QUIZ SECTION
Polymorphic Skin Eruption in a Middle-Aged Man: A Quiz
Silvia CATAPANO1,2, Niccolò GORI1,2*, Alessandro DI STEFANI1,2 and Ketty PERIS1,2
1Dermatologia, Dipartimento di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Largo A. Gemelli 8, IT-00168 Rome, Italy and 2UOC di Dermatologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario A. Gemelli – IRCCS, Rome, Italy. E-mail: niccolo.gori@unicatt.it
Citation: Acta Derm Venereol 2023; 103: adv00880. DOI https://doi.org/10.2340/actadv.v103.6548.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Published: Mar 8, 2023
INTRODUCTION
A 42-year-old man presented with a 2-year history of chronic-relapsing, asymptomatic, skin lesions. Physical examination revealed reddish-brown papules and nodules on his trunk and lower and upper limbs with involvement of the palms. Some of the papules showed central necrosis (Fig. 1A). In addition, in the previous few weeks, ery-thematous patches developed, varying in size and shape, with a scaly surface, on his trunk and upper limbs (Fig. 1B). Histological examination of a papule on the right leg revealed a superficial focally band-like dermal lymphocytic infiltrate with scattered CD30+ large cells (Fig. 2A). Skin biopsy of an erythematous patch of the abdomen showed a slight patchy-lichenoid lymphocytic infiltrate with focal epidermotropism of solitary atypical lymphocytes (Fig. 2B).
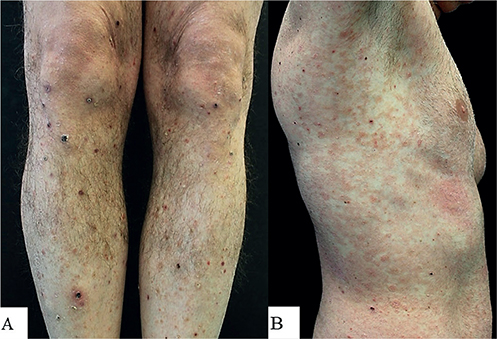
Fig. 1. Clinical image of (A) papulonodular skin lesions on lower limbs with central necrosis, and (B) erythematous small and confluent patches, with scaly surface, mainly distributed on the trunk.
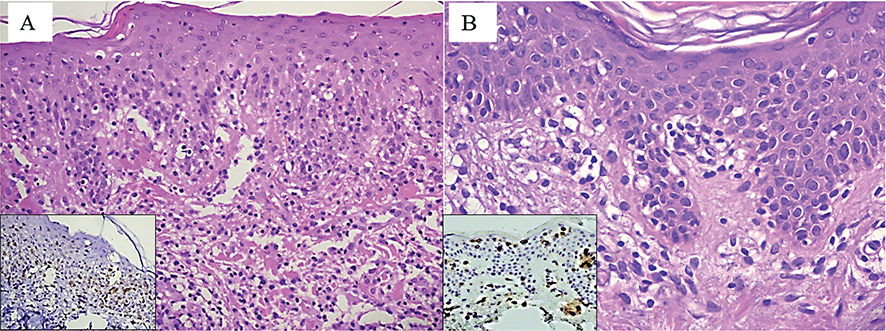
Fig. 2. Histological image, showing (A) a band-like dermal lymphocytic infiltrate with focal epidermotropism and scattered CD30+ cells (inset); (B) intraepidermal atypical lymphocytes aligned along the basal layer. (A, B: haematoxylin and eosin staining, original magnification ×100 and ×200, respectively; Insets (A and B): immunohistochemistry anti-CD30 and anti-CD4.
The patient was in overall good health, and routine blood examination resulted within the normal limits.
What is your diagnosis? See next page for answer.
ANSWERS TO QUIZ
Polymorphic Skin Eruption in a Middle-aged Man: A Commentary
Diagnosis: Lymphomatoid papulosis associated with mycosis fungoides
According to the World Health Organization (WHO) classification of tumours of haematopoietic and lymphoid tissues (1, 2), lymphomatoid papulosis (LyP) is included in the group of “CD30+” cutaneous lymphoproliferative disorders. It is a chronic relapsing, self-healing, papulonodular skin eruption, showing histological features of a cutaneous skin cell lymphoma. Notably, in 10–40% of cases, LyP is preceded, simultaneous with, or followed by, a second type of haematological malignancy (HM) (2). Recently, Melchers et al. (3) evaluated the frequency of associated HM in 504 patients affected by LyP, revealing an overall prevalence of 15.5%, with mycosis fungoides (MF) (6.1%) and anaplastic large cell lymphoma (5.8%) being the most frequently reported associated HM. In addition, the authors reported an association with other types of HM, including Hodgkin lymphoma, different B-cell neoplasms and myeloid leukaemias, in 3.8% of cases (3). The pathogenetic mechanism underlying the association of LyP with a second neoplasm are unknown. Notably, identical TCR gene rearrangements and oncogenic mutations were found in distinctive lesions of LyP and MF in the same patients, suggesting that both disorders could arise from a common lymphoid progenitor (4). Histologically LyP displays a highly polymorphic pattern, with 5 main pathological subtypes being described (A, B, C, D and E) as well as several other rarer variants. All of these subtypes can be detected during the course of disease in distinct histological specimens taken from the same patient, and occasionally features related to 2 or more variants can be detected in the same skin lesion. In the current case, histological examination revealed the simultaneous presence of features related to type B (MF-like) subtype, such as epidermotropic infiltrate and band-like dermal infiltrate. A few studies have suggested that subtypes B might be associated with a higher risk of developing a second malignancy, despite the fact that this observation was not confirmed by other researchers (2, 5). As known, in the later stage, patients with MF can occasionally develop papules and nodules with a morphological change of lymphocytes from small/medium to large dimension with possible expression of CD30 (6).
Therefore, prior to the occurrence of a papule/nodular eruption in a patient affected by MF, differential diagnosis between LyP and large cell transformation MF may be challenging and require an accurate clinical/pathological correlation. Notably, in the current patient the previous appearance of LyP lesions helped in making a confident diagnosis of the associated MF, although the histological pattern of the latter was not specific for cutaneous lymphomas.
Treatment of LyP is based on a wait-and-see approach, as well as local and systemic treatment, such as topical corticosteroids, psoralen plus ultraviolet A (PUVA), methotrexate, bexarotene or brentuximab, according to the number, size, morphology and distribution of the lesions and the disease course (2). Furthermore, it is important to perform periodic follow-up visits in order to diagnose any secondary lymphomas at an early stage.
REFERENCES
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, Jaffe ES. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019; 134: 1112.
- Martinez-Cabriales SA, Walsh S, Sade S, Shear NH. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol 2020; 34: 59–73.
- Melchers RC, Willemze R, Bekkenk MW, de Haas ERM, Horvath B, van Rossum MM, et al. Frequency and prognosis of associated malignancies in 504 patients with lymphomatoid papulosis. J Eur Acad Dermatol Venereol 2020; 34: 260–266.
- Wobser M, Roth S, Appenzeller S, Kneitz H, Goebeler M, Geissinger E, et al. Oncogenic mutations and gene fusions in cd30-positive lymphoproliferations and clonally related mycosis fungoides occurring in the same patients. JID Innov 2021; 1: 100034.
- Rongioletti F. Frequency, risk factors and prognosis of systemic haematologic malignancies, cutaneous and other neoplasms in lymphomatoid papulosis: where are we now? J Eur Acad Dermatol Venereol 2020; 34: 216–217.
- Nakahigashi K, Ishida Y, Matsumura Y, Kore-Eda S, Ohmori K, Fujimoto M, et al. Large cell transformation mimicking regional lymphomatoid papulosis in a patient with mycosis fungoides. J Dermatol 2008; 35: 283–288.