barriers to enrolment and participation. An online survey, administered 1 May to 6 June 2020 to adults (≥ 18 years) with eczema in the USA, was analysed. Among 800 patients included, mean age was 49.4 years, most respondents were female (78.1%), White (75.4%), non-Hispanic (91.4%), and geographically living in an urban/suburban area (Rural-Urban Continuum Codes (RUCC) 1–3, 90.8%). Only 9.7% of respondents reported previous participation in clinical trials, while 57.1% had considered participation and 33.2% never considered participation. Higher satisfaction with current eczema therapy, clinical trial literacy, and confidence in finding eczema trial information were all associated with clinical trial awareness, interest, and successful participation. Younger age and having atopic dermatitis were associated with increased awareness, while female gender was a barrier to interest and successful participation.
Key words: clinical trials; atopic dermatitis; eczema; health literacy; patient recruitment; biomedical translational science.
SIGNIFICANCE
Different diseases may have different needs for clinical trials, since they are unique to patient populations. This study of adult eczema patients found that few ever participated in clinical trials, yet a large subset had considered it. Higher satisfaction with current therapy, clinical trial literacy, and confidence to find clinical trial information were associated with awareness, interest, and successful participation in clinical trials. Younger age and having atopic dermatitis were associated with increased awareness, while being female was a barrier to interest and successful participation in clinical trials. Reducing barriers and increasing knowledge/awareness may lead to increased participation in eczema clinical trials.
Citation: Acta Derm Venereol 2023; 103: adv6520. DOI https://doi.org/10.2340/actadv.v103.6520.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 10, 2023; Published: Jun 20, 2023
Corr: Isabelle J. Thibau, National Eczema Association, 505 San Marin Drive, Suite B300, Novato, California 94945, USA. E-mail: isabelle@nationaleczema.org
Competing interests and funding: All authors had access to the data. The authors have no disclosures or potential conflicts of interest apart from this: ES reports personal fees from AbbVie, Amgen, Arena Pharmaceuticals, Aslan Pharma, Boston Consulting Group, Collective Acumen, LLC (CA), Dermira, Eli Lilly, Evidera, ExcerptaMedica, Forte Bio RX, Galderma, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin Pharmaceutical Development, Leo Pharm, Medscape LLC, Merck, Pfizer, Physicians World LLC, Regeneron, Roivant, Sanofi-Genzyme, Trevi therapeutics, Valeant, WebMD. These potential conflicts of interest have been reviewed and managed by OHSU. WSB is an Advisory Board Honoraria for Incyte and Pfizer (paid to institution), receives PI research grant funding from Pfizer, and is a salaried employee of NEA. ARL and IJT are also salaried employees of NEA.
Data from this manuscript were presented at the 2022 Annual American Academy of Dermatology meeting as an ePoster: “Grinich EE, Thibau IJ, Price KN, Latour E, Simpson EL, Begolka WS. 32097 Factors associated with eczema clinical trial participation in adults. J Am Acad Dermatol 2022 Sep 1; 87 (3): AB167”.
INTRODUCTION
Eczema refers to a collection of eczematous dermatoses, including, but not limited to, atopic dermatitis (AD). The patient-specific heterogeneity in symptom presentation, combined with the recurring and remitting nature of eczema, makes it difficult to treat using a “one-size fits all” approach. Investigational therapies from clinical trials (CTs) can provide new therapeutic opportunities and options for patients with recalcitrant disease, greater ability to align with patient treatment preferences, while also addressing unmet needs and advancing medical knowledge. Recent advances in understanding the pathophysiology of eczema has resulted in an unprecedented increase in the number of eczema CTs investigating novel therapeutic targets as well as other interventional approaches. In 2021, over 90 CTs related to eczema were newly initiated and/or actively recruiting participants in the USA, representing a 3.3-fold increase in eczema CTs since 2008 (ClinicalTrials.gov). In addition, there is significant movement across all CTs to engage more inclusive, representative population demographics, as well as disease-specific demographics (1–3). Despite the need for improved eczema therapies and the rapid expansion of new CTs, clinical trial participation (CTP) has remained low. A recent poll of eczema patients and caregivers revealed that 8% of respondents reported previous CTP (4).
For CTP to occur, patients must first be aware of CTs. In a nationally representative survey study, 64% of adults with no previous medical research participation reported awareness of research opportunities (5). Furthermore, patients must be interested and motivated to participate and face few or no barriers to CT enrolment. Factors that may increase CTP interest are: benefit to other eczema patients, contribution to the advancement of knowledge, and importance of the study (6) as well as side-effects from current therapy, previous CT experience and knowledge, and confidence that the trial will potentially improve disease understanding and access to health (5, 7–11). Age, race, gender, number of medications, insurance status, and employment status have not been previously associated with broad CT interest (5).
Other potential barriers to CT participation include the possibility of receiving placebo, lack of sufficient information on risks and trial procedures, invasive tests, use of extra medication, expected burden, time constraints, and “fear” (7, 12).
It is unclear how, or if, the results from studies exploring broad awareness and participation in CTs apply to patients with eczema. There remains a paucity of literature on eczema patients’ awareness and understanding of CTs. Similarly, information on drivers and barriers to CT enrolment and CTP is scant. This study aims to identify patterns of CT awareness and understanding, as well as key factors associated with CTP in patients with eczema.
MATERIALS AND METHODS
Study design and study population
A 46-question online survey was administered 1 May to 6 June 2020, to adult patients (≥ 18 years) with eczema who were US or US territory residents following a voluntary response convenience sampling strategy. Eczema was defined as 1 or more of the following self-reported diagnoses: AD, contact dermatitis, dyshidrotic eczema, hand eczema, neurodermatitis, nummular eczema, seborrhoeic dermatitis, and/or stasis dermatitis. Survey availability was communicated via the National Eczema Association (NEA) website, e-mail, and social media (see Appendix S1). This study used a past NEA survey to base sample size on given limited data published on eczema patient experiences with CTs. Potential participants were directed to online screening for eligibility and informed consent. Participants who completed the survey were entered into a drawing to win 1 of 10 US$50 e-gift cards. This study was determined exempt by Western Institutional Review Board Copernicus Group. NEA researchers developed the survey instrument, and patient volunteers pilot tested the survey instrument to give feedback on content and literacy level before the instrument was finalized.
The survey collected information on respondent demographics, understanding of, and experience with, eczema CTs, as well as drivers for and barriers to CTP. Respondents were grouped into 4 race categories (1) White, 2) Black or African American, 3) Asian or Asian American, Native American or Alaskan Native, Native Hawaiian or Pacific Islander, and 4) Other) to achieve adequate sample size. Geographical location of respondents was defined as Urban/Suburban vs Rural using Rural-Urban Continuum Codes (RUCC) (Urban/Suburban (RUCC 1–3), Rural (RUCC 4–10)) ((accessed 22 March 2022); Available online (13)). As respondents were allowed to report multiple eczema diagnoses, eczema severity was determined by selecting the diagnosis with the highest severity for each patient.
Respondents were classified into 3 analysis subgroups based on previous CT participation or interest (Fig. 1). Those who indicated previous participation in ≥ 1 CTs were denoted as, “actual CTP”. Respondents who indicated no prior CTP, but an attempt or consideration of CTP were denoted as, “considered CTP with/without attempt.” Respondents who indicated no prior CTP and no consideration of CTP were denoted as, “no CTP consideration/attempt.”
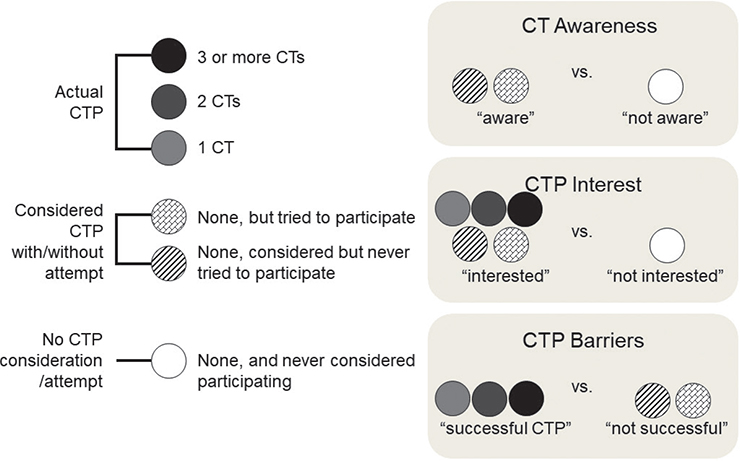
Fig. 1. Grouping of respondents based on clinical trial participation (CTP) and subsequent group comparisons. Respondents with participation in ≥1 clinical trial were grouped as, “actual CTP”. Those who either considered or tried CTP were grouped as, “Considered CTP with/without attempt”. Those with no prior CTP or attempt were grouped as, “No CTP consideration/attempt”. Appropriate groupings were then compared to determine factors associated with CTP, including awareness, interest and barriers.
Clinical trial understanding
CT understanding was determined using responses to 13 CT-related terms (see Appendix S1). Likert responses were transformed to an overall composite “understanding score”, with a possible range from 13 to 65. Given the paucity of literature on assessing CT understanding in patients, novel measurement tools were created specific to this study to address targeted aims. As such, the measurement tools have not yet been validated.
Clinical trial awareness
The considered CTP with/without attempt group was compared against the no CTP consideration/attempt group to ascertain factors associated with CT awareness. Those who already participated in ≥ 1 CTs were excluded from the “aware” group to account for the confounding factor of impact of previous participation in CTs on awareness.
Barriers to clinical trial participation interest
The actual CTP group and the considered CTP with/without attempt group were combined and compared against the no CTP consideration/attempt group in order to determine barriers to any level of interest in CTP.
Barriers to clinical trial enrolment
The actual CTP group was compared with the considered CTP with/without attempt group to determine what barriers patients face when they are already interested in CTP, but ultimately choose not to enrol or were unable to participate for other reasons.
Statistical analysis
Descriptive statistics were used to summarize demographic characteristics and survey respondent responses (means and standard deviations (SD) for continuous variables; frequencies and percentages for categorical variables). Group comparisons for categorical variables were performed using Fisher’s exact test; group comparisons with 1-way analysis of variance (ANOVA) for continuous variables. Respondents with missing data for a variable were suppressed from the analysis of that variable. Analysis was done using R: A Language and Environment for Statistical Computing (14). Significance was set at p < 0.05. Logistic regression was used to separately explore each outcome of interest: (i) CT awareness, (ii) CT interest, and (iii) CTP barriers. Multivariable logistic regression models were developed using purposeful selection (15) (see Appendix S1 for univariate logistic details). In addition, 1-way ANOVA with post-hoc Tukey test was used to determine associations between the composite understanding score and CT subgroups: 3 CTs, 2 CTs, 1 CT, tried, considered, and none.
RESULTS
A total of 1,016 adults participated in the survey (with 67.7% completion rate). Of those, 216 did not indicate their experience level with CTs and were excluded. Analysis was based on 800 patients. Mean age was 49.4 years, with females accounting for 78.1% (n = 625) of respondents. Most respondents identified as White (75.4%, n = 596), non-Hispanic (91.4%, n = 731), and living in an urban/suburban area (RUCC 1–3, 90.8%, n = 722) (Table I). The majority of respondents collectively reported no previous CTP (57.1% n = 457) with (14.5%, n = 116) or without (42.6% n = 341) attempted participation. Only 9.7% (n = 77) reported previous CTP, and (33.2%, n = 266) reported no CTP and no previous consideration/attempt.
Clinical trial understanding
Certain CT-related terms were found to be more poorly understood across all respondents regardless of CT experience. Over half reported a below average understanding of the terms: “rescue therapy,” “treatment arm,” and “washout period”. There was a significant difference in composite understanding score of CT-related terms between different levels of CTP (p = 0.013, 1-way ANOVA). Specifically, the no CTP consideration/attempt group had a lower composite score on average compared with the considered (p < 0.001), tried (p < 0.001), 1 CT (p < 0.001), and 3 CT groups (p < 0.013) (Fig. 2).
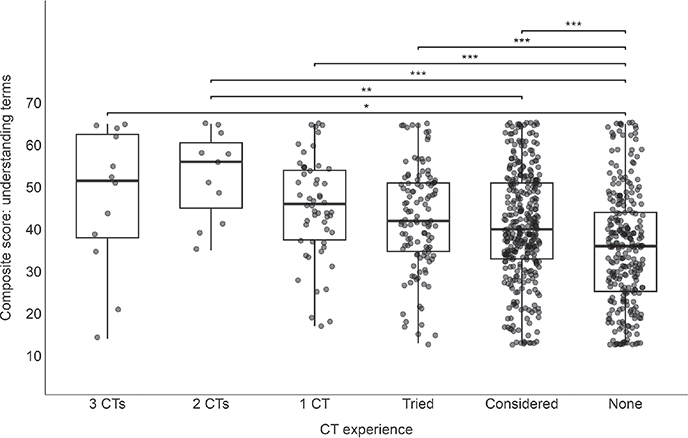
Fig. 2. Association of understanding of clinical trial (CT)-related terms with previous CT participation. One-way ANOVA with post-hoc Tukey tests were used to determine associations between the composite score of CT-related understanding terms and CT subgroups: 3 CTs, 2 CTs, 1 CT, tried, considered, and none. A significant difference in composite understanding score was seen between different levels of CTP (p = 0.013). Specifically, the no CTP consideration/attempt group had a lower composite score on average compared with the considered (p < 0.001), tried (p < 0.001), 1 CT (p < 0.001), and 3 CT groups (p < 0.013).
Factors associated with clinical trial awareness
In the multivariable model (Table II), there were no significant differences in gender, race, ethnicity, geography, number of eczema diagnoses and eczema severity between the “aware” and “not aware” CTP subgroups (Fig. 1). Those 65 years of age and older were 54.8% less likely (95% CI 30.0–71.0%) to be aware of CTs compared with those aged 18–35 years (p < 0.001). Patients with an AD diagnosis were 1.8 times more likely (95% CI 1.2–2.8) to be aware of CTs compared with all other diagnoses (p = 0.003). Those who reported being satisfied/very satisfied with their current eczema therapy were 40.3% less likely (95% CI 11.8–59.7%) to be aware of CTs compared with those who were dissatisfied/very dissatisfied (p = 0.010), and those who were extremely/very confident in their ability to find information on available eczema CT were 1.8 times more likely (95% CI 1.1–2.8) to be aware of CTs compared with those who were slightly/not confident at all (p = 0.010). Lastly, for every 1-point increase in the composite understanding score, odds of CT awareness went up by 2.8% (95% CI 1.5–4.2%, p < 0.001).
Factors associated with clinical trial participation interest
In univariable analyses, there were significant differences in age (p = 0.002) and proportion with an AD diagnosis (p < 0.001) between those who were “interested” in CTP and those who were “not interested” (Fig. 1). In the multivariable model (Table II), females were 56.0% less likely (95% CI 24.8–73.8%) to have interest in CTP than males (p = 0.002). Patients who were satisfied/very satisfied with their current eczema therapy were 3.0 times more likely (95% CI 1.6–6.1) to be interested in CTP than those who were dissatisfied/very dissatisfied (p < 0.001). This question did not distinguish those who may have had concurrent CTP. Patients who were very/extremely confident in finding information on available eczema CTs were more likely to be interested in CTP than those who were slightly or not confident at all (adjusted OR 3.2, 95% CI 1.5–7.4, p = 0.003). Finally, for every 1-point increase in the CT-related term understanding score, patients were 3.2% more likely to be interested in CTP (95% CI 1.1–5.4%, p = 0.002).
Factors associated with barriers to clinical trial participation
The subset of respondents with “successful CTP” were compared with those who considered/tried without successful CTP (Fig. 1) to examine possible external barriers to CT enrolment and participation within a group of interested respondents. In the multivariable model (Table II), females were 61.5% less likely to have actual CTP compared with males (95% CI 31.7–78.1%, p < 0.001). Participants who were satisfied/very satisfied with their current eczema therapy had 3.5 times the adjusted odds (95% CI 1.8–7.2) of actual CTP compared with those who were dissatisfied/very dissatisfied (p < 0.001). To address current CTP as a potential confounder of satisfaction, we excluded 12 respondents who had CTP in 2020 from the model and found it had little effect on satisfaction odds and no change in statistical significance. Those who were extremely/very confident in their ability to find eczema CT information had 2.4 times the odds of actual CTP compared with those who were only slightly/not very confident (95% CI 1.1–5.5, p = 0.034).
DISCUSSION
Using an online survey to characterize the adult eczema patient community awareness, understanding, and participation in eczema CT, several factors were identified that contribute to increased CT interest and engagement. Higher satisfaction with current eczema therapy, better understanding of CT-related terms, and confidence in finding information about available eczema CTs were all associated with CT awareness, interest, and successful participation. In addition, younger age and an AD diagnosis were associated with increased CT awareness, while female gender was a barrier to both interest and successful CTP.
The first step to increasing awareness of eczema CTs is increasing general understanding and awareness of CTs. A multi-specialty study of public attitudes toward CTs found a strong belief in the importance of CTs, but limited understanding of the clinical research process (16). In the current study, increased knowledge of CT-related terms was also associated with increased CT awareness. However, some terms were still poorly understood regardless of CT awareness, including “rescue therapy”, “treatment arm”, and “washout period”.
A Korean population survey study demonstrated higher income and education, middle age, male gender, and living in an urban/suburban area were positively associated with awareness of CTs (7). Another study on awareness of CTs in cancer patients confirmed that those with higher income and education were more likely to be aware of CTs, and that those who identified as Black or African American or Hispanic were less likely to be aware than those who identified as White (17). The current study found that patients 65 years and older were less likely to be aware of eczema CTs than those aged 18–35 years; however, there were no differences in awareness by race or ethnicity. Association between younger age and awareness found in the current study may be due to the classical younger onset of eczema compared with many other chronic conditions. Those with an AD diagnosis were more likely to be aware of eczema CTs compared with those with other eczema types. CTs for non-AD types of eczema are limited, which may account for the observed reduction in eczema-specific CT awareness for those patients.
Following a general knowledge of CTs and awareness of eczema-specific CTs, patients must also be interested in, or driven to, participating. Previous research has identified driving factors as: benefit to other eczema patients/altruism (6, 9, 11), dissatisfaction with current therapy, previous CT experience and knowledge, and confidence that the trial will potentially improve disease understanding and access to health (5, 7, 10). In the current study, identified driving factors for interest in CTP were male gender, higher satisfaction with current therapy, better knowledge of CT-related terms, and confidence in ability to find information on available eczema CTs. The association with higher eczema therapy satisfaction may be confounded by this study’s cross-sectional nature, namely that some patients may have continued taking beneficial medications available through CTP and are therefore currently satisfied. In addition, someone who is satisfied with their current eczema therapy may be more optimistic about new treatments than someone is not satisfied with their current therapy. Evidence for the effect of age on CT interest is conflicting. In 1 US population study, older patients were more likely to be interested in CTP (5), while in a study of women receiving routine breast cancer examinations, younger women were more likely to be interested in CTP (10). In the current study, there was no association between age and CTP interest/drive.
Finally, for CTP to occur, patients must face few barriers to enrolment and participation. One barrier to CTP in this study was being female. Female under-representation in CTs has been a pervasive issue in the past, although it has drastically improved since measures were implemented by the Food and Drug Administration (3). A recent review of dermatology-related CTs found a 45.6% representation of women and, specifically, found the expected representation in CTs for AD based on disease prevalence (2). The discrepancy between this information and the current study could be explained by the uneven gender distribution of respondents (only 21.1% male overall).
Another identified barrier to CTP was lower confidence in knowing where to find information on available eczema CTs. In fact, only 33% (n = 264) of all respondents felt that they were very/extremely confident. Although 1 previous study showed a majority (88%) of patients said they would value being informed about CTs by their healthcare provider (HCP), most heard about CTs through television commercials (53%), while only 19–21% found out through their HCP (5, 16). In the current study, 8.7% of patients said they get information about eczema CTs through their HCP. Despite patients wanting to hear about CTs from their provider, some research shows that television and radio commercials are still an effective way of increasing CT knowledge, with a 9% increase in knowledge of the term “randomized clinical trial” after a targeted media campaign (18).
While it is important to encourage participation, it is equally important to not advertise CTs as an alternative therapeutic route. Patients with a solid understanding of what benefits CTs may bring to them and/or others with eczema are more likely to be interested in CTP. For this reason, HCPs may have the best opportunity to educate and be a resource to their patients about CTs and their broader benefits, in turn building patient trust. HCPs and patient groups should consider elucidating CTs before patients even consider participation, as this lack of knowledge of the CT process and important terms could be critical barriers to participation and a contributor to misunderstanding the experimental nature of a CT. Patient trust in HCPs and medical researchers play a role in their willingness to participate in CTs. Although not included in this study’s models, previous work conducted by Johnson et al. (19) in 2023 identified having trust in the CT doctor(s)/site as the consideration with the largest proportion of high importance ratings among adult respondents.
Study strengths and limitations
Limitations of this study include potential recall and selection biases from NEA community members, including a predominantly female, white and urban/suburban population. Strengths include a large sample size obtained through diverse recruitment methods, including patients with all eczema types, allowing for a more holistic view of eczema CTP and generalizability to multiple eczema types. The inclusion of patient-reported data also gives a view of CT experience and personal considerations that cannot be collected through observational studies alone.
Conclusion
This cross-sectional study corroborates past studies’ findings of low eczema CTP rates and reveals a significant portion of eczema patients who are considering CTs with/without a previous attempt. Different diseases may have different needs for CTs as they are unique to patient populations. This study elucidates factors associated with eczema CT awareness, interest, and participation. While CT literacy was better among patients who expressed interest in CTs or had participated previously, certain CT terms were poorly understood by all respondents. Reducing barriers to participation, and an effort from all stakeholders to increase CT knowledge and awareness, may lead to increased CTP at a critical time when eczema treatment development is booming.
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
IRB approval status: Western Institutional Review Board Copernicus Group’s (WCG) IRB Affairs Department reviewed the study under the Common Rule and applicable guidance. They determined the study is exempt under 45 CFR § 46.104(d)(2), because the research only includes interactions involving educational tests, survey procedures, interview procedures, or observations of public behaviour; and any disclosure of the human subjects’ responses outside the research would not reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, educational advancement, or reputation.
REFERENCES
- Nephew LD. Accountability in clinical trial diversity: The buck stops where? EClinicalMedicine 2021; 36: 100906.
- Ding J, Zhou Y, Khan MS, Sy RN, Khosa F Representation of sex, race, and ethnicity in pivotal clinical trials for dermatological drugs. Int J Womens Dermatol 2021; 7: 428–434.
- Administration USD of H&. HSFAD, US Department of Health & Human Services; Food and Drug Administration. Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. PsycEXTRA Dataset. 1993. [accessed March 1 2022] Available from: https://www.fda.gov/media/75648/download.
- McCleary KK. The more than skin deep ”voice of the patient” report. [cited 2023 Jun 1]. Available from: https://www.morethanskindeep-eczema.org/uploads/1/2/5/3/125377765/mtsd_report_-_digital_file_1.pdf.
- Davis MM, Clark SJ, Butchart AT, Singer DC, Shanley TP, Gipson DS. Public participation in, and awareness about, medical research opportunities in the era of clinical and translational research. Clin Transl Sci 2013; 6: 88–93.
- Patel KR, Silverberg JI. Willingness to participate in atopic dermatitis studies and clinical trails. Dermatitis 2020; 31: e9–e11.
- Chu SH, Kim EJ, Jeong SH, Park GL. Factors associated with willingness to participate in clinical trials: a nationwide survey study. BMC Public Health 2015; 15: 10.
- Peay HL, Biesecker BB, Wilfond BS, Jarecki J, Umstead KL, Escolar DM, Tibben A. Barriers and facilitators to clinical trial participation among parents of children with pediatric neuromuscular disorders. Clin Trials 2018; 15: 139–148.
- van de Glind EM, Vural EM, Scholten E, Hooft L, Portegijs E, van Munster BC, et al. Barriers to and facilitators of participation of older adults in a placebo-controlled randomized clinical trial. J Am Geriatr Soc 2013; 61: 1421–1422.
- Ellis PM, Butow PN, Tattersall MH, Dunn SM, Houssami N. Randomized clinical trials in oncology: understanding and attitudes predict willingness to participate. J Clin Oncol 2001; 19: 3554–3561.
- Tohid H, Choudhury SM, Agouba S, Aden A, Ahmed LHM, Omar O, et al. Perceptions and attitudes to clinical research participation in Qatar. Contemp Clin Trials Commun 2017; 8: 241–247.
- Joshi V, Kulkarni AA. Public awareness of clinical trials: a qualitative pilot study in Pune. Perspect Clin Res 2012; 3: 125–132.
- USDA Economic Research Service. Rural-Urban Continuum Codes. 2020 [accessed 2022, April 22]. Available from: https://www.ers.usda.gov/dataproducts/rural-urban-continuum-codes.asp.
- R Core Team (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Hosmer, David W. and Lemeshow, Stanley. Applied logistic regression. John Wiley and Sons, 2000.
- Anderson A, Borfitz D, Getz K. Global public attitudes about clinical research and patient experiences with clinical trials. JAMA Netw Open 2018; 1: e182969.
- Lara PN Jr, Paterniti DA, Chiechi C, Turrell C, Morain C, Horan N, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol 2005; 23: 9282–9289.
- Mackenzie IS, Wei L, Rutherford D, Findlay EA, Saywood W, Campbell MK, Macdonald TM. Promoting public awareness of randomised clinical trials using the media: the “Get Randomised” campaign. Br J Clin Pharmacol 2010; 69: 128–135.
- Johnson JK, Loiselle A, Thibau IJ, Smith Begolka W. Factors related to eczema clinical trial participation among adult patients and caregivers. Contemp Clin Trials Commun 2023 33: 101138.