ORIGINAL REPORT
PsoBarrier EU study: a Multicentre, Cross-sectional Survey Investigating the Quality of Psoriasis Care in Four European Countries
Anna LANGENBRUCH1, Nicole MOHR1, Valerie ANDREES1, Ihno KESSENS1, Adam REICH2, Magdalena CZARNECKA-OPERACZ3, Luis PUIG4, Esteban DAUDEN5, Lars IVERSEN6 and Matthias AUGUSTIN1
1Institute for Health Services Research in Dermatology and Nursing (IVDP), University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany, 2Department of Dermatology, Institute of Medical Sciences, Medical College of Rzeszow University, Rzeszów, 3Department of Dermatology, Medical University of Poznan, Poznan, Poland, 4Department of Dermatology, Hospital de la Santa Creu I Sant Pau, Barcelona, 5Department of Dermatology, Hospital Universitario de la Princesa, Madrid, Spain and 6Department of Dermatology, Aarhus University Hospital, Aarhus, Denmark
Enhanced treatment options for psoriasis and growing use of guidelines increased the potential to better quality of psoriasis care in Europe. The aim of the PsoBarrier EU study is to compare the quality and processes of psoriasis care in four European countries with different healthcare systems, based on validated quality indicators. This cross-sectional survey was conducted in dermatology centres in Denmark, Germany, Poland and Spain on 1,304 patients, using standardized patient and physician questionnaires. Measured by quality of psoriasis care indicators, patients in Poland had the most critical outcomes, such as the highest disease severity (Psoriasis Area and Severity Index; PASI) and lowest health-related quality of life (Dermatology Life Quality Index; DLQI). This indicates differences in psoriasis care, with Polish participants experiencing more severe psoriasis and its consequences. Differences in the healthcare systems, which create barriers to accessing treatments, could explain variations in quality of care.
Key words: health services research; health care quality; psoriasis; quality of life.
SIGNIFICANCE
Analysis of healthcare quality in psoriasis in four European countries (Denmark, Germany, Poland and Spain) revealed marked differences in patient-relevant indicators, which may result from different healthcare systems. Lack of access to guideline-compliant treatment for psoriasis could be the major barrier explaining higher reductions in quality of life for patients with psoriasis in Poland. These data indicate variations in healthcare within the European Union, which should be the target for improvements.
Citation: Acta Derm Venereol 2023; 103: adv6532. DOI https://doi.org/10.2340/actadv.v103.6532.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jul 11, 2023; Published: Aug 31, 2023
Corr: Anna Langenbruch, Institute for Health Services Research in Dermatology and Nursing, University Medical Center Hamburg, Martinistraße 52, DE-20246 Hamburg, Germany. E-mail: a.langenbruch@uke.de
Competing interests and funding: LP received consulting fees from Abbvie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB. He received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Janssen, Lilly, Novartis, UCB. He received support for attending meetings and/or travel from Janssen and UCB. His institution received grants or contracts from Abbvie, Almirall, Amgen, Boehringer Ingelheim, Leo-Pharma, Lilly, Novartis, Pfizer, Sanofi, UCB. MA has served as advisor and/or paid speaker for and/or participated in research projects sponsored by Abbott/AbbVie, Almirall, Amgen, Beiersdorf, Biogen Idec, BMS, Boehringer Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Fresenius, Galderma, GSK, Hexal, Incyte, Janssen-Cilag, LEO Pharma, Lilly, Medac, Merck, MSD, Mylan, Novartis, Pfizer, Regeneron, Sandoz, Sanofi-Aventis, Stiefel, UCB and Xenoport. ED received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Almirall, Janssen-Cilag, Leo-Pharma, Novartis, Lilly, UCB, Boehringer-Ingelheim. He received support for attending meetings and/or travel from UCB, Lilly, Janssen, LEO Pharma. He participated on a Data Safety Monitoring Board or Advisory Board of Abbvie, Almirall, Janssen-Cilag, Novartis, Lilly, UCB, Brystol-Myers and Boehringer-Ingelheim. LI received grants or contracts from Eli Lilly, Janssen Cilag, Leo Pharma, Novartis, Regranion. He received consulting fees from AbbVie, BMS, Boehringer Ingelheim, Eli Lilly, Janssen Cilag, Leo Pharma, Novartis, Regranion, Samsung, Union Therapeutics UCB. He received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AbbVie, BMS, Boehringer Ingelheim, Eli Lilly, Janssen Cilag, Leo Pharma, Novartis, Samsung, UCB as well as support for attending meetings and/or travel from AbbVie, Eli Lilly, Janssen Cilag, Leo Pharma, Novartis, UCB. He participated on a Data Safety Monitoring Board or Advisory Board of BMS. AR received personal and institutional grants or contracts from Abbvie, Alvotech, Amgen, AnaptysBio, Argenx, Biothera, BMS, Celgene, Celltrion, Dermira, Galderma, Inflarx, Janssen, Kiniksa, Kymab, Leo Pharma, Novartis, Pfizer, Trevi Therapeutics, UCB. He received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Chema Rzeszow, Eli Lilly, Leo Pharma, Novartis, Sandoz, Takeda. He participated on a Data Safety Monitoring Board or Advisory Board of Abbvie, Galderma, Sandoz and Sanofi Aventis. AL, VA, NM, IK, MC-O have no conflicts of interest to disclose.
The study received financial support from Sandoz. Sandoz was not involved in study design, data collection, data analysis, interpretation of data, and writing of the paper.
INTRODUCTION
Psoriasis in adults has a prevalence of 1.07–3.46% in Western Europe, and is one of the most common immune-mediated disorders (1). Currently psoriasis is incurable and can affect not only physical health but also psychological well-being (2, 3), cause significant sleep problems (4) and impairments in health-related quality of life (HRQoL) (5, 6), noticeable direct costs of illness, work impairments and reduced productivity (7, 8).
Quality of psoriasis care can be measured using validated quality indicators. A set of such indicators was introduced 2009 (9) based on the German national S3 guideline (10). It constitutes of parameters of outcomes quality, such as clinical and patient-reported outcomes. HRQoL belongs to these quality indicators, since the concept takes into account that patients experience their health limitations and medical treatment differently (5). This subjectively different experience must be considered in medical decision-making, and thus also in quality of care indicators.
A series of nationwide studies in Germany over recent years have focused on psoriasis (PSO) care. These studies indicate the positive effects of treatment guidelines, since healthcare quality indicators had improved after the implementation of the German national S3 guideline (10, 11).
Regarding the European context, a few population-based multicentre studies on PSO and its burden exist. However, they focus mainly on patients with a particular severity grade, which impedes comparisons with the German data (12–17).
Since comparable data on quality of PSO care are lacking in Europe, the aim of this study was to generate data on PSO care in dermatological centres from four European countries (Denmark, Poland, Spain, and Germany). These countries were chosen since they represent healthcare systems that vary in the way they are organized, funded and managed (Table SI). The proportion of gross domestic product (GDP) that was spent on healthcare in 2019 is similar in Denmark, Germany, and Spain (10.0%, 11.7%, 9.1%) but markedly lower in Poland, at approximately 6.5% (18–21).
Not surprisingly, the national guidelines for psoriasis treatment in Spain, Germany, Denmark, and Poland have many similarities, as they are all related to the European Dermatology Forum (EDF) guideline (22), are evidence-based, and aim to provide healthcare professionals with recommendations for the diagnosis and management of psoriasis (10, 23–25). The main goals of psoriasis management in all guidelines are to reduce symptoms, improve quality of life, and prevent complications. This typically involves topical and systemic therapies (or a combination of both), lifestyle modifications, psychological interventions, and patient education. In addition, a global report published by the World Health Organization (WHO) called for government action to improve the situation of people with psoriasis, to ensure that they receive early diagnosis and appropriate treatment, and to limit stigmatization (26).
MATERIALS AND METHODS
Study design
The PsoBarrier-EU study is a multicentre cross-sectional survey study conducted to gain information on routine care in adult patients with psoriasis. Data were collected via dermatologists in Denmark, Germany, Poland, and Spain. The study included statements from the dermatologists about their patients, as well as self-reports from the patients.
Participants
Patients were enrolled from January 2016 to December 2017 in Germany, from August 2016 to July 2018 in Poland, from August 2016 to November 2018 in Spain, and from June 2018 to February 2020 in Denmark. Inclusion criteria were confirmed psoriasis vulgaris according to the clinical diagnosis, a minimum age of 18 years and speaking the main language of the country in order to answer the questionnaire. The patient and their treating dermatologist were each asked to complete a questionnaire to obtain a subjective and clinical perspective (respectively) on the patient’s situation.
Recruitment
The recruitment of study sites was organized by a local coordinator in each country. The participating dermatologists were encouraged to ask each adult patient with PSO to participate in the survey study, as they presented to their clinic. The aim was to obtain a sample as reflective as possible of patients with PSO in the respective country treated by dermatologists.
Materials
The patient questionnaire comprised 15 pages, and the questionnaire for the dermatologist comprised 6 pages. Both versions were pilot-tested in a single centre (Hamburg) before large-scale use. The original questionnaire was in German, but was translated by professional translators into the language of the participating countries.
The questions referred to a broad spectrum of clinical and patient-reported outcomes (Appendix S1).
Quality of care indicators
Only one set of quality indicators for psoriasis healthcare assessments was published in the literature. These were agreed within the framework of an expert consensus in Germany (9):
- Severity (Psoriasis Area and Severity Index (PASI) (27) and PASI > 20)
- HRQoL (Dermatology Life Quality Index (DLQI) (28) and DLQI >10)
- Systemic therapy in the last five years
- Hospital treatment in the last five years
- Absence from work due to PSO in the last 12 months.
Since the guideline recommendations for psoriasis in the four countries investigated are largely based on the European guideline, and since the published indicators correspond with the EU guideline, the same set of quality indicators was applied in all countries.
Statistical analysis
All data were described using standard statistical parameters: absolute and relative frequencies for categorical data; mean value and standard deviation (SD) for continuous data. χ2 tests for independence were performed to compare the four countries in terms of frequency differences in disease- and quality-of-care-related aspects (e.g. in terms of severe HRQoL limitations, high severity of PSO, PSO-related absence from work). One-way analyses of variance (ANOVA) were calculated to compare the mean differences; for example, in disease-related aspects and quality of care criteria (mean DLQI, mean PASI) between the four countries. In case the Levene’s test indicated heterogeneity of variance, Welch ANOVA was interpreted. The α-level was set at 5%. Missing values were not replaced. All statistical analyses were performed using SPSS Statistics v.27 (IBM, Armonk, NY, USA).
RESULTS
Characterization of the participating patients
In total, 1,304 patients were included in the PsoBarrier EU study. Per country, the study received 497 complete questionnaire sets from Germany, 511 from Poland, 161 from Denmark, and 135 from Spain. The number of study sites (usually one responsible practitioner in a dermatologist office) ranged from 5 in Denmark to 6 in Spain, to 8 in Poland and to 29 study sites in Germany. Fewer women than men participated in all four countries: 41.4% in Germany, 34.1% in Poland, 31.4% in Denmark, and 38.0% in Spain. The mean age was similar in all countries (from 46 years in Poland to 51 years in Spain). A body mass index (BMI) of > 30 kg/m2 was more frequent in Denmark (38.5%) than in the other countries (χ2 (3) = 8.3, p < 0.05). Fewer patients in Spain reported being in employment (χ2 (3) = 18.0, p < 0.001, Table I). The countries differed in the influence their PSO had on career-related aspects such as keeping the job (χ2 (3) = 45.7, p < 0.001). Patients reported more often in Spain and Poland that PSO had a negative impact on their career (Table II).
Psoriasis patient journey
When the first symptoms appeared, most patients in Denmark and Spain consulted a general practitioner (GP). By contrast, in Germany and Poland most patients first visited a dermatologist (Fig. 1). More than 75% of the patients reported that their PSO was detected during their first visit. The mean time between the first skin changes and diagnosis differed between countries (Welch’s F (3, 257.1) = 10.0, p < 0.001). It was shortest in Poland (0.9 ± 2.2 years). At the time of diagnosis most patients had a mild or moderate PSO. The proportion of patients with severe PSO at first diagnosis was highest in Spain (26.1%, χ2 (3) = 16.9, p < 0.01). The most common first therapy prescribed was topical therapy in all countries. Most patients have changed their therapy since the first diagnosis, Danish patients reported to have changed their therapy most often (6.0 ± 6.9 times, Welch’s F (3, 288.7) = 5.6, p < 0.01).
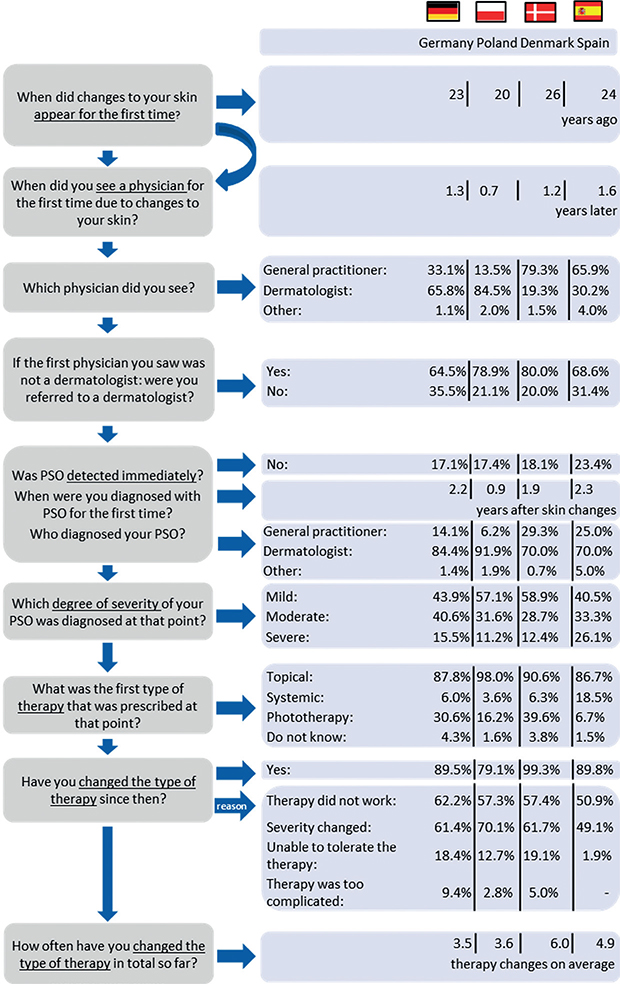
Fig. 1. Journey through psoriasis care. PSO: psoriasis. Germany n=497; Poland n=511; Denmark n=161; Spain n=135, missing values vary per item.
Distance and time-related barriers to seeing a dermatologist
The waiting time for the first appointment with the current dermatologist differed between the countries (χ2 (3) = 178.0, p < 0.001). It was longer than two months for 41.6% of the patients in Denmark, 37.8% in Spain, 6.4% in Germany, and 4.8% in Poland. Willingness to wait for a PSO specialist appointment differed between the countries (Welch’s F (3, 299.6) = 61.6, p < 0.001). Patients reported being willing to wait 2.0 ± 1.4 weeks in Poland, 3.4 ± 2.4 weeks in Germany, 3.9 ± 2.8 weeks in Denmark, and 4.5 ± 3.9 weeks in Spain. The distance to the current dermatologist differed (Welch’s F (3, 313.4) = 34.4, p < 0.001). It was longest in Denmark, with a mean length of 53.9 ± 52.6 km (median 40.0 km), followed by Poland (35.7 ± 49.8 km, median 15.0 km), Spain (19.3 ± 29.4 km, median 9.0 km) and Germany (18.2 ± 21.8 km, median 10.0 km). The maximum distance that patients would be willing to travel to the dermatologist also differed (Welch’s F (3, 247.4) = 10.0, p < 0.001). It was also longest in Denmark, at 99.8 ± 115.2 km (median 70.0 km), and shortest in Germany, at 50.4 ± 59.1 km (median 30.0 km).
Current therapy
The most common current therapies (except for topical treatment) according to the physician were non-biological systemic antipsoriatics in Germany (39.7%), phototherapy in Poland (31.5%), biologics in Denmark (53.8%) and Spain (65.6%) (Table III).
Additional support
The proportion of patients who reported ever having received psychological or psychotherapeutic help for the emotional burden caused by their PSO differed between the countries (χ2 (3) = 37.5, p < 0.001). It was most often experienced by Spanish patients (23.5%), followed by Poland (9.7%), Germany (8.8%) and Denmark (2.5%). The participation in patient training classes differed between the countries (χ2 (3) = 19.9, p < 0.001). It was most often reported in Denmark with 11.4% of the patients, followed by Germany (10.0%), Spain (7.5%), and Poland (4.0%).
Treatment burden and treatment satisfaction
Patients from Poland reported more often than patients from the other countries that more than 30 minutes are required for their daily skin treatment (χ2 (3) = 232.0, p < 0.001, Table SII). In addition, more patients from Poland largely or completely agreed with the statement that their treatment is a burden to them (χ2 (3) = 88.3, p < 0.001, Table SIII).
The mean rating of treatment of the last 12 months (scale from 1 “very satisfied” to 4 “very dissatisfied”) differed between the countries (Welch’s F (3, 400.4) = 27.3, p < 0.001). Patients were most satisfied with their treatment in Denmark and Spain (1.4 ± 0.7 and 1.6 ± 0.8) and least satisfied in Poland (2.0 ± 0.8) (2 = ”rather satisfied”). The proportion of patients who rated the PSO care as at least “good” also differed (χ2 (3) = 54.9, p < 0.001). It was highest in Spain (90.9%) and Denmark (86.7%), and smaller in Germany (68.5%) and Poland (65.1%).
Quality of psoriasis care according to the German consensus on quality indicators (9)
The mean PASI and the proportion of patients with PASI > 20 was highest in Poland and lowest in Denmark and Spain. The mean DLQI and the proportion of patients with DLQI > 10 was highest in Poland and lowest in Denmark. The proportion of patients with at least one hospital stay due to PSO was highest in Poland and lowest in Denmark. The proportion of patients with at least one day absent from work due to PSO was highest in Poland and lowest in Spain. The proportion of patients who reported having been treated with at least one systemic agent in the last five years was lowest in Poland and highest in Spain (Table IV).
| Germany | Poland | Denmark | Spain | p - value* | |
| PASI, (n) mean ± SD | (492) 6.9 ± 8.4 | (504) 10.6 ± 8.3 | (159) 4.1 ± 6.0 | (130) 3.8 ± 5.1 | 0.000 |
| DLQI, (n) mean ± SD | (491) 6.1 ± 6.8 | (500) 11.5 ± 8.3 | (158) 4.3 ± 5.2 | (131) 6.3 ± 6.7 | 0.000 |
| PASI > 20, (n) % | (45) 9.1 | (69) 13.7 | (4) 2.5 | (1) 0.8 | 0.000 |
| DLQI > 10, (n) % | (105) 21.4 | (257) 51.4 | (19) 12.0 | (30) 22.9 | 0.000 |
| Systemic therapy in the last 5 years (at least once), (n) % | (285) 61.2 | (272) 54.1 | (113) 74.8 | (115) 87.8 | 0.000 |
| Hospital treatment in the last 5 years (at least once), (n) % | (50) 14.0 | (290) 62.0 | (7) 5.1 | (11) 12.8 | 0.000 |
| Absence from work in the last 12 months due to PSO (% at least 1 day, only patients with a job), (n) % | (20) 7.1 | (91) 33.2 | (9) 8.6 | (3) 5.7 | 0.000 |
| Germany total n = 497; Poland total n = 511; Denmark total n = 161; Spain total n = 135, missing values vary per item. | |||||
| PASI: Psoriasis Area and Severity Index (possible range from 0 = no severity to 72 = maximum severity), DLQI: Dermatology Life Quality Index (possible range from 0 = no health-related quality of life (HRQoL) impairments to 30 = maximum HRQoL impairments). | |||||
| *Differences between the countries according to Welch analysis of variance (ANOVA) or χ². | |||||
DISCUSSION
The article outlines psoriasis care in the four European countries Denmark, Germany, Poland and Spain with a special focus on the patient perspective. The PSO care quality indicators developed by a German consensus (9) indicate that healthcare in Poland is worse than in Denmark, Spain and Germany.
The data collection was designed to obtain a sample of typical patients with PSO under dermatological care in each country. The mean age and the gender proportions are similar to previous publications from routine care in these countries, suggesting external validity of our cohort (14, 16, 17).
The selected countries reflect different healthcare systems in Europe. All four countries have healthcare systems covering access to care for the majority of their population. While there is no gatekeeping system in Germany, for patients in Poland, Denmark, and Spain, access to secondary care is only possible after a referral by the GP. However, the gatekeeping system in Poland was introduced only recently. Consequently, most patients in Poland and in Germany consulted a dermatologist directly regarding their first skin changes. Most patients in Denmark and Spain consulted the GP first.
The number of dermatologists per 1 million inhabitants in 2020 was highest in Germany (n = 75) (29) followed by Poland (n = 69) (30), Spain (n = 46, year 2021) (31) and Denmark (n = 41) (32). The results of the current study reflect these differences, showing that waiting and travel times in Denmark are highest. It is notable that only 4.8% of the Polish patients state that their waiting time for a first appointment was longer than 2 months.
The data reveal some differences between the populations with regard to lifestyle parameters. For example, in Denmark fewer patients report that they smoke, which might indicate a healthier lifestyle compared with the other countries. Nevertheless, the proportion of smokers in all countries among people with PSO is higher than in the general population (2017: Germany 34.1% vs 18.8%, Denmark 23.1% vs 16.9%, Spain 30.2% vs 22.1%; Poland 40.3% vs 22.7%; comparative data from the Organisation for Economic Co-operation and Development (OECD)) (33).
Regarding the patient pathway, it is noticeable that patients from Poland did not show a higher severity of PSO at the time of initial diagnosis. Regarding treatment, significant differences can be illustrated. In Poland, the proportion of patients treated with phototherapy is higher than in the other countries, whereas the proportion of those treated with biologics is smaller. The strongest influence on occupation is reported by Spanish and Polish patients. One aspect that could play a role, especially in warmer regions such as Spain, is the visibility of the disease, which is more often hidden by clothes in cooler regions. In case of a stigmatizing assessment of the visible disease manifestations by the society or the patient, this could lead to emotional stress. This relates to studies from Poland and Germany, which recently also identified stigmatization as a severe problem among patients with PSO (34, 35). The (according to the current study results) higher number of patients seeking psychotherapeutic help in Spain could also be an indication of emotional burden caused by stigmatization. Given the substantial burden on patients with psoriasis, it is surprising that patients from Germany, Poland and Denmark in particular rarely report ever having received psychological support. A systematic review found promising effects of psychological interventions in patients with psoriasis, including cognitive behavioural therapy, mindfulness-based interventions and motivational interviewing (36). This indicates promising effects of a holistic approach in the treatment of patients with psoriasis.
With regard to healthcare quality indicators, patients from Poland reported the most critical results, such as the lowest HRQoL. Moreover, patients recruited in Poland display a higher severity of PSO. Both are comparable to the recent study on patients with psoriasis in Poland by Purzycka-Bohdan et al. (16). The fact that Polish patients are treated in well-trained, qualified centres and receive dermatological specialist treatment reduces the probability of minor quality of care due to lack of physician experience. Satisfaction with the medical consultation could be one reason why the Polish patients show a moderate level of satisfaction with their therapy that is similar to the German patients, despite poorer medical care. It is evident that there is a limited reimbursement of modern drugs in Poland, whereas a large number of biological drugs can be assessed by the patients in the other countries. One reason for higher satisfaction in Denmark and Spain could be the referral system, which has a stronger gatekeeping effect in these countries compared with Germany.
Strengths and limitations
Since all data were obtained before the onset of the SARS-CoV-2 (COVID-19) pandemic, the bias of different SARS-CoV-2 epidemiology between countries has been avoided.
The selection of patients presenting to a dermatologist by referral from the GP is probably higher and might lead to a certain bias. With the local coordinators in the recruitment process, it was not possible to achieve regionally evenly located study sites in each country. This should be taken into account when discussing the representativeness of data. Nevertheless, it should be emphasized that the pattern of patient recruitment for this study was the same in all countries. However, it cannot be excluded that more specialized centres participated, and therefore a disproportionate number of severely affected patients and patients with an above-average treatment were recruited. Furthermore, data collection did not take place at exactly the same time in all countries. Conditions could therefore have changed in the meantime, in particular the availability of innovative drugs. Implications for future research and implications for practice can be found in Appendix S2.
Conclusion
The systematic collection of comparable primary data from four European countries with different healthcare systems provides evidence of differences in healthcare between these countries. Overall, the data on quality of care indicators suggest that Polish participants are more burdened by psoriasis and its consequences than patients in other countries, despite being rather satisfied with the healthcare they receive. In addition, various barriers were also identified in the other countries, which differ in nature and extent. Differences in the healthcare system, leading to limitations in access to certain treatment options, could be one reason why patients are affected differently by psoriasis depending on the country.
ACKNOWLEDGEMENTS
The authors thank all patients and physicians involved in this study and the Scientific Communication Team of the IVDP, in particular Sara Tiedemann and Mario Gehoff, for copyediting.
All participants provided written informed consent. A first approval of the ethics commission of the local medical association in Hamburg, Germany (PV4996) was obtained. Consecutive local approvals were obtained from ethics commissions in Denmark, Poland, and Spain.
REFERENCES
- Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 2020; 369: m1590.
- Ferreira BIRC, Da Abreu JLPC, Reis JPGD, Da Figueiredo AMC. Psoriasis and associated psychiatric disorders: a systematic review on etiopathogenesis and clinical correlation. J Clin Aesthet Dermatol 2016; 9: 36–43.
- Singh S, Taylor C, Kornmehl H, Armstrong AW. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 425–440.e2.
- Halioua B, Chelli C, Misery L, Taieb J, Taieb C. Sleep disorders and psoriasis: an update. Acta Derm Venereol 2022; 102: adv00699.
- Augustin M, Radtke MA. Quality of life in psoriasis patients. Expert Rev Pharmacoecon Outcomes Res 2014; 14: 559–568.
- Korte J de, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc 2004; 9: 140–147.
- Jungen D, Augustin M, Langenbruch A, Zander N, Reich K, Strömer K, et al. Cost-of-illness of psoriasis – results of a German cross-sectional study. J Eur Acad Dermatol Venereol 2018; 32: 174–180.
- Mustonen A, Mattila K, Leino M, Koulu L, Tuominen R. Psoriasis causes significant economic burden to patients. Dermatol Ther (Heidelb) 2014; 4: 115–124.
- Radtke MA, Reich K, Blome C, Kopp I, Rustenbach SJ, Schäfer I, et al. Evaluation of quality of care and guideline-compliant treatment in psoriasis. Development of a new system of quality indicators. Dermatology (Basel) 2009; 219: 54–58.
- Nast A, Altenburg A, Augustin M, Boehncke W-H, Härle P, Klaus J, et al. Deutsche S3-Leitlinie zur Therapie der Psoriasis vulgaris, adaptiert von EuroGuiDerm - Teil 1: Therapieziele und Therapieempfehlungen. J Dtsch Dermatol Ges 2021; 19: 934–951.
- Augustin M, Eissing L, Langenbruch A, Enk A, Luger T, Maaßen D, et al. The German National Program on Psoriasis Health Care 2005-2015: results and experiences. Arch Dermatol Res 2016; 308: 389–400.
- Daudén E, Pujol RM, Sánchez-Carazo JL, Toribio J, Vanaclocha F, Puig L, et al. Demographic characteristics and health-related quality of life of patients with moderate-to-severe psoriasis: the VACAP study. Actas Dermosifiliogr 2013; 104: 807–814.
- Tribó MJ, Turroja M, Castaño-Vinyals G, Bulbena A, Ros E, García-Martínez P, et al. Patients with moderate to severe psoriasis associate with higher risk of depression and anxiety symptoms: results of a multivariate study of 300 Spanish individuals with psoriasis. Acta Derm Venereol 2019; 99: 417–422.
- Ruiz Genao DP, Carretero G, Rivera-Diaz R, Carrascosa JM, Sahuquillo-Torralba A, Herrera-Acosta E, et al. Differences in epidemiology, comorbidities and treatment choice between plaque psoriasis and pustular psoriasis: results from the BIOBADADERM registry. Br J Dermatol 2022; 187: 817–820.
- Loft ND, Egeberg A, Rasmussen MK, Bryld LE, Gniadecki R, Dam TN, et al. Patient-reported outcomes during treatment in patients with moderate-to-severe psoriasis: a Danish nationwide study. Acta Derm Venereol 2019; 99: 1224–1230.
- Purzycka-Bohdan D, Kisielnicka A, Zabłotna M, Nedoszytko B, Nowicki RJ, Reich A, et al. Chronic plaque psoriasis in Poland: disease severity, prevalence of comorbidities, and quality of life. J Clin Med 2022; 11: 1254.
- Schwarz CW, Loft N, Rasmussen MK, Nissen CV, Dam TN, Ajgeiy KK, et al. Predictors of response to biologics in patients with moderate-to-severe psoriasis: a Danish nationwide cohort study. Acta Derm Venereol 2021; 101: adv00579.
- OECD/European Observatory on Health Systems and Policies, editor. Denmark: Country Health Profile 2021, State of Health in the EU. Paris/Brussels: OECD Publishing; 2021.
- OECD/European Observatory on Health Systems and Policies, editor. Germany: Country Health Profile 2021, State of Health in the EU. Paris/Brussels: OECD Publishing; 2021.
- OECD/European Observatory on Health Systems and Policies, editor. Poland: Country Health Profile 2021, State of Health in the EU. Paris/Brussels: OECD Publishing; 2021.
- OECD/European Observatory on Health Systems and Policies, editor. Spain: Country Health Profile 2021, State of Health in the EU. Paris/Brussels: OECD Publishing; 2021.
- Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol 2020; 34: 2461–2498.
- Danish Health Authority. National clinical guideline on psoriasis: quick guide; 2016 [cited 2023 May 10]. Available from: https://www.sst.dk/en/English/publications/2016/National-clinical-guideline-on-psoriasis.
- Reich A, Adamski Z, Chodorowska G, Kaszuba A, Krasowska D, Lesiak A, et al. Psoriasis. Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Part 1. pd 2020; 107: 92–108.
- Carrascosa JM, Puig L, Belinchón Romero I, Salgado-Boquete L, Del Alcázar E, Andrés Lencina JJ, et al. Actualización práctica de las recomendaciones del Grupo de Psoriasis de la Academia Española de Dermatología y Venereología (GPS) para el tratamiento de la psoriasis con terapia biológica. Parte 1. “Conceptos y manejo general de la psoriasis con terapia biológica”. Actas Dermosifiliogr 2022; 113: 261–277.
- WHO. Global report on psoriasis. Geneva: World Health Organization; 2016.
- Fredriksson T, Pettersson U. Severe psoriasis – oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
- Bundesärztekammer. Ergebnisse der Ärztestatistik zum 31.12.2020: Tabelle 3: Ärztinnen/Ärzte nach Bezeichnungen und Tätigkeitsarten. Available from: https://www.bundesaerztekammer.de/baek/ueber-uns/aerztestatistik/aerztestatistik-2020.
- Naczelna Izba Lekarska. Central Register of Physicians [cited 2020 Mar 3]. Available from: https://nil.org.pl/uploaded_files/1583227918_zestawienie-nr-04.pdf.
- Barber-Pérez P, González López-Valcárcel B. Informe Oferta-Necesidad de Especialistas Médicos 2021-2035: EcoSalud. Universidad de Las Palmas de Gran Canaria; 2022. Available from: https://www.sanidad.gob.es/areas/profesionesSanitarias/profesiones/necesidadEspecialistas/docs/2022Estudio_Oferta_Necesidad_Especialistas_Medicos_2021_2035V3.pd.f
- Danish Health Data Agency. Arbejdsstyrken af sundhedsuddannede: Opgørelserne viser arbejdsstyrken af antal sundhedsuddannede fordelt på år, faggrupper, herkomst, speciale, alder og køn, uddannelsesland, brancher og sektorer, region (2000-2020); 2020. Available from: https://www.esundhed.dk/Emner/Beskaeftigede-i-sundhedsvaesnet/Arbejdsstyrken-af-sundhedsuddannede.
- OECD. Health at a Glance 2019: OECD indicators. Paris: OECD; 2019.
- Jankowiak B, Kowalewska B, Krajewska-Kułak E, Khvorik DF. Stigmatization and Quality of Life in Patients with Psoriasis. Dermatol Ther (Heidelb) 2020; 10: 285–296.
- Sommer R, Topp J, Mrowietz U, Zander N, Augustin M. Perception and determinants of stigmatisation of people with psoriasis in the German population. J Eur Acad Dermatol Venereol 2020; 34: 2845–2855.
- Qureshi AA, Awosika O, Baruffi F, Rengifo-Pardo M, Ehrlich A. Psychological therapies in management of psoriatic skin disease: a systematic review. Am J Clin Dermatol 2019; 20: 607–624.